Introduction
Multiple myeloma (MM) is a type of cancer that
results from the malignant proliferation of plasma cells in the
bone marrow (1,2). MM is the second most common B-cell
neoplasm of the blood system. The incidence rate in the Chinese Han
population is 1/10 million, and the incidence rate in developed
countries is 4/10 million (3). The
number of new cases in the United States in 2015 was ~28,000, with
the number of new cases increasing year by year. (4). Myeloma accounts for 13.4% of all
hematologic malignancies, 19% of all mortality due to hematological
malignancies and 2% of all tumor-associated mortalities (5). In the United States, there were
~10,000 mortalities caused by myeloma in 2010 (6,7). The
overall prognosis of patients with myeloma is poor. The median
survival of patients receiving conventional therapy is 3–4 years
(8). Certain new drugs have also
been combined with conventional therapies in the clinic, which may
further improve the prognosis and survival rate. However, the
current results suggest that almost all patients will eventually
relapse and it remains incurable, with a median overall survival of
4 years (9,10). Thus, there is urgent need to
develop novel strategies for the treatment of MM and to improve the
current treatments.
Immunotherapy can be used to control or eliminate
minimal residual disease, which can consolidate the efficacy of
chemotherapy or stem cell transplantation in patients (10). Myeloma cells secrete monoclonal
immunoglobulins with unique antigenic determinants, which may
become a tumor-specific antigen (10). A previous study reported the
extraction and immunization of patients with idiotype
immunoglobulin in the patient's plasma for immunotherapy, but the
results are not satisfactory (11). This is partly due to the fact that
this idiotypic immunoglobulin is less immunogenic and the antigen
in each patient is unique. Other patients cannot be vaccinated or
mediated by the idiotype immunoglobulin vaccine. The cytotoxic T
cells benefit from the antimyeloma effect (10). Therefore, there is an urgent need
for a new tumor-associated antigen to target in myeloma
immunotherapy. This target should be an antigen common to most
myeloma cells and can induce a strong immune response in most
patients.
Heat shock proteins (HSPs) are a class of
tumor-specific antigens with these characteristics. These HSPs
include HSP27, HSP70 and HSP90. HSPs are highly expressed in
numerous inflammatory disorders and tumors (12,13).
High expression of HSP also indicates poor patient prognosis and
increased drug resistance (2). In
addition, HSPs are essential for the survival of tumor cells.
Downregulation or inhibition of HSP expression leads to significant
apoptosis of myeloma cells. HSP90 inhibitors have been used in
clinical trials (12,14). These properties of HSPs make it a
suitable for investigation as new tumor-associated antigen
candidate. HSPs have been reported to be highly expressed in MM,
but exhibit low expression in normal tissues, and to have a crucial
role in the survival of myeloma cells (2). Studies have shown that HSP90
complexes are activated in cancer cells, but are inactive in normal
tissues (15–17). All these features make HSPs an
excellent target for tumor therapy. Since HSP27, the small HSP that
is an ATP-independent chaperone, is reported to be overexpressed in
several caner types including MM (18–22),
it can be investigated as a potential therapeutic target for the
treatment of MM.
Bortezomib, a first-in-class proteasome inhibitor,
is used in for treatment of MM (23). Currently, bortezomib is approved
for the treatment of myeloma in relapsed patients post-transplant
or as a second line treatment in patients unsuitable for
transplantation. In the present study, HSP27 expression was
analyzed and compared in samples of 50 patients with MM following
treatment with bortezomib combination with vincristine, doxorubicin
and dexamethasone (VAD) traditional chemotherapy, or VAD
chemotherapy alone. Further, we also performed the treatment in the
myeloma cell line to determine the regulation of bortezomib on
HSP27 expression. In addition, we also explore the correlation of
HSP27 with apoptosis related genes.
Materials and methods
Case collection and grouping
Between February 2016 and February 2017, samples
were obtained from 50 healthy subjects and 50 patients with MM at
Rizhao People's Hospital (Rizhao, China). The ratio of men and
women (M/F) was 23/27, and the average age was 58.85±3.06 years in
patients with MM. For the healthy subjects the ratio of men and
women was 30/20 and the average age was 57.58±7.41 years. After
medical diagnosis and graded examination, 17 patients with MM (M/F,
7/10) were in the stage I MM group, 15 patients were stage II (M/F,
8/7) and 18 patients were stage III (M/F, 8/10). All diagnoses and
classifications were based on the International Myeloma Working
Group (IMWG) diagnostic criteria for MM (2014) (24) and the Revised International Staging
System (ISS) international prognostic staging criteria (25). The nature and purpose of the study
was explained to each subject and informed consent was signed. The
study was approved by the Ethics Committee of Rizhao People's
Hospital. Inclusion criteria for patients set for the study were as
follows: i) Compliance with relevant diagnostic criteria set by the
IWMG, confirmed by bone marrow analysis, X-ray film, blood image
analysis and laboratory examination; ii) accordance with ISS
standards and Durier-Salmon staging system staging criteria
(26); iii) patients were newly
diagnosed; iv) patients had received >3 courses of regular
chemotherapy; and v) patients exhibited an expected survival of
>3 months. Exclusion criteria were as follows: i) Patients were
diagnosed with severe heart, liver, kidney, lung or blood system
diseases; ii) patients exhibited secondary plasma cell enlargement;
iii) patient presented increased primary disease and organ failure;
iv) there were missing data or patients missed follow-ups.
The 50 MM cases were randomly divided into two
groups (n=25/group), control group and observation group (clinical
trial no. ChiCTR1900023172). VAD chemotherapy was performed in
control group (NC group) by intravenous infusion of vincristine 0.5
mg/day for 1–4 days, intravenous infusion of doxorubicin 10 mg/day
for 1–4 days, and oral administration of dexamethasone 40 mg/day
for 1–4 days. Bortezomib in combination with VAD chemotherapy was
administered to the observation group. On the basis of VAD
chemotherapy, intravenous bortezomib 1.3 mg/m2 was
administered on days 1, 4, 8 and 11. A period of treatment was
defined as 28 days; a total of three periods of treatment were
performed.
Determination of HSP27 expression in
MM patients by ELISA
All samples were collected on patients with an empty
stomach. Bone marrow (5 ml) was collected on the day prior to
treatment and the first day following treatment, then sodium
citrate was added to samples to prevent coagulation. Ficoll
gradient centrifugation was performed to obtain the myeloma cells.
The cells were centrifuged at 10,000 × g for 5 min at 4°C, then
HSP27 was detected via ELISA according to the manufacturer's
protocols (cat. no. 69-40173; MSK Bio). The optical density (OD)
value was measured at 450 nm using a microplate reader (RT-6100;
Rayto Life and Analytical Sciences Co., Ltd.).
