Introduction
The incidence of ischemic heart disease is
increasing yearly, and heart failure, as a resulting condition, is
a worldwide problem that seriously threatens the survival and
quality of life of the global population (1). Due to the minimal regenerative
capacity of the adult heart, it forms scar tissue to replace the
contractile cardiomyocytes upon injury, eventually leading to heart
failure (2). The only feasible
treatment for patients with end-stage heart failure is heart
transplantation, but this is not widely available due to the
limited number of donor organs. At present, myocardial cell
transplantation is a popular topic of study in the treatment of
ischemic heart disease. Following myocardial cell transplantation,
the development of myocardial tissue engineering has generated new
possibilities for the treatment of ischemic heart disease.
Myocardial tissue engineering aims to reconstruct
ideal myocardial tissue by combining cells with scaffolding
polymers to replace and repair the damaged myocardium (3–8). It
involves 3 stages: Isolation of the donor cells; development of the
scaffold material; and construction of the engineered myocardial
tissue. The source and species of seed cells are the key factors in
myocardial tissue engineering. At present, there are a number of
data concerning heart autologous stem cells, pluripotent stem
cells, embryonic stem cells, skeletal myoblasts and bone marrow
mononuclear cells (BMMSCs) (9–11).
Of these cell types, BMMSCs are widely used for cardiac repair due
to their easy accessibility and availability. For scaffold design,
the pore size, distribution and interconnectivity are critical
factors that determine mass transfer of oxygen and nutrients to
support effective vascular ingrowth within scaffolds.
Polylactic-co-glycolic acid (PLGA) scaffolds possess high porosity,
and good biocompatibility and biodegradability (12), all of which assist to induce tissue
formation through cell migration and nutritional diffusion.
At present, there are two predominant methods used
for myocardial tissue engineering. The first method involves
directly injecting cardiomyocytes or cardiac-like cells into the
heart muscle. A number of studies have confirmed that BMMSCs
implantation may induce cardiac regeneration and improve cardiac
function through myocardial angiogenesis (13–15).
However, cell implantation studies are usually limited by
restricted cardiomyogenic potential and low cell survival (16–19).
The second method involves transplantation of three-dimensional
heart grafts. Li et al (20) reconstructed myocardial cells in
three-dimensional structures using a preformed biodegradable
gelatin mesh. A previous study has suggested that cell sheet
transplantation may treat heart failure in animal models (21). The present study aimed to construct
engineered myocardial tissues in vitro using a PLGA scaffold
and cardiomyocyte-like cells derived from BMMSCs, which may support
the endogenous ability of induced cells to form a cardiac
tissue-like structure.
Materials and methods
Isolation and culture of BMMSCs
A total of 40 male Sprague-Dawley rats (age,
4-weeks-old;) were purchased from the Laboratory Animal Center of
Fourth Military Medical University (Xi'an, China). The housing
temperature ranged between 18 and 26°C, the humidity ranged between
40 and 70%. All rats had ad libitum access to food and
water, with a 12-h light/dark cycle. All rats were anesthetized and
sacrificed by cervical dislocation. The femurs and tibias of rats
were removed under sterile conditions. The marrow cavities were
washed with Dulbecco's modified Eagle's medium (DMEM; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), and the mixed suspension
was added slowly to the Percoll® solution (1.073 g/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for centrifugation
at 2,000 × g for 20 min at room temperature. The enriched cells in
the middle layer were collected and mixed with incomplete culture
medium for centrifugation at 1,500 × g for 15 min at room
temperature. Following removal of the supernatant, the cells were
re-suspended with complete DMEM-low glucose medium supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 µg/ml streptomycin, 100 U/ml
penicillin, 10 mmol/l HEPES and 300 mg/l L-glutamine, and then
inoculated in a 25 cm2 culture flask at 37°C in a
humidified atmosphere of 5% CO2. The non-adherent cells
were discarded after 3 days. The culture medium was changed every 3
days. When the cells reached 80% confluence, the cells were
digested with 2.5 g/l trypsin and passaged at a 1:2 ratio. The
study was approved by the Ethical Review Committee of Shaanxi
Provincial People's Hospital (Xi'an, China). All procedures
involving animals were in accordance with the ethical standards of
the Institutional Research Committee of Shaanxi Provincial People's
Hospital.
Induction and differentiation of
BMMSCs by 5-azacytidine (5-aza)
BMMSCs at third passage were induced using complete
DMEM-low glucose medium containing 10 µmol/l 5-aza (Sigma-Aldrich;
Merck KGaA). After 24 h, the induction medium was removed and the
medium was changed to complete DMEM-low glucose medium without
5-aza at room temperature. The medium was changed every 3 days.
After 4 weeks of culture, the cells were prepared for subsequent
experiments.
Scaffolds preparation
The PLGA scaffolds consisted of 50% polylactic acid
and 50% polyglycolic acid. The porous microstructure was prepared
by a particulate extraction method with particle diameter of
150–200 µm, as described previously (22), and the porosity was 90%. The PLGA
polymer was cut into squares (10×10×1 mm). Co60
irradiation was used to sterilize the PLGA scaffolds prior to cell
inoculation, and then the scaffolds were soaked in PBS for 1 h and
in DMEM for 1 h, and finally for the next step of the experiment
after water drainage.
Detection of cell adhesion rate
A total of 12 pieces of spare PLGA scaffolds were
placed in a 24-well culture plate. Then, 1 ml cardiomyocyte-like
cells suspension (1×109 cells/ml) was added to each of
the PLGA scaffolds. Following incubation at 37°C in a
CO2 incubator for 4, 12, 24 and 48 h, PBS was added
slowly to flush non-adherent cells. This step was repeated twice.
The cells were digested by 2.5 g/l trypsin and then counted using a
counting board under optical inverted phase contrast microscope
with ×100 magnification. The cell adhesion rate was calculated as
follows: Number of adherent cells/total number of cells ×100%.
