Introduction
Chronic rhinosinusitis (CRS) is a chronic
inflammatory disease involving sinonasal mucosa (1). CRS affects approximately 8% of the
Chinese population, and results in serious health consequences
(2). Although endoscopic sinus
surgery (ESS) can improve the prognosis of patients with CRS in the
case of unsuccessful medical therapy, a refractory course presents
in a minority of cases. Therefore, the pathogenesis of CRS requires
further investigation.
Epithelial-mesenchymal transition (EMT) is a
biological process, in which epithelial cells acquire mesenchymal
properties and lose their epithelial phenotype (3). The features of EMT include decreased
expression of epithelial makers, such as E-cadherin and
cytokeratin, and increased expression of mesenchymal markers,
namely α-smooth muscle actin (α-SMA), vimentin and fibronectin. EMT
plays key roles in crucial cell functions including organogenesis,
tumor formation and fibrosis (4,5).
Recently, EMT was reported to be involved in a number of airway
diseases (6–9). The pathological basis of the symptoms
in CRS includes tissue remodeling, which is characteristic of the
abnormal deposition of the extracellular matrix (ECM). Currently, a
limited number of studies have been conducted that have examined
the association between EMT and CRS (10–16).
However, these results were controversial and further investigation
on this topic is necessary. The evidence from primary nasal
epithelial cells (NECs) in patients with CRS remains limited
(14–16), whereas the association between EMT
markers and clinical features in CRS has been scarcely explored
(16). Therefore, the involvement
of EMT in CRS is essential for improving the prognosis of these
patients.
The present study aimed to examine the expression of
EMT markers in sinonasal specimens from CRS and control patients.
CRS was divided into two types, namely chronic rhinosinusitis
without nasal polyps (CRSsNP) and chronic rhinosinusitis with nasal
polyps (CRSwNP) (17). In
addition, EMT features were evaluated in primary NECs following
transforming growth factor (TGF)-β1 stimulation. The associations
between the expression levels of α-SMA and the clinical
characteristics of CRS were also determined.
Materials and methods
Subjects
In the present study, 39 patients were recruited,
including 23 females and 16 males, aged 11–72 years old
(45.85±14.88). All patients underwent endoscopic sinus surgery
(ESS) from September 2009 to February 2011 at the Department of
Otolaryngology of the Affiliated Eye Ear Nose and Throat Hospital
(AEENTH) of Fudan University. Patients with immunodeficiency
diseases (human immunodeficiency virus, diabetes, renal disease)
were excluded. CRS subjects were diagnosed according to the
EPOS-2007 criteria (17). The
patients undergoing ESS for benign diseases other than CRS were
employed as the control subjects.
Clinical information was recorded prior to surgery,
including age, sex, disease duration, sinus surgical history,
smoking history, co-existence of allergic rhinitis, asthma, aspirin
sensitivity and gastroesophageal reflux disease (GERD). The
Lund-Mackay CT score was estimated preoperatively. The visual
analogue scale (VAS), the rhinosinusitis outcome measure-31
(RSOM-31) and the Lund-Kennedy endoscopy scores were assessed pre-
and post-operatively as previously described (18–20).
The samples were obtained from the ethmoid sinus
with edema during surgery or during discharge. These samples were
representative of CRS, whereas the samples from the nasal cavity
were used as controls. Each sample was divided into 4 specimens for
the detection of mRNA and protein expression, for hematoxylin and
eosin (H&E) staining and for immunofluorescence staining,
respectively.
Reverse transcription-quantitative PCR
(qPCR)
Total RNA was extracted from tissues with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and subsequently reverse transcribed into cDNA with a
SuperScript™ first strand synthesis kit (Invitrogen; Thermo Fisher
Scientific, Inc.). RT-qPCR was performed with a thermal cycler
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Amplification
conditions for the RT reaction were 42°C for 50 min and 70°C for 15
min. qPCR was then performed using an SYBR ExScript kit (Takara
Biotechnology Co., Ltd.) and conducted on a Mx3000 Real-Time PCR
system (Stratagene, La Jolla, CA). Conditions of the PCR
amplification were: 94°C (5 min), then 30 cycles of 94°C for 30
sec, 56°C for 30 sec and 72°C for 60 sec, with a final extension at
72°C for 10 min. Each sample was measured in triplicate. The mRNA
levels were determined using the 2−ΔΔCq method (21). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was selected as the control gene. The
primer sequences are presented in Table SI.