Curative effect judgment
International unified curative effect standard was
used for curative effect judgment. i) Complete remission (CR):
Results of urine and blood immunofixation electrophoresis were
negative, no plasmacytoma was present, and the proportion of plasma
cells in bone marrow was <5%. ii) Very good partial response
(VGPR): Urine, blood electrophoresis results were negative, but
immunofixation electrophoresis results were positive, serum M
protein levels decreased >90% and urinary M protein levels
<0.1 g/24 h. iii) Partial remission (PR): A decrease in serum M
protein content >50% and a urinary M protein level <0.2 g/24
h. iv) Stable condition (SD): Those who did not meet CR, VGPR and
PR criteria. v) Progression of disease (PD): The increase in serum
or urinary M protein levels was >25%, or the number of bone
marrow plasma cells increased significantly. For those that met the
criteria for PR or above, treatment was considered to be effective
and the remission rate (%) was calculated as (CR+VGPR+PR)/total
number of cases ×100.
Cell culture and grouping
Human multiple myeloma U266 cells were purchased
from the American Type Culture Collection. Under aseptic
conditions, U266 cells were cultured in RPMI-1640 medium containing
15% FBS (both Beijing Solarbio Science & Technology Co., Ltd.)
in a 37°C and 5% CO2 incubator. The cells in the
logarithmic growth phase were used for experiments. The cells were
collected for analysis when at 80% confluency. The cells were
divided into three groups: Control group without treatment,
bortezomib group (10 nM bortezomib treatment) and HSP27 inhibitor
group (200 nmol/l OGX-427 treatment). OGX-427 was obtained from
OncoGenex Pharmaceuticals. OGX-427 is an anti-sense inhibitor
targeting the HSP27 translation initiation site
(5′-GGGGACGCGCGCTCGGTCAT-3′).
CCK-8 assay for cell
proliferation
Logarithmic growth phase U266 cells were harvested
and seeded in 96-well plates at a density of 5×104
cells/ml (100 µl per well). Cells treated as aforementioned were
cultured at 37°C in a 5% CO2 incubator for 0, 24, 48, 72
and 96 h. CCK-8 solution (10 µl; Engreen) was added to each well,
mixed and culture was continued for 4 h. The microplate reader was
shaken and zeroed using a blank control well. The absorbance of
each well was measured at 450 nm. The proliferation of the cells
was calculated according using formula: Cell proliferation
inhibition rate (%)=(1-OD experimental group/OD control group)
×100.
Detection of apoptosis by Annexin
V-FITC/propidium iodide (PI) double staining
Cells treated as aforementioned were cultured for 24
h and then harvested. Cells (5×105/ml) were then stained
using the Annexin V-FITC/PI Apoptosis Detection kit (eBioscience;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The stained cells were analyzed using a flow
cytometer (Beckman Coulter, Inc.) and then the results assessed
using CellQuest software version 6.0 (BD Biosciences).
Determination of HSP27 in myeloma
cells by ELISA
The contents of HSP27 in myeloma cells were detected
in accordance with the ELISA kit instructions (cat. no.
ELH-HSP27-1; RayBiotech, Inc.), and the OD value was measured at
450 nm using a microplate reader (RT-6100; Rayto Life and
Analytical Sciences Co., Ltd.).
Detection of HSP27, Bax and Bcl-2 mRNA
expression levels by reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using the TRIzol kit
(Invitrogen; Thermo Fisher Scientific, Inc.; OD260/OD280 indicated
between 1.8 and 2.0 for RNA purity). RT of cDNA was performed using
SuperScript III Reverse Transcriptase (Thermo Fisher Scientific,
Inc.); the reaction was performed at 37°C for 1 h. qPCR was
performed using an SYBR Green kit (Invitrogen; Thermo Fisher
Scientific, Inc.) in a Master EP realplex2 (Eppendorf). The
reaction conditions were as follows: 95°C for 5 min, then 95°C for
15 sec and 60°C for 15 sec (40 cycles). Data were processed using
the 2−ΔΔCq method (27)
and relative expression levels were calculated using β-actin mRNA
as an internal reference. The primer sequences were as follows:
HSP27, forward 5′-GACGTCCAGAGCAGAGTCAGCCAG-3′, reverse
5′-GGTGGTTGCTTGAACTTTATTTGAG-3′; Bax, forward
5′-GACACCTGAGCTGACCTTGG-3′, reverse 5′-GAGGAAGTCCAGTGTCCACC-3′;
Bcl-2, forward 5′-ATCGCTCTGTGGATGACTGAGTAC-3′, reverse
5′-AGAGACAGCCAGGAGAATCAAAC-3′; and β-actin, forward
5′-GTCCACCTTCCAGCAGATGTG-3′ and reverse
5′-GCATTTGCGGTGGACGAT-3′.
Western blot analysis of HSP27, Bax
and Bcl-2 protein expression
Cells were lysed and centrifuged at 14,000 × g for
20 min, the the supernatant was removed and cell pellets were
collected. The protein concentration was measured by using a BCA
kit (Beijing Solarbio Science & Technology Co., Ltd.). Protein
samples (4 µl) were added to 5X SDS loading buffer, and then
separated by SDS-PAGE on 10% gels. Electrophoresis was performed at
80 V, and semi-dry electrotransfer to PVDF membranes (Merck KGaA)
was performed at 20 V for 30 min. The membrane was washed and
blocked with 5% nonfat milk for 2 h at room temperature. Rabbit
anti-human HPS27 (1:1,000; cat. no. ab5579; Abcam), rabbit
anti-human Bcl-2 (1:200; cat. no. ab32124; Abcam), rabbit
anti-human Bax (1:200; cat. no. ab32503; Abcam) and rabbit
anti-β-actin (1:1,000; cat. no. ABIN2854709; antibodies-online
GmbH) antibodies were added and incubated overnight at 4°C. After
washing, membranes were incubated with goat anti-rabbit IgG-
horseradish peroxidase (1:1,000; cat. no. 7074; Cell Signaling
Technology, Inc., USA) for 30 min at 37°C. The results were
observed and recorded using a Roche Elecsys-2010 chemiluminometer
(Roche Diagnostics). Protein expression levels were normalized to
β-actin and quantified using ImageJ software version 1.46 (National
Institutes of Health).