Construction of engineered myocardial
tissues in vitro
The cardiomyocyte-like cells induced by 5-aza were
digested by 2.5 g/l trypsin, and suspended at a concentration of
1×109 cells/ml. The suspension was slowly instilled onto
the prepared PLGA scaffold, and moved into a 5% CO2
incubator at 37°C. After 4 h, the initial gelation was observed in
the PLGA-cardiomyocyte-like cells compound, and 2 ml culture medium
containing 10% FBS was carefully added to completely submerge the
compound. The PLGA-cardiomyocyte-like cells compounds were
transferred to a 5% CO2 incubator at 37°C for subsequent
culture. The medium was changed every 2 days. The total culture
time was 14 days.
Immunofluorescence staining of the
cardiomyocyte-like cells
The immunofluorescence staining of cardiac troponin
I (cTnI; cat. no. sc-8118; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) was performed to identify whether BMMSCs induced by 5-aza
had differentiated into cardiomyocytes. The cells were fixed using
4% polyoxymethylene at 4°C for 20 min, incubated in 3% hydrogen
peroxide and methanol for 10 min at room temperature, blocked in
normal goat serum (Abcam, Cambridge, UK) at 37°C for 30 min, and
incubated with cTnI antibody [polyclonal goat anti-mouse
immunoglobulin G (IgG); 1:25 dilution] overnight at 4°C. Subsequent
to washing with PBS, the cells were stained by fluorescent
isothiocyanate-conjugated rabbit anti-goat IgG (cat. no. sc-2777;
Santa Cruz Biotechnology, Inc.; 1:50 dilution) for 40 min at 37°C.
Finally, the cells were counterstained with Hoechst 33258 at a
1:1,000 dilution, at room temperature, for 10 min, to visualize the
nuclei. Fluorescence microscopy (Olympus BX-51; Olympus
Corporation, Tokyo, Japan), at an ×200 magnification, was used to
observe the cells and record the results.
H&E and immunohistochemical
staining
After 14 days of culture in vitro, the
engineered myocardial tissues were washed with PBS, fixed in 4%
paraformaldehyde at 4°C for 24 h, dehydrated on an ascending series
of ethanol, embedded in paraffin, sectioned into 10 µm slices and
finally were stained with hematoxylin for 10 min and 0.5% eosin for
1 min at room temperature (H&E). For the immunohistochemical
staining of cTnI, the sections were incubated with the primary
antibodies directed against cTnI (polyclonal goat anti-mouse IgG;
cat. no. sc-8118; Santa Cruz Biotechnology, Inc.; 1:25 dilution) at
4°C overnight, and then incubated with secondary antibody (rabbit
anti-goat IgG; cat. no. sc-2768; Santa Cruz Biotechnology, Inc.;
1:1,000 dilution concentration) at 37°C for 30 min. A total of 1
mg/ml DAB reagent was added to the cells for 5–10 min at room
temperature. The sections were sealed with neutral gum for