Western blot analysis
The tissues were incubated with lysis buffer
(Beyotime Institute of Biotechnology) on ice. The protein
concentration was quantified using a BCA protein concentration
assay kit (Beyotime Institute of Biotechnology). The proteins were
separated by SDS-PAGE and were loaded on the 10% gels in equal
amounts (40 µg). Following electrophoresis, they were transferred
to PVDF membranes. The membranes were blocked using 5% BSA
(Beyotime Institute of Biotechnology) for 1 h at room temperature,
and immunoblotted with mouse monoclonal antibodies against α-SMA
(1:1,000; sc-130617; Santa Cruz Biotechnology, Inc.), TGF-β1
(1:1,000; ab64715; Abcam), E-cadherin (1:1,000; ab1416; Abcam),
fibronectin (1:1,000; ab26245; Abcam) and GAPDH (1:1,000; cat. no.
97166; Cell Signaling Technology) overnight at 4°C. The following
morning, the membranes were incubated with anti-mouse IgG
conjugated to horseradish peroxidase (1:2,000; cat. no. 7076; Cell
Signaling Technology). The blots were visualized by Amersham ECL™
Select (GE Healthcare Life Sciences) with X-OMAT BT film
(Carestream Health). Quantity One 2.0 (Bio-Rad Laboratories, Inc.)
was used for the quantification of protein expression. The protein
level of control group was set as 1; all data were normalized to
the control group.
Immunofluorescence and H&E
staining
The tissue samples were fixed with 4%
paraformaldehyde for 24 h at room temperature and embedded in
paraffin. Embedded tissues were cut into 5-µm sections and
deparaffinized with xylene and hydrated with a series of ethanol
solutions in a decreasing concentration gradient. The sections were
permeabilized with 0.1% Triton X-100, blocked with 1% BSA for 1 h
at room temperature, and subsequently incubated with primary
antibodies overnight at 4°C, including mouse monoclonal antibodies
against TGF-β1 (1:500; ab64715; Abcam), matrix metalloproteinase
(MMP)-9 (1:500; ab58803; Abcam) and fibronectin (1:100; ab26245;
Abcam). The sections were probed with goat anti-mouse
cyanine3-labeled secondary antibody (1:400; M30010, Invitrogen;
Thermo Fisher Scientific, Inc.) for 2 h at room temperature, and
subsequently mounted with DAPI for 20 min at room temperature.
Fluorescence was measured over 10 fields per sample at
magnification, ×400 (×40 objective lens) by fluorescence microscopy
(Leica Microsystems GmbH).
For double-labeling immunofluorescence, the primary
antibodies used were the following: mouse monoclonal antibody
against α-SMA (1:200; sc-130617; Santa Cruz Biotechnology, Inc.)
and rabbit monoclonal antibody against pan-cytokeratin (1:200;
ab234297; Abcam). FITC-conjugated rabbit anti-mouse (1:100; F9137;
Sigma-Aldrich; Merck KGaA) and cyanine3-conjugated goat anti-rabbit
secondary antibodies (1:200; A10520; Thermo Fisher Scientific,
Inc.) were applied respectively.
Furthermore, the pathological classification of
Hellquist was conducted for CRSwNP based on H&E staining, as
described previously (22).
Isolation of primary NECs
Each tissue was cut into small pieces and incubated
with collagenase for 12–24 h at 4°C, as soon as the sinonasal
mucosa was removed from the patients with CRS. The suspension was
vortexed and filtered (50 µm) to remove the clumps. Magnetic cell
sorting method was used to obtain NECs. The cells were resuspended
in degassed buffer [phosphate-buffered saline (PBS) pH 7.2, 0.5%
FCS, 2 ml EDTA] at a density of 107 cells/80 µl and
incubated with microbeads conjugated to monoclonal mouse anti-human
CD326 antibodies (Miltenyi Biotec, Inc.) at a bead-to-total cell
ratio of 20 µl:107 cells for 20 min at 4°C. The
supernatant was aspirated by collecting the beads using an MS
Column and a MACS Separator (Miltenyi Biotec, Inc.). The cells were
resuspended in BEBM media and seeded in a culture flask (Fig. S1A).
The purity of NECs was verified via two methods.