Statistical analysis
SPSS 19.0 statistical software was used to analyze
the data. The data are expressed as the mean ± SD. Multiple
comparisons were evaluated by one-way ANOVA with the least
significant difference test used for follow-up analysis. Spearman's
test was used for correlation analysis. Fisher's exact test was
used to analyze the difference of total efficacy in age and gender
groups, separately. The χ2 test was used to analysis the
differences in clinical efficacy between the NC group and
observation group. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of HSP27 in patients with
MM at different stages
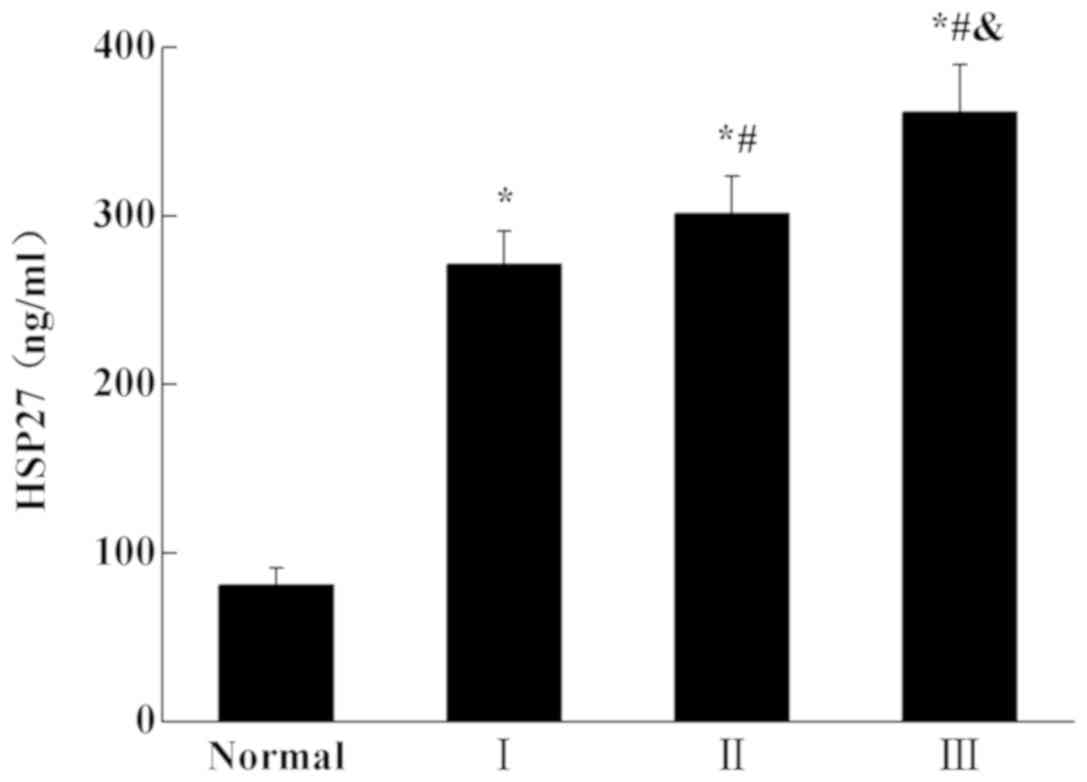
The HSP27 level was determined using ELISA
(Fig. 1). Compared with the normal
(healthy patients) group, the expression level of HSP27 in was
significantly increased in patients with MM (P<0.05). The
expression of HSP27 in patients with stage III MM was significantly
higher than that in patients with stage II MM (P<0.05). The
expression of HSP27 in patients with stage III and stage II MM was
also significantly higher than that patients with stage I
(P<0.05). The results indicated that the expression of HSP27
increased with the development of the disease.
Association between HSP27 and the
curative effect after different treatments
As shown in Table
I, 25 patients with MM ≤60 years old in the bortezomib-treated
group had a mean age of 45.75±9.57, with eight male patients
(75.0%), and six female patients (25.0%). Patients with MM >60
years old had an average age of 69.8±3.97, with five males (26.7%)
and six females (73.3%). These results demonstrated that there was
no significant difference in the treatment effect in the different
age and gender groups (P>0.05).
 | Table I.Effect of age and sex on clinical
efficacy in bortezomib treatment group. |
Table I.
Effect of age and sex on clinical
efficacy in bortezomib treatment group.
| Group | CR | VGPR | PR | SD | PD | Total efficacy
(%) | Fisher's test | P-value |
|---|
| Age (years) |
|
|
|
|
|
| 1.857 | 0.209 |
| ≤60
(n=14) | 4 | 6 | 3 | 1 | 0 | 13 (92.9) |
|
|
| >60 (n=11) | 1 | 3 | 4 | 1 | 2 | 8
(72.7) |
|
|
| Sex |
|
|
|
|
|
| 1.009 | 0.328 |
| Male
(n=13) | 2 | 5 | 3 | 2 | 1 | 10 (76.9) |
|
|
| Female
(n=12) | 3 | 4 | 4 | 0 | 1 | 11 (91.7) |
|
|
As shown in Table
II, the effective rate of the NC group was 56% and the
effective rate of the observation group was 84%. The expression of
HSP27 in patients with MM was decreased after routine treatment
(NC) and bortezomib treatment (Fig.
2). The decrease the in bortezomib treatment group was
significantly greater than that in routine treatment (NC) group.
Compared with the PD group, the expression of HSP27 in the patients
with total effective treatment was significantly decreased
(P<0.05). These results suggest that bortezomib treatment
significantly inhibited the expression of HSP27 in patients with
MM.
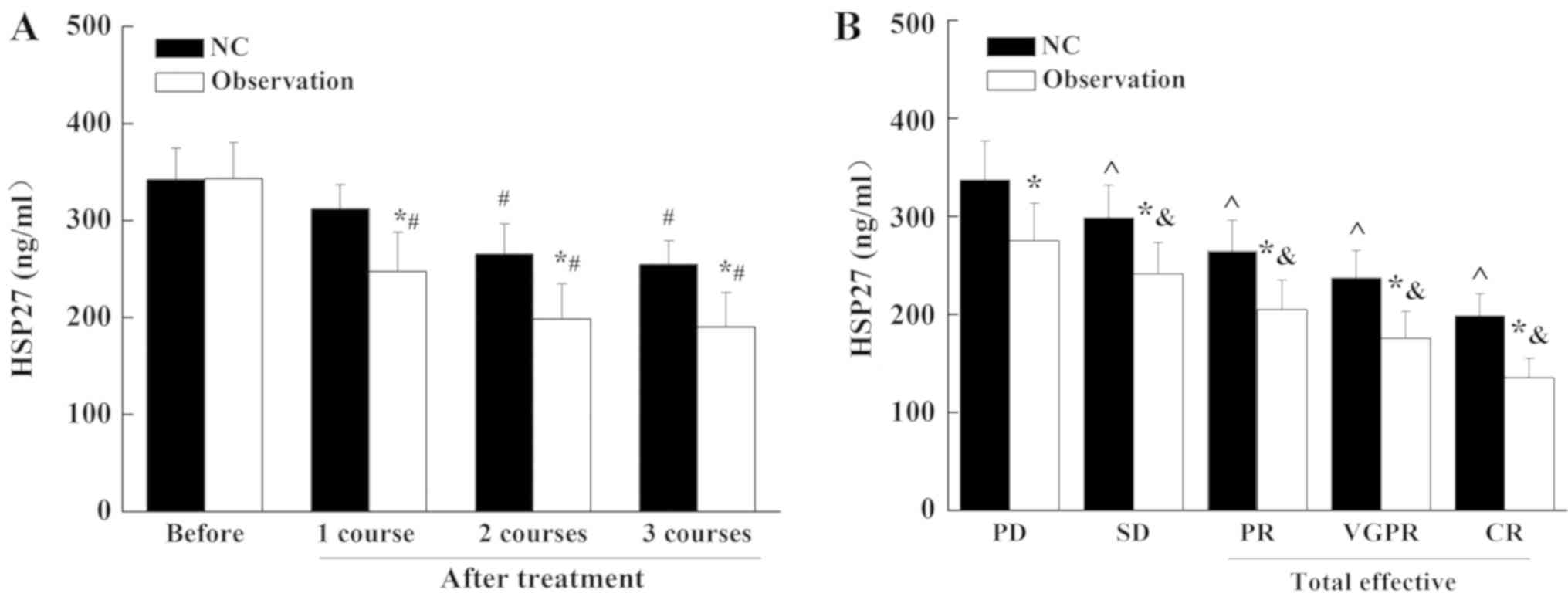 | Figure 2.Expression of HSP27 in different
therapeutic treatment groups. (A) ELISA was performed to determine
the expression of HSP27 before and after different treatment
strategies. (B) ELISA was performed to determine the HSP27
expression levels in patients with different therapeutic outcomes
following standard chemotherapy or bortezomib treatment. *P<0.05
vs. NC group; #P<0.05 vs. before treatment;
&P<0.05 vs. PD in Observation; ^P<0.05 vs. PD
in NC group. HSP27, heat shock protein 27; NC, VAD chemotherapy
control group; observation, bortezomib in combination with VAD
chemotherapy; VAD, vincristine, doxorubicin and dexamethasone; PD,
progression of disease; SD, stable condition; PR, partial remission
stable condition; VGPR, very good partial response; CR, complete
remission. |
 | Table II.Clinical efficacy of different
treatment groups. |
Table II.