microscopic examination under optical inverted phase contrast
microscope at ×200 magnification. Cells with brown granular DAB
reaction product in the cytoplasm were considered positive for the
protein.
Scanning electron microscopy
After 14 days of culture in vitro, the
engineered myocardial tissues were fixed in 3% glutaraldehyde at
4°C for 2 h. Samples were then coated with a thin layer of gold for
5 min in a Sputter-coater JFC-1100. The specimen was viewed using a
Hitachi S-3400N scanning electron microscope operating at a typical
5 kV accelerating voltage, 20°C and 10−5 Torr.
Transmission electron microscopy
After 14 days of culture in vitro, the
engineered myocardial tissues were initially fixed in 3%
glutaraldehyde at 4°C for 2 h, post-fixed in 1% osmium tetroxide at
4°C for 20 min and embedded in epoxy resin at 45°C for 12 h.
Ultrathin sections of 1 µm were prepared and double stained with 2%
uranyl acetate at room temperature for 15 min and 6% lead citrate
at room temperature for 15 min. The cellular ultrastructure was
observed with a JEM-2000EX transmission electron microscope (JEOL,
Ltd., Tokyo, Japan).
Statistical analysis
Data are presented as mean ± standard deviation.
Data were compared using standard or repeated measures analysis of
variance where appropriate. Pairwise comparisons between groups
were performed using the least significant difference test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cellular morphological changes of
BMMSCs induced by 5-aza
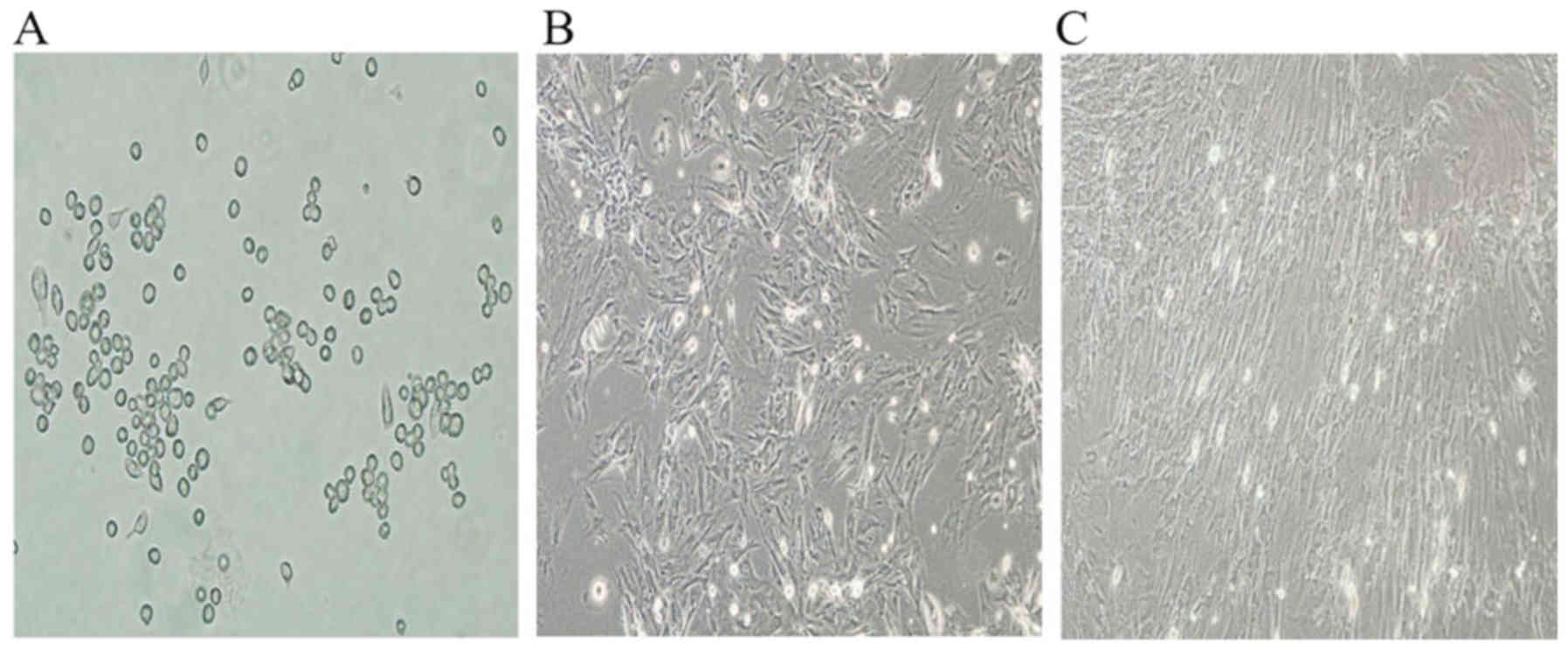
After 3 days of primary culture, the adhered BMMSCs
were observed to have round or short spindle morphologies (Fig. 1A). Following subculture, the cells
became polygonal or long spindle-shaped (Fig. 1B). Subsequent to induction by
5-aza, a proportion of cells died, but the surviving cells began to
proliferate and differentiate. Then, the cells aggregated and
gradually increased in size 4 weeks later; the adherent cells were
completely in contact with neighboring cells and arranged in a
uniform direction (Fig. 1C).
Adhesion rate of cardiomyocyte-like
cells
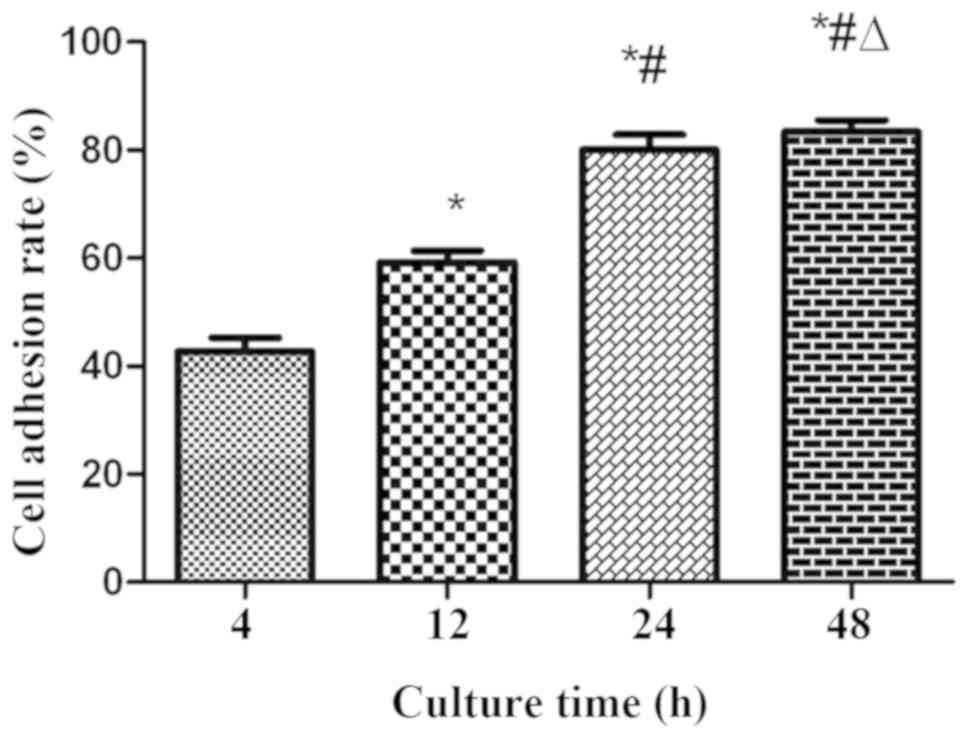
The adhesion rates of cardiomyocyte-like cells in
PLGA scaffolds were as demonstrated in Fig. 2. It was identified that the
adhesion rates at 12, 24 and 48 h were markedly increased compared
with that at 4 h (P<0.01), and the adhesion rates at 24 and 48 h
were increased compared with that at 12 h (P<0.05). However, the
adhesion rate of 48 h was not statistically significant compared
with 24 h (P>0.05). It was indicated that the adhesion rate of
the cardiomyocyte-like cells increased gradually with increases in
culture time. The majority of the cardiomyocyte-like cells had
adhered to PLGA scaffolds at 24 h.