First, immunofluorescence was performed on cells, which were fixed
and stained with pan-cytokeratin as described above. The
proportions of the NECs (cytokeratin-positive cells) in ten fields
were recorded under a fluorescence microscope. Second, flow
cytometry was performed. Cells were digested with 0.25% trypsin at
37°C for 5 min and collected via centrifugation at 1,000 × g for 5
min at 4°C. Following two washes with ice-cold PBS, the cells were
re-suspended in 50 µl of PBS, blocked with anti-human IgG (1:1,000;
cat. no. ab195574; Abcam) at room temperature for 15 min, and
subsequently incubated with mouse phycoerythrin-conjugated
anti-pan-cytokeratin (1:1,000; cat. no. SAB4700668; Sigma-Aldrich;
Merck KGaA) for 30–45 min at 4°C in the dark. The NECs were
identified using an EPICS XL flow cytometer (Beckman Coulter, Inc.)
and analyzed by Cell Quest software version FCS2.0 (BD
Biosciences). Cytokeratin-positive cells were considered NECs. The
purity of the NECs reached 89.3% by immunofluorescence or 92.1% by
flow cytometry, respectively (Fig.
S1B and C).
EMT induction and assessment
For EMT induction, the cells were incubated with
TGF-β1 (1, 5 and 10 ng/ml) for 1, 2 and 3 days, respectively. The
morphology of the cells was observed using a phase contrast
microscope. Double-labeling immunofluorescence of α-SMA and
cytokeratin was applied. The expression levels of E-cadherin and
α-SMA were determined by western blot analysis, as described
above.
Statistical analysis
Statistical analyses were carried out using SPSS
14.0 (SPSS, Inc.). The data are presented as mean ± standard
deviations (SD). The differences were analyzed by one-way ANOVA
followed by Bonferroni post hoc test among multiple groups. The
Kruskal-Wallis test was employed for data with skewed distribution.
The correlations between α-SMA levels and clinical parameters of
CRS were calculated by the Pearson correlation method. P<0.05
was used to indicate significant differences.
Results
Basic characteristics
A total of 26 patients with CRS and 13 control
subjects were enrolled in the present study. Patients with CRS
included 13 CRSwNP and 13 CRSsNP cases. The controls involved 3
cases with paranasal sinus cyst, 3 cases with cerebrospinal fluid
rhinorrhea, 2 cases with nasal septum deviation, 2 cases with
traumatic optic neuropathy, 1 case with pituitary adenoma, 1 case
with thyroid eye disease and 1 case with frontal sinus osteoma. The
basic characteristics are documented in Table SII.
Expression of EMT markers in tissues
of patients with CRS and control subjects
The mRNA levels of E-cadherin in CRS group were
significantly increased compared with in the control group,
whereas, no significant differences were observed between the two
groups in the mRNA levels of other markers including α-SMA, TGF-β1,
MMP-9, fibronectin and vimentin (Table SIII). The mRNA levels of
E-cadherin, α-SMA and TGF-β1 in CRSsNP were
significantly higher than those noted in the control subjects.
CRSwNP exhibited decreased mRNA levels of α-SMA and vimentin
compared with the expression levels of these markers in the control
subjects. CRSsNP tissues had elevated expression of
E-cadherin, α-SMA, TGF-β1, fibronectin and
vimentin when compared with CRSwNP. MMP-9 mRNA levels
exhibited no difference between these two groups (Fig. 1A-C).