Clinical efficacy of different
treatment groups.
| Group | CR | VGPR | PR | SD | PD | Total efficacy
(%) | χ2
test | P-value |
|---|
| NC group
(n=25) | 3 | 6 | 5 | 8 | 3 | 14 (56) | 4.667 | 0.031a |
| Observation group
(n=25) | 5 | 9 | 7 | 2 | 2 | 21 (84) |
|
|
Effect of bortezomib treatment on the
growth and apoptosis of U266 cells
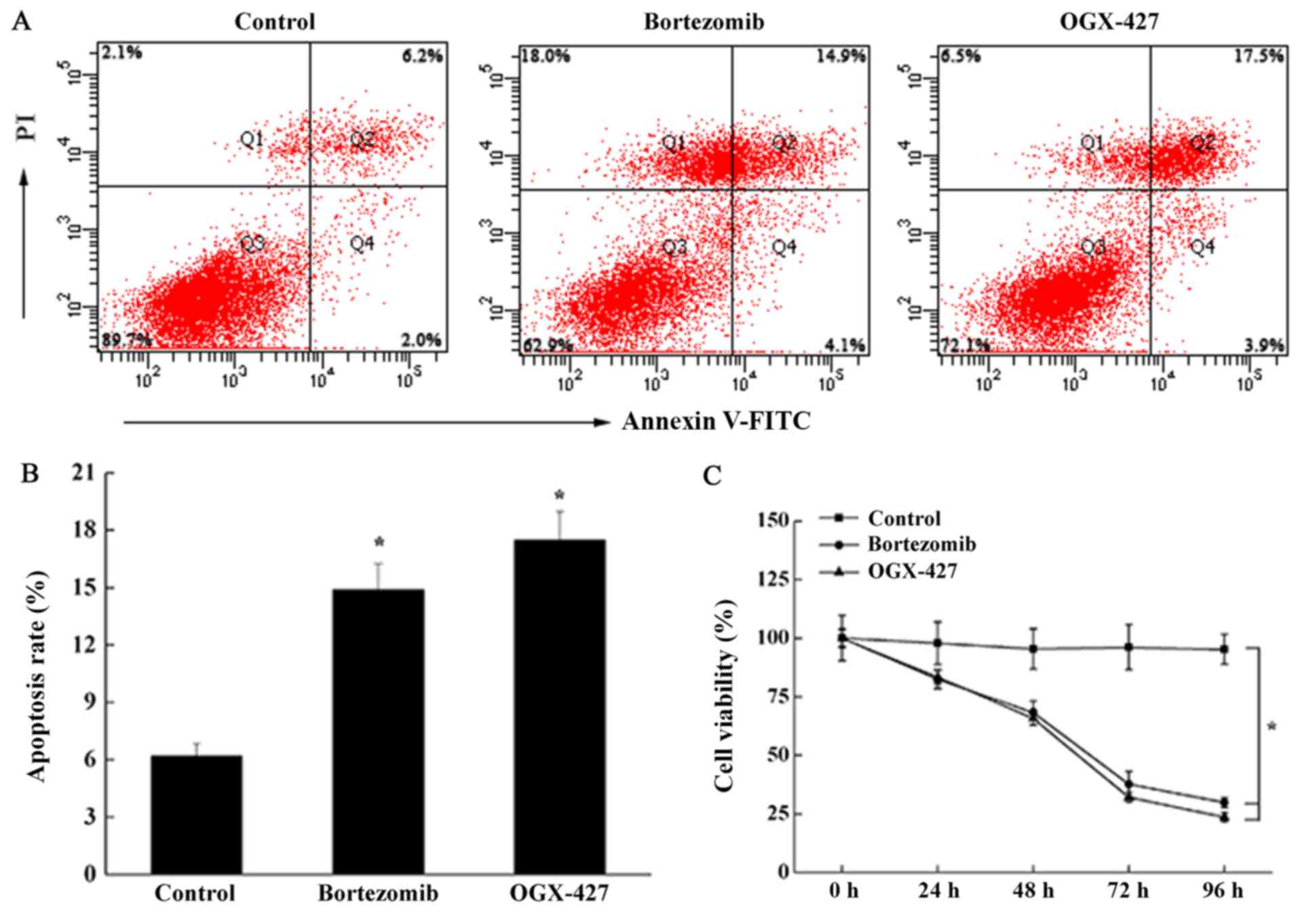
The results of the CCK-8 assay showed that
bortezomib treatment inhibited the growth of U266 cells, and that
the effect was increase over time (Fig. 3C). Compared with the control group,
after U266 cells were treated with bortezomib or OGX-427 treatment
for 48 h, U266 cell proliferation rate was significantly reduced
(P<0.05). Cell proliferation rate in OGX-427 group was not
significantly different to the bortezomib treatment group
(P>0.05). Apoptosis of the cells was detected using the Annexin
V/PI double staining method (Fig. 3A
and B). Compared with the control group, the apoptosis of the
cells was significantly increased by bortezomib treatment or
OGX-427 treatment (P<0.05) but there was no significant
difference in apoptosis between the bortezomib and OGX-427
treatment groups. These data demonstrated that bortezomib treatment
could inhibit the proliferation of U266 cells and promote
apoptosis.