Immunofluorescence staining for
cTnI
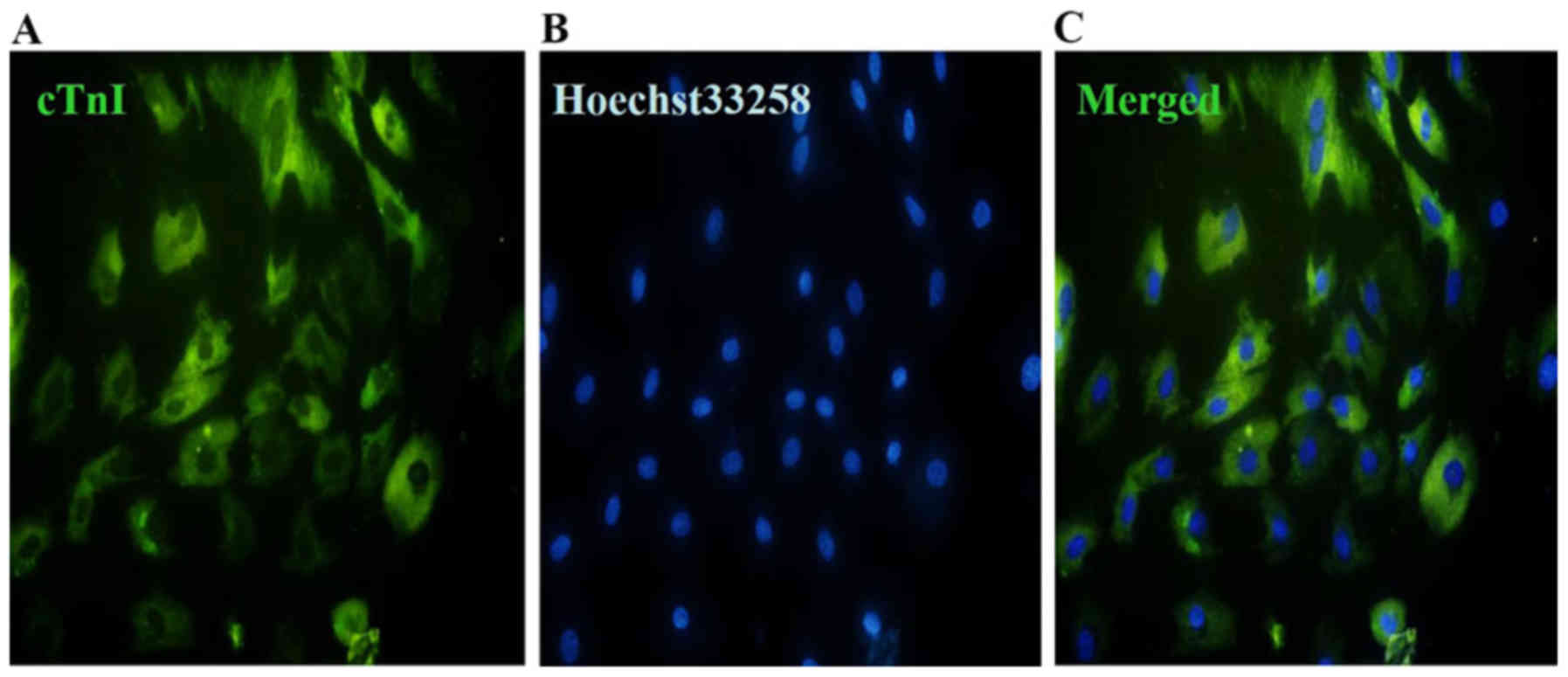
The expression of cTnI was estimated by
immunofluorescence staining following 5-aza induction. The results
suggested that the cells at 4 weeks after induction by 5-aza
exhibited green fluorescence (Fig.
3A). The nuclei of the cells exhibited blue fluorescence
(Fig. 3B). Fig. 3C represents the merged data from
Fig. 3A and B.
H&E and immunohistochemical
staining of the engineered myocardial tissue
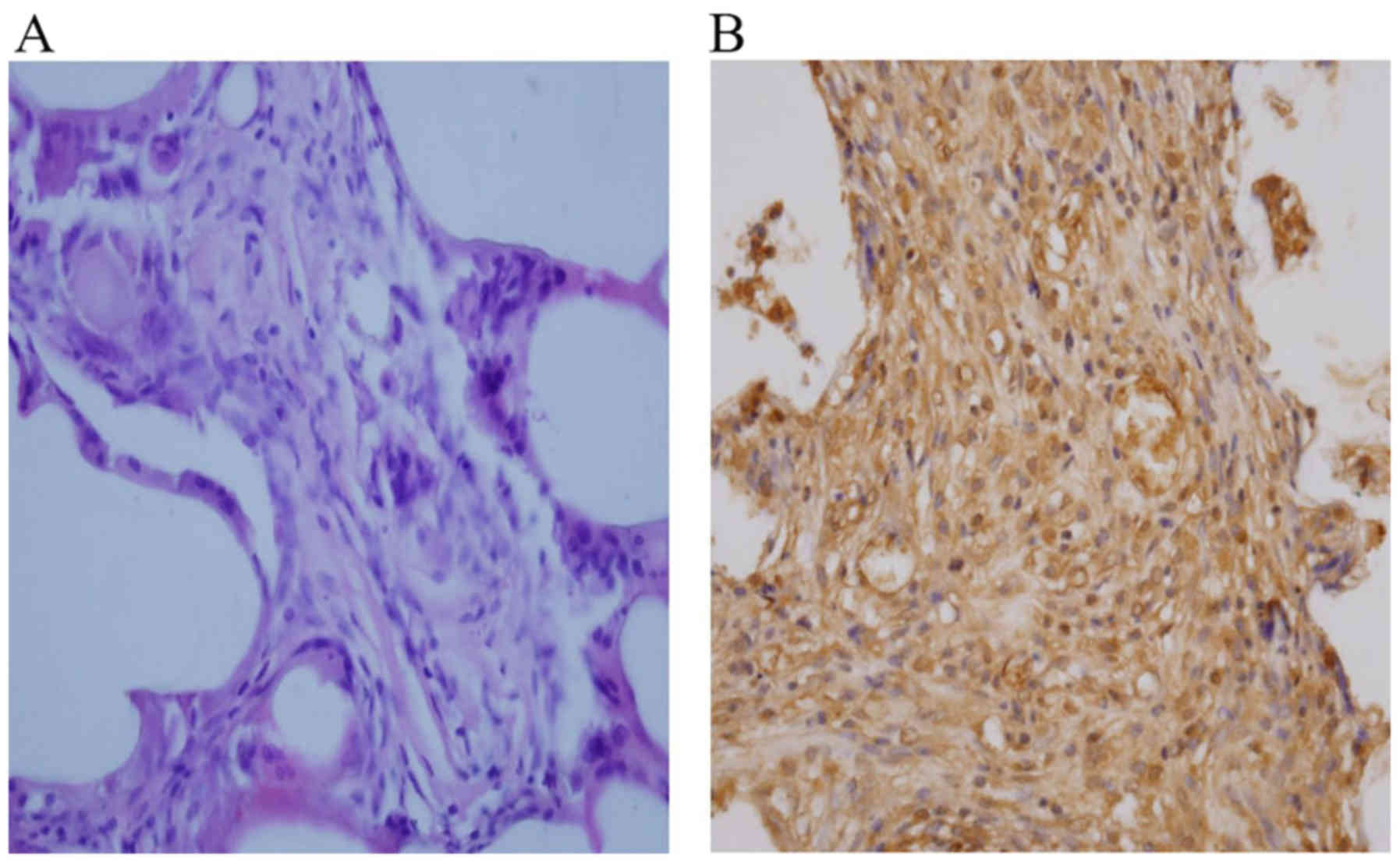
H&E staining demonstrated the presence of an
elongated pattern, marked organized striations, and a low nucleus:
Cytoplasm ratio (Fig. 4A), which
indicated the structural maturation of the engineered myocardial
tissue. Immunohistochemical staining revealed that the cells in the
engineered myocardial tissues contained a number of differentiated
cardiomyocytes that were positive for cTnI (Fig. 4B), revealing the presence of
cardiomyocyte-like cells within PLGA scaffolds.