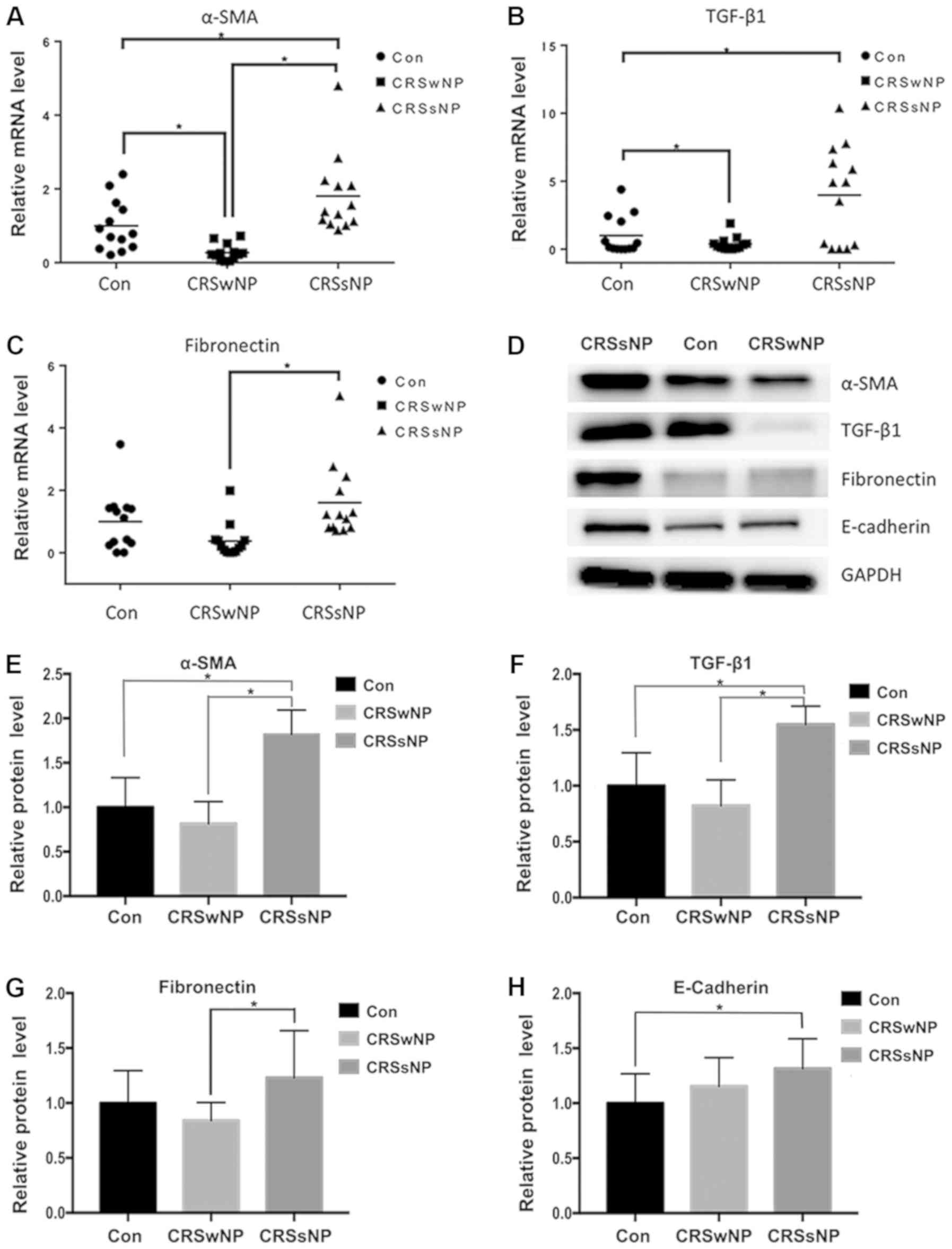 | Figure 1.Relative mRNA and protein levels of
EMT markers in CRSsNP, CRSwNP and control subjects. (A-C) Relative
mRNA levels of α-SMA, TGF-β1, and fibronectin in the
three groups. (D) Protein levels of EMT markers in the three
groups. (E-H) Quantified data of western blot analysis. *P<0.05.
EMT, epithelial-mesenchymal transition; CRS, chronic
rhinosinusitis; α-SMA, α-smooth muscle actin; TGF-β1, transforming
growth factor-β1; CON, control group; CRSsNP, CRS without nasal
polyps; CRSwNP, CRS with nasal polyps. |
The protein levels of E-cadherin in CRS were
significantly higher than those noted in the control subjects. The
protein levels of α-SMA, TGF-β1 and E-cadherin in CRSsNP were
significantly increased compared with the control group. The
protein levels of α-SMA, TGF-β1 and fibronectin in CRSsNP were
significantly upregulated compared with CRSwNP (Fig. 1D-H). The protein levels of the EMT
markers are presented in Table
SIV.
The protein levels of α-SMA, TGF-β1, fibronectin and
MMP-9 were increased in CRSsNP, as demonstrated by
immunofluorescence assays (Fig.
2). In addition, the cells that co-expressed α-SMA and
cytokeratin were detected in the mucosal epithelium in both CRSsNP
and CRSwNP using double-labeling immunofluorescence assays
(Fig. 2A).