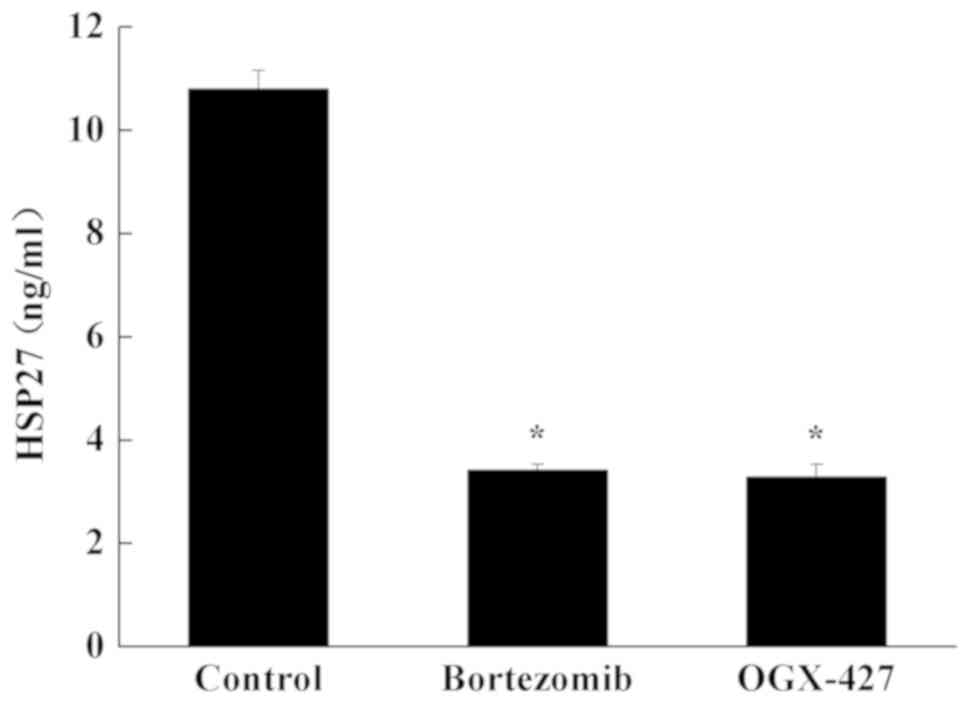
HSP27 expression in myeloma cells
ELISA was performed to determine the HSP27 protein
level in U266 cells. Compared with control group, the content of
HSP27 in U266 cells was significantly decreased in the bortezomib
group and OGX-427 group (P<0.05; Fig. 4). Compared with the bortezomib
treatment group, the content of HSP27 in the OGX-427 group was
unchanged (P>0.05; Fig. 4).
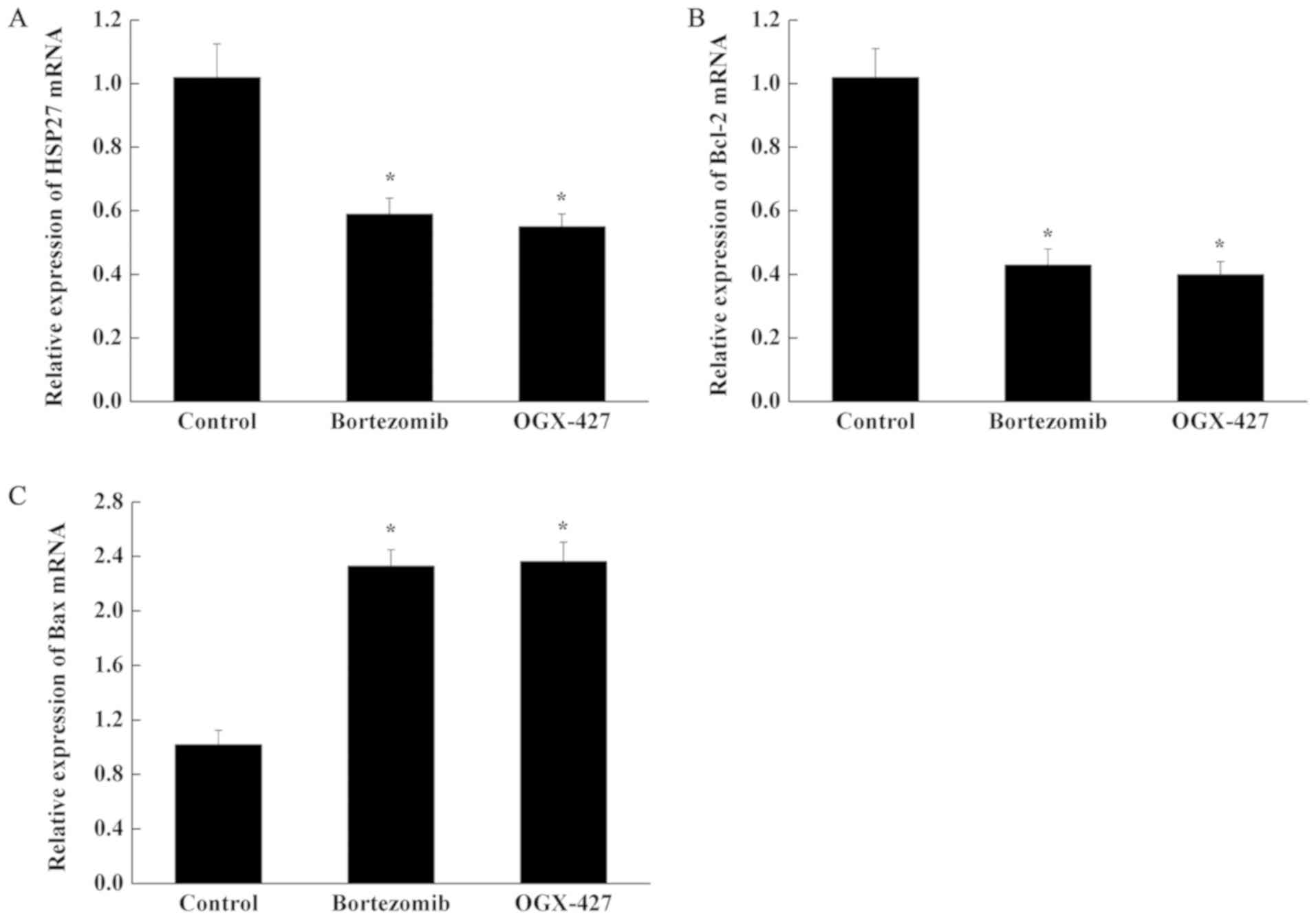
Expression of HSP27, Bcl-2 and Bax
mRNA in U266 cells
Compared with control group, the expression of HSP27
mRNA and Bcl-2 mRNA in U266 cells was decreased significantly in
the bortezomib and OGX-427 treatment groups, while the expression
of Bax mRNA was increased significantly (P<0.05; Fig. 5). Compared with the Bortezomib
group, the mRNA levels of three genes in the OGX-427 group were
unchanged (P>0.05; Fig. 5).
Effect of bortezomib on the expression
of HSP27 mRNA and Bcl-2, Bax mRNA in U266 cells
As shown in Fig.