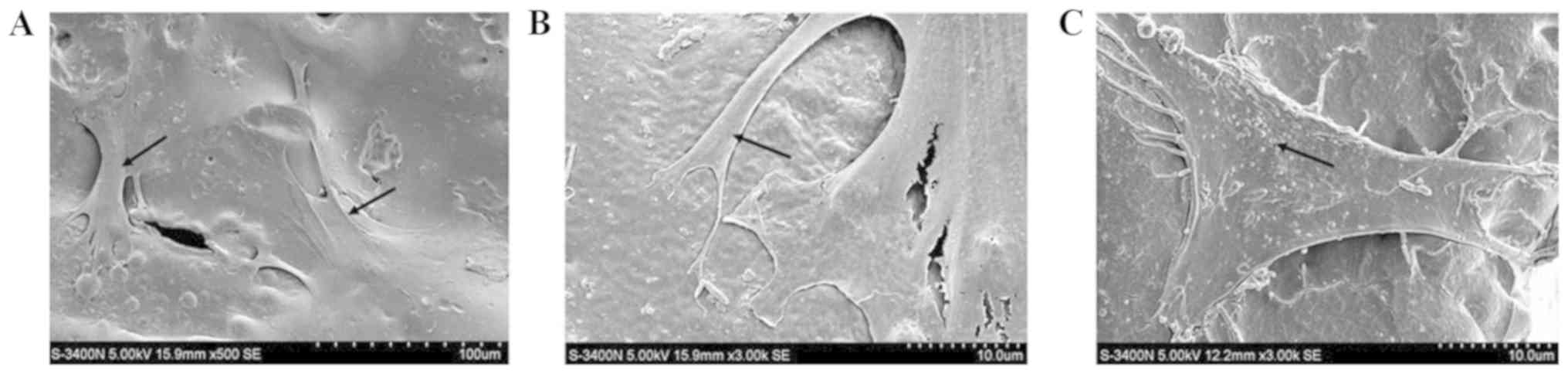
Scanning electron micrographs of the
engineered myocardial tissue
The results of the scanning electron microscopy
suggested that the inoculated the cells were well attached to PLGA,
and the cells grew and proliferated in 3 dimensions in the
engineered myocardial tissues (Fig.
5A). A large number of cell enations in the surface of PLGA
were observed, indicating good adhesion of these cells (Fig. 5B). In particular, the cells were
able to secrete a large number of extracellular matrix proteins,
which are necessary for cell differentiation (Fig. 5C).
Transmission electron micrographs of
the engineered myocardial tissue
The results of the transmission electron microscopy
demonstrated the presence uniformly distributed myofilaments
(Fig. 6A) and clearly-defined Z
line-like structures (Fig. 6B),
which are the signs of mature cardiomyocytes. In addition, the
presence of specialized junctional structures, including desmosomes
and gap junctions, was observed (Fig.
6C and D), which were responsible for electromechanical
coupling between neighboring cardiomyocytes.
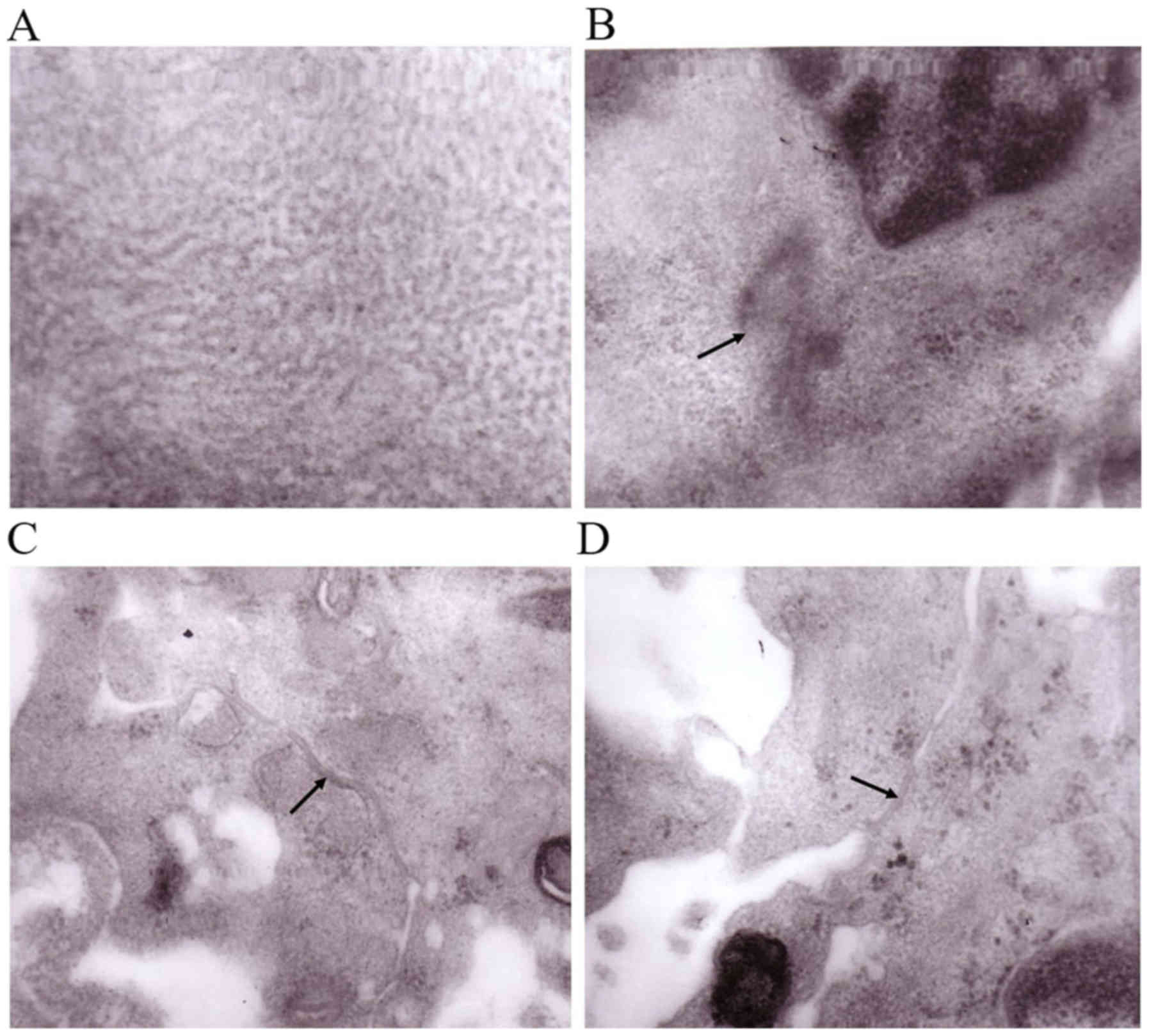 | Figure 6.Transmission electron microscopy
images of the engineered myocardial tissue. (A) Uniformly
distributed myofilaments (magnification, ×200,000). (B) Z line-like
structures, as indicated by the black arrow (magnification,
×60,000). (C) Desmosomes, as indicated by the black arrow
(magnification, ×80,000). (D) Gap junctions, as indicated by the
black arrow (magnification, ×80,000). |
Discussion
Tissue engineering has become a promising adjunct
therapy to the treatment of cardiovascular disease (23–25).
It employs certain principles from bioengineering and life sciences
fields to generate tissue-like replacements to restore
physiological function. The aim of generating engineered myocardial
tissue is to repair or regenerate myocardial tissues associated
with cardiovascular disease. At present, neonatal cardiac cells are
used in myocardial tissue engineering. However, the cardiac cells
do not proliferate in high enough numbers to repopulate
bio-engineered cardiac tissue. In previous years, stem cells have
become an important area of study, as they are multipotent cells
with self-renewing ability. Under certain conditions, they are able
to differentiate into multiple types of functional cells, including
cardiomyocytes. Among these, BMMSCs have become the most promising
seed cells, and have been termed the ‘repair stem cell’ (26). BMMSCs are multipotential cells and
are easily obtained. In addition, BMMSCs produce cytokines and
certain growth factors, which serve important roles in their
differentiation and proliferation abilities (27). In addition, BMMSCs have exhibited
immunomodulatory abilities, as demonstrated in bone marrow
Graft-verses-host disease (28–30).
BMMSCs are able to differentiate into cardiomyocytes under certain