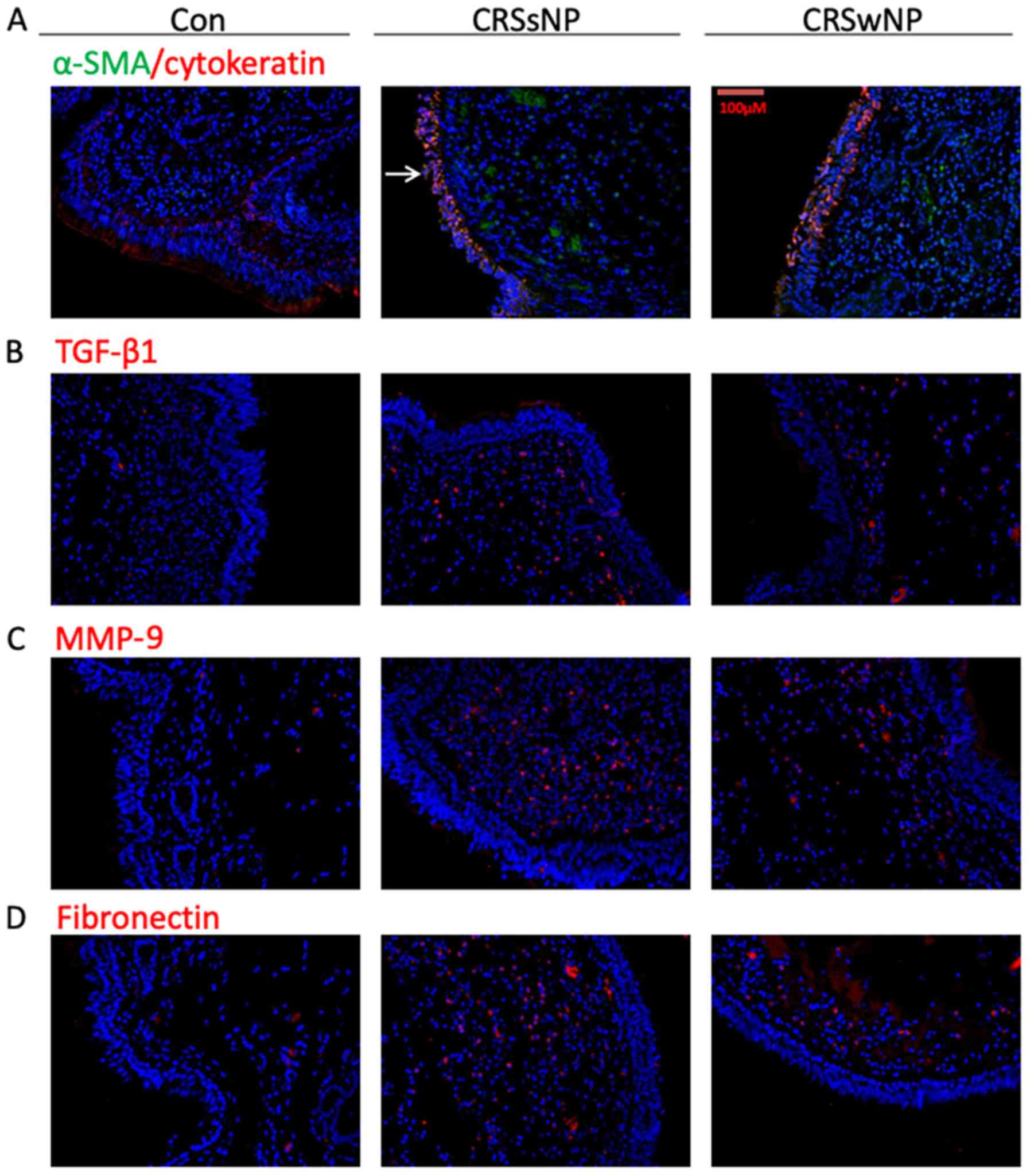 | Figure 2.Immunofluorescence staining of the
tissues from CRSsNP, CRSwNP and the control subjects. (A) Red
fluorescence labeling denotes α-SMA, green fluorescence labeling
keratin and yellow fluorescence is indicative of the cells in the
EMT process (arrow). (B) Red fluorescence labeling of TGF-β1. (C)
Red fluorescence labeling of MMP-9. (D) Red fluorescence labeling
of fibronectin. EMT, epithelial-mesenchymal transition; CRS,
chronic rhinosinusitis; α-SMA, α-smooth muscle actin; TGF-β1,
transforming growth factor-β1; MMP-9, matrix metallopeptidase 9;
CON, control group; CRSsNP, CRS without nasal polyps; CRSwNP, CRS
with nasal polyps. |
Characteristics of EMT in primary NECs
following TGF-β1 treatment
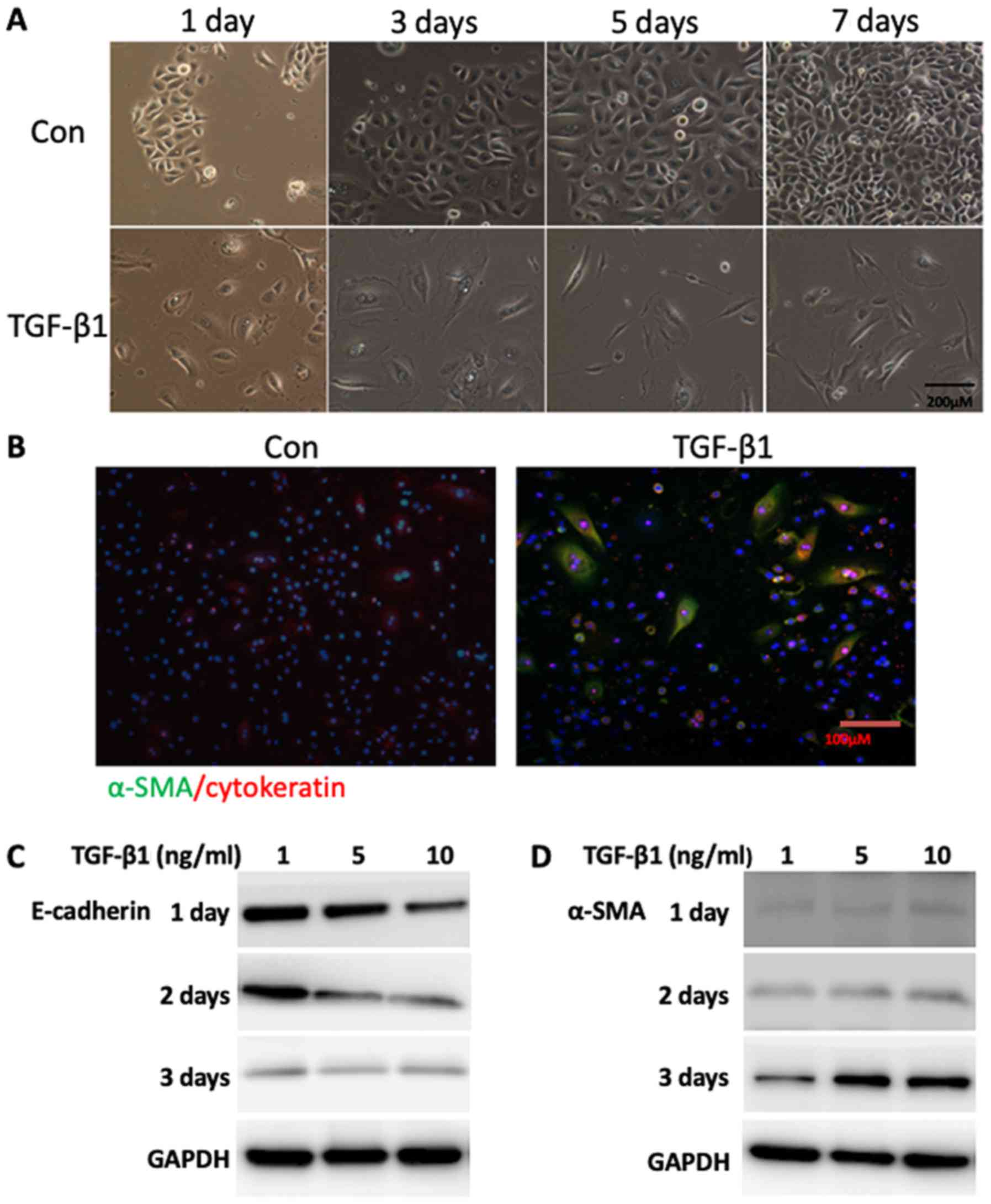
Following incubation with TGF-β1, the NECs acquired
a mesenchymal phenotype as demonstrated by phase contrast
microscopy. After 24 h of induction by TGF-β1, the junctions among
epithelial cells were interrupted, and the cells became dispersed
and flat. After 3 days, the cytoplasm began to show polarity,
extending in two opposite directions like tentacles. After 7 days,
over half of the cells presented typical long spindle fibroblasts
(Fig. 3A). In addition, NECs that
co-expressed α-SMA and cytokeratin were detected following TGF-β1
treatment by double-labeling immunofluorescence staining (Fig. 3A and B). In addition, the protein
levels of α-SMA were increased, whereas the levels of E-cadherin
were decreased following TGF-β1 treatment (Fig. 3C).