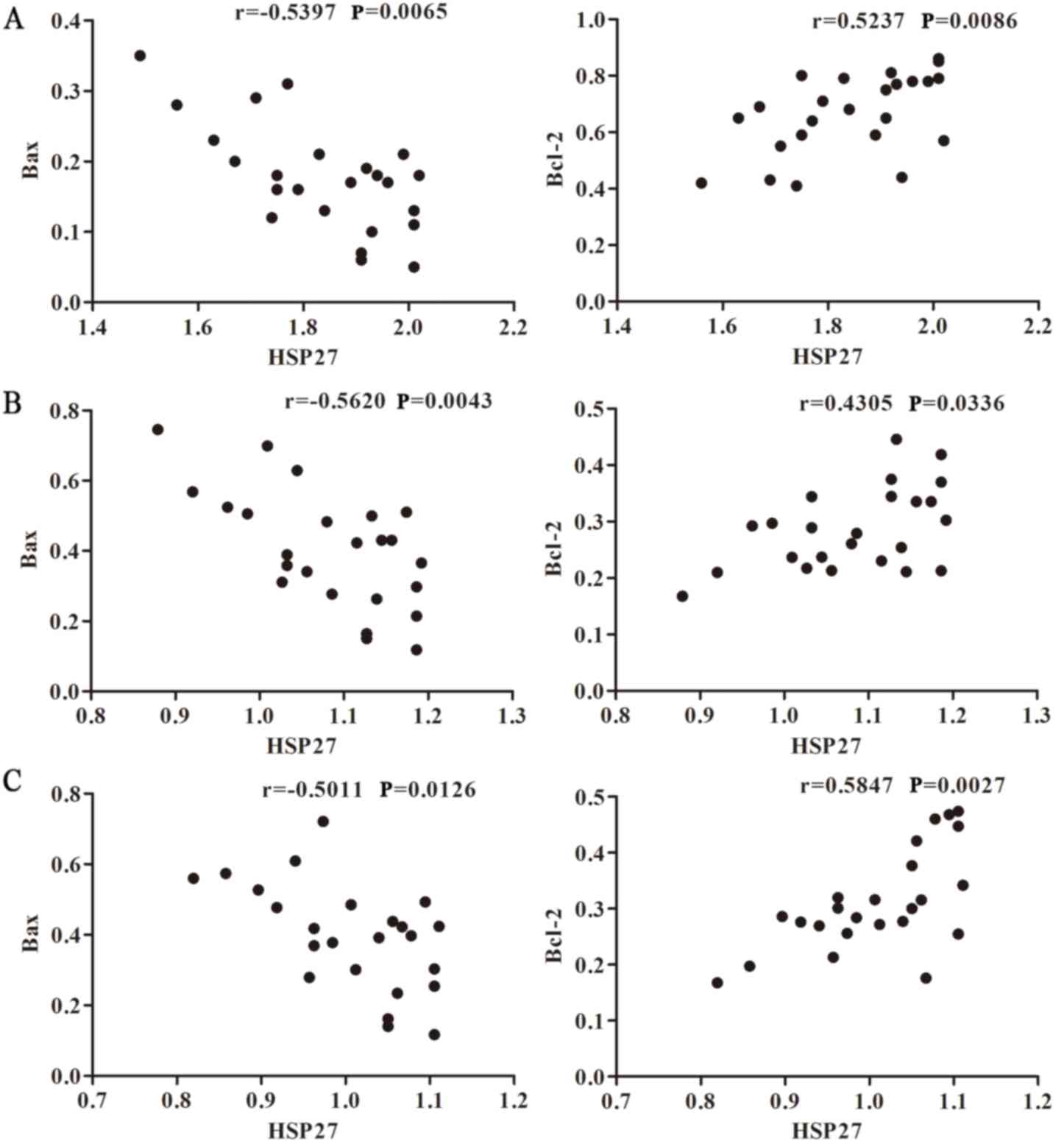
6A, Spearman's analysis was used to verify the correlation
between HSP27 and Bax and Bcl-2 prior to bortezomib treatment. The
results revealed that HSP27 was negatively correlated with Bax
expression (r=−0.5397; P=0.0065), but positively correlated with
Bcl-2 expression (r=0.5237; P=0.0086). Following bortezomib
treatment (Fig. 6B), the mRNA
expression levels of HSP27, Bax and Bcl-2 in U266 cells were
monitored. Spearman's analysis revealed a negative correlation
between HSP27 and Bax, with a correlation coefficient of r=−0.562
(P=0.0043). There was a positive correlation between HSP27 mRNA and
Bcl-2 mRNA, with a correlation coefficient of r=0.4305 (P=0.0336).
Additionally, the correlations between HSP27, and Bax and Bcl-2
expression after adding OGX-427 inhibitor were analyzed. The
correlation coefficients were −0.5011 (P=0.0126) and 0.5847
(P=0.0027), respectively (Fig.
6C). These results indicated that the expression of HSP27 is
associated with apoptosis-related genes, Bcl-2 and Bax.
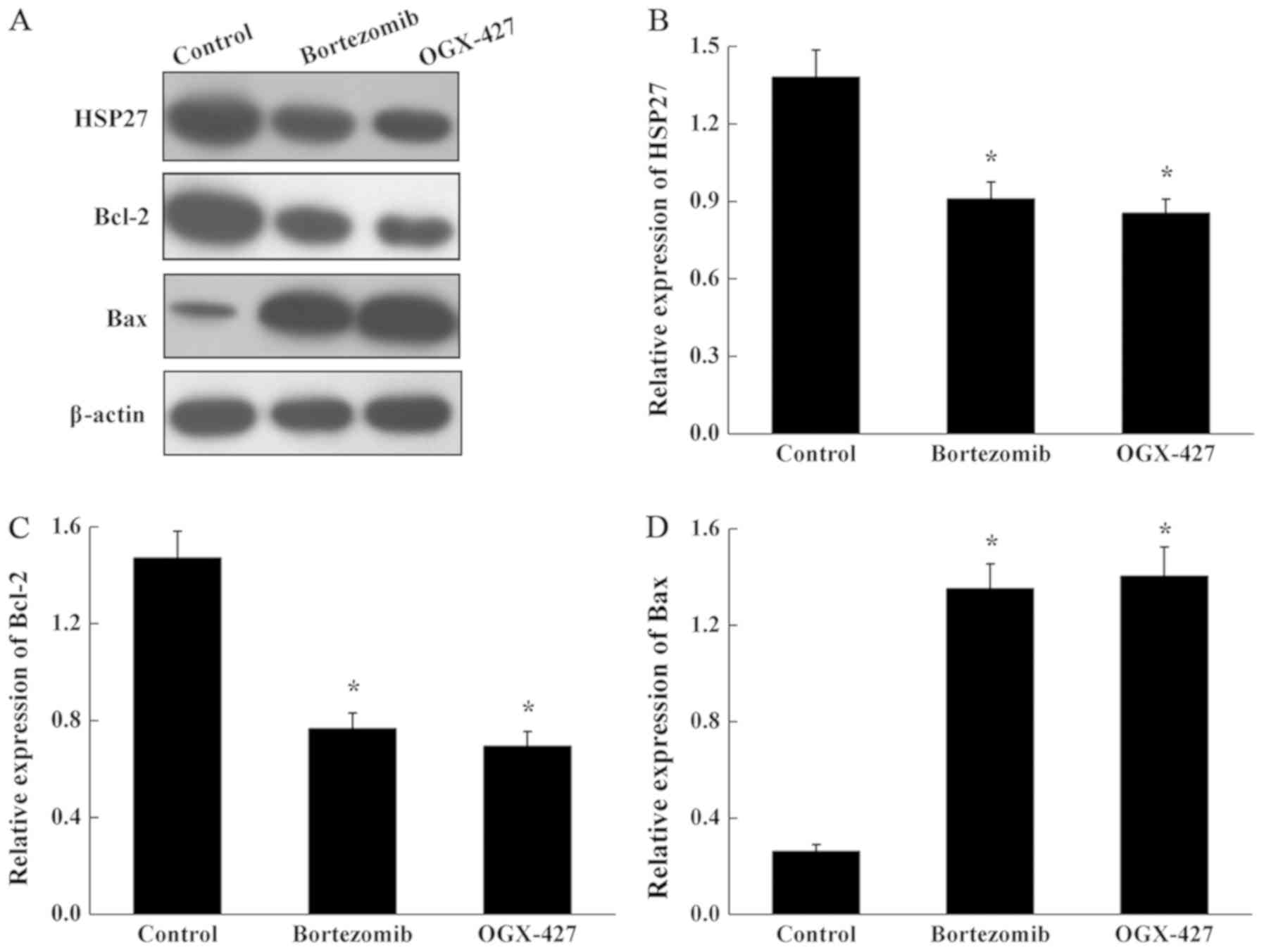
Effect of bortezomib on the expression
of HSP27, Bcl-2 and Bax protein in U266 cells
As shown in Fig. 7,
compared with the control group, the expression levels of HSP27 and
Bcl-2 protein were significantly decreased in the bortezomib and
OGX-427 treatment groups, and the expression of Bax protein was
significantly increased in the in bortezomib and OGX-427 treated
cells (P<0.05). The expression level of three proteins was not
significantly altered in the in OGX-427 group compared with the
bortezomib group (P>0.05).
Discussion
MM is a cancer of plasma cells, which are a type of
white blood cell (28). The cause
of MM is still unknown and the risk factors include alcohol,
obesity, radiation exposure, family history and certain chemicals.
The mechanism of MM involves the production of abnormal antibodies
by plasma cells, which causes kidney problems and overly thick
blood (8). MM is typically
diagnosed based on bloods analysis, urine tests, bone marrow biopsy
and medical imaging. MM is considered to be treatable, but not
incurable, despite the rapid development of treatment strategies.
Thus, novel therapeutic treatments re urgently needed for patients
with MM.
HSPs are a family of molecular chaperones that have
ac crucial role in protein folding and cellular protein
homeostasis. In addition to these function, HSPs also have
important role in the cancer development and often high expressed
in a series of cancers (2). In the
recent years, HSPs have been explored as a therapy target in cancer
treatment, including MM. HSP90 inhibitors have been used in
clinical trial for the treatment of MM (11–13,29).
HSP70 inhibitors were also reported to induce MM tumor cell death
(30). HSP27, a small HSP that is
an ATP-independent chaperone protein, is reportedly overexpressed
in several cancer types, including colorectal, breast and ovarian
cancer, and and MM. HSP27 has an important role in inhibiting the
release of the pro-apoptotic mitochondrial protein Smac in MM
(19–22). Thus, HSPs can be explored as a
therapeutic and treatment target in cancers that have high
expression of HSPs. Bortezomib is an antitumor drug used as a first
therapeutic proteasome inhibitor in certain human cancer types,
including MM. The current study analyzed the effect of bortezomib
on HSP27 expression when used in combination with traditional
chemotherapy to investigate the role of HSP27 in MM, and thus