specific conditions (31–34). Makino et al (35) demonstrated that BMMSCs may
differentiate into cells with a cardiac phenotype following
treatment with 5-aza. Tomita et al (36) revealed that the optimal
concentration for cardiomyogenic differentiation was 10 µM for 24
h. Therefore, the present study selected BMMSCs as the seed cells,
and induced cardiomyogenic differentiation in these cells using
5-aza.
The ideal biomaterial should have the following
characteristics: i) Good biocompatibility without causing
inflammatory and toxic reactions; ii) good absorption and the
ability to be completely replaced by host tissue; iii) good
plasticity; and iv) appropriate degradability according to the rate
of tissue regeneration (37). PLGA
scaffold is a synthetic polymer material, which has the advantages
of being non-toxic, with high porosity, and good biocompatibility
and biodegradability. In addition, PLGA promotes good cellular
interaction, which is useful in cell migration and nutritional
diffusion (38). In the present
study, cardiomyocyte-like cells differentiated from BMMSCs were
seeded on PLGA scaffolds and cultured together to form the
engineered myocardial tissue in vitro.
It is well-known that cTnI exists only in the atrial
and ventricular muscles, and it is a specific protein for the
identification of cardiac myocytes. Therefore, the present study
detected the expression of cTnI following 5-aza induction in order
to verify the differentiation of BMMSCs to cardiomyocytes. Using
immunofluorescence staining, it was identified that the
cardiomyocyte-like cells differentiated from BMMSCs subsequent to
5-aza induction were positive for cTnI, indicating that the
differentiated cardiomyocytes contained myocardium-specific
proteins, which may then be used for the construction of engineered
myocardial tissue.
The results of cell adhesion rates and scanning
electron microscopy demonstrated good compatibility of the
cardiomyocyte-like cells and PLGA. Transmission electron microscopy
revealed that the ultrastructure of the engineered myocardial
tissues constructed in vitro was essentially uniform. The
existence of Z line-like structures, myofilaments, gap junctions
and desmosomes may assist in explaining the ability of the cells
for growth and migration within engineered myocardial tissues
(39). The contraction of
engineered myocardial tissues constructed in vitro would
require electrophysiological integration with host tissues, so it
is important to note the development of gap junctions between the
engineered myocardial tissues.
At present, the mechanism of engineered myocardial
tissue remains unclear (40–42).
Previous studies have hypothesized that the paracrine effects of
the implanted cells are more likely to affect the survival and
growth of engineered myocardial tissues (43–45).
However, there are a number of doubts that require additional
confirmation.