Correlation between α-SMA protein
expression and clinical features of CRS
A negative correlation was evident between α-SMA
protein expression and smoking. No association existed between
α-SMA levels and the remaining clinical characteristics (Table I).
 | Table I.Correlation analysis between α-SMA and
CRS characteristics. |
Table I.
Correlation analysis between α-SMA and
CRS characteristics.
|
|
| Correlation with
α-SMA (WB) |
|---|
|
|
|
|
|---|
| Item | Proportion or mean ±
SD | r | P-value |
|---|
| Age (years) | 46.04±15.82 | −0.280 | 0.167 |
| Sex (M/F) | 16/10 | −0.270 | 0.183 |
| Course of CRS
(years) | 7.06±6.63 | −0.223 | 0.273 |
| Allergic rhinitis
(+/-) | 11/15 | 0.013 | 0.952 |
| Asthma (+/-) | 2/24 | −0.281 | 0.165 |
| Smoking (+/-) | 7/19 | −0.402 | 0.042 |
| Aspirin allergy
(+/-) | 0/26 | / | / |
| Gastroesophageal
reflux (+/-) | 2/24 | 0.018 | 0.931 |
| History of sinus
surgery (+/-) | 3/23 | −0.363 | 0.068 |
With regard to preoperative parameters, no
correlation was noted between α-SMA levels and the symptoms noted
in RSOM-31 (Table SV). The α-SMA
levels were inversely correlated with the Lund-Kennedy score, while
they did not correlate with specific endoscopic signs (Table II). α-SMA levels were negatively
correlated with CT scores of anterior ethmoid sinus, while no
correlation was noted with the other paranasal sinuses, indicating
that CRS simply involving anterior ethmoid were less likely to
undergo EMT (Table SVI).
 | Table II.Correlation analysis between α-SMA
and preoperative endoscopic scores of CRS. |
Table II.
Correlation analysis between α-SMA
and preoperative endoscopic scores of CRS.
|
|
| Correlation with
α-SMA |
|---|
|
|
|
|
|---|
| Item | Mean score ±
SD | r | P-value |
|---|
| Polyps | 3.19±1.47 | −0.082 | 0.691 |
| Edema | 3.42±0.99 | −0.335 | 0.095 |
| Discharge | 3.23±1.11 | −0.363 | 0.068 |
|
Scarring/adhesion | 0.04±0.20 | 0.133 | 0.518 |
| Crusting | 0.23±0.65 | −0.369 | 0.064 |
| Endoscopic
score | 10.12±2.61 | −0.408 | 0.038 |
Correlation between α-SMA protein
expression and surgical outcomes of CRS
The levels of α-SMA were negatively correlated with
RSOM-31 symptoms including hyposmia, hearing loss, fatigue, and
reduced productivity (Table
III). A trend towards negative correlation was observed between
α-SMA levels and VAS score, although no significance was found.
Negative correlations were present between α-SMA levels and
endoscopic score, and between α-SMA levels and endoscopic signs,
including polyps, discharge and crusting (Table IV).
 | Table III.Correlation analysis between α-SMA
and postoperative symptom scores of the patients with CRS. |
Table III.
Correlation analysis between α-SMA
and postoperative symptom scores of the patients with CRS.