determine the mechanism of how this combination treatment may
benefit patients with MM.
Bortezomib is a synthetic reversible proteasome
inhibitor that can inhibit tumors in multiple manners (31). It has been reported that bortezomib
promotes BAX protein expression, thereby promoting tumor cell
apoptosis (32). HSP27 exerts
regulatory effects on apoptosis, proliferation and migration,
including in tumor cells (33). It
has been reported that HSP27 is overexpressed in various malignant
tumors, such as breast cancer and leukemia (34,35).
Therefore, the expression levels of HSP27 may be a useful indicator
for the clinical evaluation of MM. The present results demonstrated
that HSP27 was highly expressed in patient serum samples, and that
expression levels increased as the cancer progressed. Following
treatment with bortezomib combination with traditional chemotherapy
or conventional treatment, HSP27 expression was significantly
decreased at the mRNA and protein level. Notably, the combination
treatment significantly increased the effective rate and the HSP27
expression was decreased in the bortezomib treatment group compared
with the conventional treatment group. It has been previously
reported that cell apoptosis is associated with the expression
levels of HSPs (36). The data of
the current study indicate that bortezomib treatment significantly
promoted cell apoptosis and downregulated of the expression of
HSP27. Further analysis demonstrated that HSP27 expression was
positively correlated with the anti-apoptotic gene Bcl-2, and
negatively correlated with the pro-apoptotic gene Bax. These
results suggest the HSP27 has a role within the apoptotic pathways.
Thus, in the future, patients may benefit from the combination
treatment. HSP27 also can be explored as a potential therapeutic
target.
In conclusion, bortezomib treatment downregulates
HSP27 and alters the expression of apoptosis-regulating proteins
Bcl-2 and Bax, and thus inhibits the proliferation and promotes the
apoptosis of myeloma cells. All these results provide a potential
method for the treatment of patients with MM.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JL and JQL participated in the design of the study.
JL, XMZ, JYS, JG, XLW and JQL conducted the assays and performed
the statistical analysis. JL, XMZ, JYS, JG, XLW and JQL drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Rizhao People's Hospital (clinical trial no. ChiCTR1900023172). All
patients provided signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang L, Fok JH and Davies FE: Heat shock
proteins in multiple myeloma. Oncotarget. 5:1132–1148. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vos T, Allen C, Arora M, Barber RM, Bhutta
ZA, Brown A, Carter A, Casey DC, Charlson FJ, Chen AZ, et al:
Global, regional, and national incidence, prevalence, and years
lived with disability for 310 diseases and injuries, 1990–2015: A
systematic analysis for the Global Burden of Disease Study 2015.
Lancet. 388:1545–1602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michels TC and Petersen KE: Multiple
myeloma: Diagnosis and treatment. Am Fam Physician. 95:373–383.
2017.PubMed/NCBI
|
|
5
|
Mahindra A, Hideshima T and Anderson KC:
Multiple myeloma: Biology of the disease. Blood Rev. 24 (Suppl
1):S5–S11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan SA and Cohen AD: Experimental
approaches in the treatment of multiple myeloma. Ther Adv Hematol.