Although significant progress has been made in the
study of myocardial tissue engineering, certain problems and
technical difficulties remain. For example, the thickness of the
three-dimensional myocardium does not match the in vivo
measurements, and the differentiation rate of BMMSCs is not ideal.
In addition, certain limitations exist regarding the angiogenesis
of engineered myocardial tissue. The generation of a stable culture
system is also required, in order to derive more reliable and more
mature engineered myocardial tissue. Furthermore, the function of a
myocardial infarction model requires validation using the
engineered myocardial tissues constructed in vitro to repair
the injured myocardium.
In summary, the present study successfully
constructed engineered myocardial tissues in vitro with
cardiomyocyte-like cells derived from BMMSCs following 5-aza
induction and PLGA scaffolds, which are likely to share some
structural similarities with the original cardiac tissue. The
present study also provides encouraging evidence for the general
feasibility of engineered myocardial tissue in vitro and
suggests the potential application for tissue repair in the future.
Despite the challenges that remain, the generation of functional
engineered myocardial tissue represents a promising supportive
therapy in postsurgical heart recovery therapy.
Acknowledgements
The authors would like to thank Professor Xiaofeng
Huang, who works in The Fourth Military Medical University (Xi'an,
China), for his assistance in electron microscopy.
Funding
The present study was supported by the Natural
Science Fundamental Research Project of Shaanxi Province (grant no.
2018JQ8076), the Incubation Fund for Science and Technology
Development of Shaanxi People's Hospital (grant no. 2018YXQ-01) and
the Natural Science Foundation of China (grant no. 30972557).
Authors' contributions
YJX, BYS and JKW conceived and designed the
experiments. SS performed the cell culture and induction. YZ
performed PLGA scaffold preparation and detection of cell adhesion
rate. FQL performed the hematoxylin-eosin staining and
immunohistochemical staining. YJX and LZ performed the
immunofluorescence staining, scanning electron microscopy detection
and transmission electron microscope detection. YJX, SS, YZ, BYS
analyzed the data. YJX wrote the paper. All authors read and
approved the final manuscript.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Ethics approval and consent to
participate
The present study was approved by the Ethical Review
Committee of Shaanxi Provincial People's Hospital (Xi'an, China).
All procedures performed in studies involving animals were in
accordance with the ethical standards of the institutional research
committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
burden of disease study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laflamme MA and Murry CE: Heart
regeneration. Nature. 473:326–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jara Avaca M and Gruh I: Bioengineered
cardiac tissue based on human stem cells for clinical application.
Adv Biochem Eng Biotechnol. 163:117–146. 2018.PubMed/NCBI
|
|
4
|
Wendel JS, Ye L, Tao R, Zhang J, Zhang J,
Kamp TJ and Tranquillo RT: Functional effects of a
tissue-engineered cardiac patch from human induced pluripotent stem
cell-derived cardiomyocytes in a rat infarct model. Stem Cells
Transl Med. 4:1324–1132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wissing TB, Bonito V, Bouten CVC and Smits
AIPM: Biomaterial-driven in situ cardiovascular tissue
engineering-a multi-disciplinary perspective. NPJ Regen Med.
2:182017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roberts EG, Lee EL, Backman D,
Buczek-Thomas JA, Emani S and Wong JY: Engineering myocardial
tissue patches with hierarchical structure-function. Ann Biomed
Eng. 43:762–773. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karikkineth BC and Zimmermann WH:
Myocardial tissue engineering and heart muscle repair. Curr Pharm
Biotechnol. 14:4–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jackman CP, Ganapathi AM, Asfour H, Qian
Y, Allen BW, Li Y and Bursac N: Engineered cardiac tissue patch
maintains structural and electrical properties after epicardial
implantation. Biomaterials. 159:48–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murry CE and Keller G: Differentiation of
embryonic stem cells to clinically relevant populations: Lessons
from embryonic development. Cell. 132:661–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park IH, Zhao R, West JA, Yabuuchi A, Huo
H, Ince TA, Lerou PH, Lensch MW and Daley GQ: Reprogramming of
human somatic cells to pluripotency with defined factors. Nature.
451:141–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mironov AV, Grigoryev AM, Krotova LI,
Skaletsky NN, Popov VK and Sevastianov VI: 3D printing of PLGA
scaffolds for tissue engineering. J Biomed Mater Res A.
105:104–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toma C, Pittenger MF, Cahill KS, Byrne BJ
and Kessler PD: Human mesenchymal stem cells differentiate to a
cardiomyocyte phenotype in the adult murine heart. Circulation.
105:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hao W, Shi S, Zhou S, Wang X and Nie S:
Aspirin inhibits growth and enhances cardiomyocyte differentiation
of bone marrow mesenchymal stem cells. Eur J Pharmacol.
827:198–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi B, Wang Y, Zhao R, Long X, Deng W and
Wang Z: Bone marrow mesenchymal stem cell-derived exosomal miR-21
protects C-kit+ cardiac stemcells from oxidative injury through the
PTEN/PI3K/Akt axis. PLoS One. 13:e01916162018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muller-Ehmsen J, Whittaker P, Kloner RA,
Dow JS, Sakoda T, Long TI, Laird PW and Kedes L: Survival and
development of neonatal rat cardiomyocytes transplanted into adult
myocardium. J Mol Cell Cardio. 34:107–116. 2002. View Article : Google Scholar
|
|
17
|
Balsam LB, Wagers AJ, Christensen JL,
Kofidis T, Weissman IL and Robbins RC: Hematopoietic stem cells
adopt mature hematopoietic fates in ischemic myocardium. Nature.
428:668–673. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gruh I, Beilner J, Blomer U, Schmiedl A,
Schmidt-Richter I, Kruse ML, Haverich A and Martin U: No evidence
of transdifferentiation of human endothelial progenitor cells into
cardiomyocytes after coculture with neonatal rat cardiomyocytes.
Circulation. 113:1326–1334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murry CE, Soonpaa MH, Reinecke H, Nakajima
H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH,
Poppa V, et al: Haematopoietic stem cells do not transdifferentiate
into cardiac myocytes in myocardial infarcts. Nature. 428:664–668.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi
A and Yau TM: Survival and function of bioengineered cardiac
grafts. Circulation. 100 (19 Suppl):II63–II69. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masuda S, Shimizu T, Yamato M and Okano T:
Cell sheet engineering for heart tissue repair. Adv Drug Deliv Rev.