2:213–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Costa LJ, Gonsalves WI and Kumar S: Early
mortality in multiple myeloma: Risk factors and impact on
population outcomes. Blood. 124:13202014.PubMed/NCBI
|
|
8
|
Peña C, Rojas C, Rojas H, Soto P, Cardemil
D, Aranda S, Contreras C, La Roca G, Russo M, Pérez C, et al:
Survival of 1,103 Chilean patients with multiple myeloma receiving
different therapeutic protocols from 2000 to 2016. Rev Med Chil.
146:869–875. 2018.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Landgren O: Shall we treat smoldering
multiple myeloma in the near future? Hematology Am Soc Hematol Educ
Program. 2017:194–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Briqle K and Roqers B: Pathobiology and
diagnosis of multiple myeloma. Semin Oncol Nurs. 33:225–236. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abdalla AO, Kokhaei P, Hansson L,
Mellstedt H and Osterborg A: Idiotype vaccination in patients with
myeloma reduced circulating myeloma cells (CMC). Ann Oncol.
19:1172–1179. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khandia R, Munjal AK, Iqbal HMN and Dhama
K: Heat shock proteins: Therapeutic perspectives in inflammatory
disorders. Recent Pat Inflamm Allergy Drug Discov. 10:94–104. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calderwood SK and Gong J: Heat shock
proteins promote cancer: It's a protection racket. Trends Biochem
Sci. 41:311–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Campanella C, Rappa F, Sciumè C, Marino
Gammazza A, Barone R, Bucchieri F, David S, Curcurù G, Caruso
Bavisotto C, Pitruzzella A, et al: Heat shock protein 60 levels in
tissue and circulating exosomes in human large bowel cancer before
and after ablative surgery. Cancer. 121:3230–3229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Richardson PG, Chanan-Khan AA, Alsina M,
Albitar M, Berman D, Messina M, Mitsiades CS and Anderson KC:
Tanespimycin monotherapy in relapsed multiple myeloma: Results of a
phase 1 dose-escalation study. Br J Haematol. 150:438–445.
2010.PubMed/NCBI
|
|
16
|
Richardson PG, Chanan-Khan AA, onial S,
Krishnan AY, Carroll MP, Alsina M, Albitar M, Berman D, Messina M
and Anderson KC: Tanespimycin and bortezomib combination treatment
in patients with relapsed or relapsed and refractory multiple
myeloma: Results of a phase 1/2 study. Br J Haematol. 153:729–740.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stühmer T, Zöllinger A, Siegmund D,
Chatterjee M, Grella E, Knop S, Kortüm M, Unzicker C, Jensen MR,
Quadt C, et al: Signalling profile and antitumour activity of the
novel Hsp90 inhibitor NVP-AUY922 in multiple myeloma. Leukemia.
22:1604–1612. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Z, Zhi J, Peng X, Zhong X and Xu A:
Clinical significance of HSP27 expression in colorectal cancer. Mol
Med Rep. 3:953–958. 2010.PubMed/NCBI
|
|
19
|
Cornford PA, Dodson AR, Parsons KF,
Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y and
Foster CS: Heat shock protein expression independently predicts
clinical outcome in prostate cancer. Cancer Res. 60:7099–7105.
2000.PubMed/NCBI
|
|
20
|
Conroy SE, Sasieni PD, Amin V, Wang DY,
Smith P, Fentiman IS and Latchman DS: Antibodies to heat-shock
protein 27 are associated with improved survival in patients with
breast cancer. Br J Cancer. 77:1875–1879. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chauhan D, Li G, Hideshima T, Podar K,
Mitsiades C, Mitsiades N, Catley L, Tai YT, Hayashi T, Shringarpure
R, et al: Hsp27 inhibits release of mitochondrial protein Smac in
multiple myeloma cells and confers dexamethasone resistance. Blood.
102:3379–3386. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arts HJ, Hollema H, Lemstra W, Willemse
PH, De Vries EG, Kampinga HH and Van der Zee AG:
Heat-shock-protein-27 (hsp27) expression in ovarian carcinoma:
Relation in response to chemotherapy and prognosis. Int J Cancer.
84:234–238. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Richardson PG, Hideshima T and Anderson
KC: Bortezomib (PS-341): A novel, first-in-class proteasome
inhibitor for the treatment of multiple myeloma and other cancers.
Cancer Control. 10:361–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rajkumar SV, Dimopoulos MA, Palumbo A,
Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E,
Richardson P, et al: International Mye-loma Working Group updated
criteria for the diagnosis of multiple myeloma. Lancet Oncol.
15:e538–e548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Greipp PR, San Miguel J, Durie BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Durie BG and Salmon SE: A clinical staging
system for multiple myeloma correlation of measured myeloma cell
mass with presenting clinical features, response to treatment and
survival. Cancer. 36:842–854. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raab MS, Podar K, Breitkreutz I,
Richardson PG and Anderson KC: Multiple myeloma. Lancet.
374:324–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakashima T, Ishii T, Tagaya H, Seike T,
Nakagawa H, Kanda Y, Akinaga S, Soga S and Shiotsu Y: New molecular
and biological mechanism of antitumor activities of KW-2478, a
novel nonansamycin heat shock protein 90 inhibitor, in multiple
myeloma cells. Clin Cancer Res. 16:2792–2802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eugênio AIP, Fook-Alves VL, de Oliveira
MB, Fernando RC, Zanatta DB, Strauss BE, Silva MRR, Porcionatto MA
and Colleoni GWB: Proteasome and heat shock protein 70 (HSP70)
inhibitors as therapeutic alternative in multiple myeloma.
Oncotarget. 8:114698–114709. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boccadoro M, Morgan G and Cavenagh J:
Preclinical evaluation of the proteasome inhibitor bortezomib in
cancer therapy. Cancer Cell Int. 5:182005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adams J: Proteasome inhibition in cancer:
Development of PS-341. Semin Oncol. 28:613–619. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ciocca DR and Vargas-Roig LM: Hsp27 as a
prognostic and predictive factor in cancer. Prog Mol Subcell Bill.
28:205–218. 2002. View Article : Google Scholar
|
|
34
|
Wei L, Liu TT, Wang HH, Hong HM, Yu AL,
Feng HP and Chang WW: Hsp27 participates in the maintenance of
breast cancer stem cells through regulation of
epithelial-mesenchymal transition and nuclear factor-κB. Breast
Cancer Res. 13:R1012011. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gonzalez-Mejia ME, Voss OH, Murnan EJ and
Doseff AI: Apigenin-induced apoptosis of leukemia cells is mediated
by a bimodal and differentially regulated residue-specific
phosphorylation of heat-shock protein-27. Cell Death Dis.
1:e642010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lanneau D, Brunet M, Frisan E, Solary E,
Fontenay M and Garrido C: Heat shock proteins: Essential proteins
for apoptosis regulation. J Cell Mol Med. 12:743–761. 2008.
View Article : Google Scholar : PubMed/NCBI
|