60:277–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong SI, Kim SH, Kim YH, Jung Y, Kwon JH,
Kim BS and Lee YM: Manufacture of elastic biodegradable PLCL
scaffolds for mechano-active vascular tissue engineering. J
Biomater Sci Polym Ed. 15:645–660. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Lee SJ, Cheng HJ, Yoo JJ and Atala
A: 3D bioprinted functional and contractile cardiac tissue
constructs. Acta Biomater. 70:48–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong X, Yuan Y, Sun X, Zhou M, Guo G,
Zhang Q, Hescheler J and Xi J: Skeletal extracellular matrix
supports cardiac differentiation of embryonic stem cells: A
potential scaffold for engineered cardiac tissue. Cell Physiol
Biochem. 45:319–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shiekh PA, Singh A and Kumar A:
Engineering bioinspired antioxidant materials promoting
cardiomyocyte functionality and maturation for tissue engineering
application. ACS Appl Mater Interfaces. 10:3260–3273. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Minguell JJ and Erices A: Mesenchymal stem
cells and the treatment of cardiac disease. Exp Biol Med.
231:39–49. 2006. View Article : Google Scholar
|
|
27
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Circ Res.
95:9–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rasmusson I: Immune modulation by
mesenchymal stem cells. Exp Cell Res. 31:1815–1822. 2006.
|
|
29
|
Kaundal U, Bagai U and Rakha A:
Immunomodulatory plasticity of mesenchymal stem cells: A potential
key to successful solid organ transplantation. J Transl Med.
16:312018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Petinati NA, Kuz'mina LA, Parovichnikova
EN, Liubimova LS, Gribanova EO, Shipunova IN, Drize NI and
Savchenko VG: Treatment of acute host-versus-graft reaction in
patients after allogeneic hematopoietic cell transplantation with
multipotent mesenchymal stromal cells from a bone marrow donor. Ter
Arkh. 84:26–30. 2012.(In Russian). PubMed/NCBI
|
|
31
|
Guo X, Bai Y, Zhang L, Zhang B, Zagidullin
N, Carvalho K, Du Z and Cai B: Cardiomyocyte differentiation of
mesenchymal stem cells from bone marrow: New regulators and its
implications. Stem Cell Res Ther. 9:442018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ramesh B, Bishi DK, Rallapalli S, Arumugam
S, Cherian KM and Guhathakurta S: Ischemic cardiac tissue
conditioned media induced differentiation of human mesenchymal stem
cells into early stage cardiomyocytes. Cytotechnology. 64:563–575.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeyaraman MM, Rabbani R, Copstein L,
Sulaiman W, Farshidfar F, Kashani HH, Qadar SMZ, Guan Q, Skidmore
B, Kardami E, et al: Autologous bone marrow stem cell therapy in
patients with ST-elevation myocardial infarction: A systematic
review and Meta-analysis. Can J Cardiol. 33:1611–1623. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Furenes EB, Opstad TB, Solheim S, Lunde K,
Arnesen H and Seljeflot I: The influence of autologous bone marrow
stem cell transplantation on matrix metalloproteinases in patients
treated for acute ST-elevation myocardial infarction. Mediators
Inflamm. 2014:3859012014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Makino S, Fukuda K, Miyoshi S, Konishi F,
Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, et al:
Cardiomyocytes can be generated from marrow stromal cells in vitro.
J Clin Invest. 103:697–705. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tomita S, Li RK, Weisel R, Mickle DA, Kim
EJ, Sakai T and Jia ZQ: Autologous transplantation of bone marrow
cells improves damaged heart function. Circulation 100 (19 Suppl).
II247–II256. 1999.
|
|
37
|
Sensharma P, Madhumathi G, Jayant RD and
Jaiswal AK: Biomaterials and cells for neural tissue engineering:
Current choices. Mater Sci Eng C Mater Biol Appl. 77:1302–1315.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu Y, Kim CS, Saylor DM and Koo D: Polymer
degradation and drug delivery in PLGA-based drug-polymer
applications: A review of experiments and theories. J Biomed Mater
Res B Appl Biomater. 105:1692–1716. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang J, Sha H, Wang G, Bao G, Lu S, Luo Q
and Tan Q: Isolation and characterization of ex vivo
expanded mesenchymal stem cells obtained from a surgical patient.
Mol Med Rep. 11:1777–1783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pahnke A, Conant G, Huyer LD, Zhao Y,
Feric N and Radisic M: The role of Wnt regulation in heart
development, cardiac repair and disease: A tissue engineering
perspective. Biochem Biophys Res Commun. 473:698–703. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Golpanian S, Wolf A, Hatzistergos KE and
Hare JM: Rebuilding the damaged heart: Mesenchymal stem cells,
cell-based therapy, and engineered heart tissue. Physiol Rev.
96:1127–1168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fujita B and Zimmermann WH: Myocardial
tissue engineering for regenerative applications. Curr Cardiol Rep.
19:782017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xing YJ, Lü AL, Wang LI and Yan XB:
Construction of engineered myocardial tissues with
polylactic-co-glycolic acid polymer and cerdiomyocyte-like cells in
vitro. J Clin Rehabil Tissue Engineering Res. 14:2875–2878.
2010.
|
|
44
|
Xing Y, Lv A, Wang L, Yan X, Zhao W and
Cao F: Engineered myocardial tissues constructed in vivo using
cardiomyocyte-like cells derived from bone marrow mesenchymal stem
cells in rats. J Biomed Sci. 19:62012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mytsyk M, Isu G, Cerino G, Grapow MTR,
Eckstein FS and Marsano A: Paracrine potential of adipose stromal
vascular fraction cells to recover hypoxia-induced loss of
cardiomyocyte function. Biotechnol Bioeng. 116:132–142. 2019.
View Article : Google Scholar : PubMed/NCBI
|