|
|
| Correlation with
α-SMA |
|---|
|
|
|
|
|---|
| Item | Mean score ±
SD | r | P-value |
|---|
| VAS score | 22.77±20.41 | −0.364 | 0.067 |
| RSOM - 31
score | 43.04±42.86 | −0.343 | 0.086 |
| Rhinobyon | 2.27±2.75 | −0.233 | 0.252 |
| Non-purulent nasal
mucus | 2.46±2.39 | −0.190 | 0.354 |
| Sneezing | 1.42±1.98 | 0.024 | 0.909 |
| Olfactory
decline | 4.27±5.77 | −0.472 | 0.015 |
| Nose backflow | 1.19±1.98 | 0.016 | 0.937 |
| Purulent nasal
mucus | 1.69±2.92 | 0.349 | 0.081 |
| Eye itching | 1.19±2.71 | 0.246 | 0.225 |
| Sore eyes | 0.54±1.14 | 0.103 | 0.618 |
| Difficulty falling
asleep | 0.69±1.93 | −0.161 | 0.432 |
| Night
awakening | 0.65±2.37 | −0.249 | 0.220 |
| Poor sleep | 1.42±2.34 | −0.300 | 0.136 |
| Wake up tired | 1.35±1.98 | −0.153 | 0.456 |
| Ear swelling | 0.50±1.66 | −0.240 | 0.237 |
| Tinnitus | 2.58±5.12 | −0.372 | 0.061 |
| Dizziness | 0.69±1.74 | −0.261 | 0.198 |
| Ear pain | 0.46±1.66 | −0.232 | 0.255 |
| Hearing loss | 1.73±3.65 | −0.413 | 0.036 |
| Weak | 2.15±2.63 | −0.537 | 0.005 |
| Loss of
efficiency | 0.88±1.90 | −0.420 | 0.033 |
| Lose focus | 1.38±2.86 | −0.030 | 0.884 |
| Headache | 0.88±2.76 | −0.037 | 0.859 |
| Facial pain | 0.23±0.51 | −0.301 | 0.135 |
| Cough | 1.31±1.83 | 0.230 | 0.259 |
| Dyspnea | 0.46±0.91 | −0.085 | 0.678 |
| Need tissue
frequently | 2.69±3.11 | −0.121 | 0.556 |
| Frequently rubbing
nose and eyes | 1.42±3.24 | −0.039 | 0.849 |
| Often blow
nose | 3.00±3.11 | −0.229 | 0.260 |
| Ozostomia | 0.96±1.59 | 0.072 | 0.728 |
| Irritable | 1.15±1.91 | −0.270 | 0.183 |
| Frustrated | 0.62±1.55 | −0.017 | 0.934 |
| Embarrassed | 0.77±1.61 | −0.206 | 0.313 |
 | Table IV.Correlation analysis between α-SMA
and postoperative endoscopic scores of the patients with CRS. |
Table IV.
Correlation analysis between α-SMA
and postoperative endoscopic scores of the patients with CRS.
|
|
| Correlation with
α-SMA |
|---|
|
|
|
|
|---|
| Item | Mean score ±
SD | r | P-value |
|---|
| Polyps | 0.19±0.57 | −0.439 | 0.025 |
| Edema | 0.65±0.94 | 0.129 | 0.529 |
| Discharge | 1.19±1.30 | −0.421 | 0.032 |
|
Scarring/adhesion | 0.00±0.00 | / | / |
| Crusting | 0.81±0.98 | −0.709 | <0.001 |
| Endoscopic
score | 2.81±2.23 | −0.640 | <0.001 |
Discussion
Chronic rhinosinusitis (CRS) is a prevalent disease
in otolaryngology. It affects the quality of life (QOL) of patients
and imposes a heavy burden on socioeconomic resources (23). The abnormal deposition of
extracellular matrix (ECM) in the mucosa is one of the pathological
causes of CRS. Epithelial-mesenchymal transition (EMT) plays
crucial roles in tissue remodeling in several pathophysiological
processes (24). Recently, the
induction of EMT was reported in CRS by several studies (10–16).
Nevertheless, inconsistent results were provided. A limited number
of studies have indicated that EMT is involved in patients with CRS
with nasal polyps (CRSwNP) and not in patients with CRS without
polyps (CRSsNP) (10–12), while Hupin et al
demonstrated increased vimentin expression levels in both groups
(16). The majority of these
studies explored the association of CRSwNP with the aforementioned
markers, while a limited number of studies focused on CRSsNP.
Therefore, additional evidence is required to unravel the
involvement of EMT markers in CRSsNP. In the present study, the
cells were stained using double-labeling with α-SMA and
cytokeratin, indicating the induction of EMT in CRS. To the best of
our knowledge, this is the first study that utilized double-label
staining to assess EMT induction in CRS. Double-label staining is
an intuitive evidence for EMT that illustrates whether cells are
undergoing this process. Several double-label stained cells were
observed in both CRSsNP and CRSwNP, although the fluorescence noted
was weak in CRSwNP. EMT can elicit loss of epithelial markers and
gain of mesenchymal markers. Increased expression of epithelial
(E-cadherin) and mesenchymal markers (α-SMA, vimentin, fibronectin,
MMP-9) was noted in both CRSsNP and CRSwNP. This is inconsistent
with the findings of Konnecke et al suggesting that the
induction of EMT was partially present in CRS (15). Partial EMT was proposed in oncology
to describe an intermediate stage of EMT, which generates cancer
cells with a higher degree of malignancy than those with a complete
EMT phenotype (25,26). However, the underlying mechanisms
require further investigation.
In contrast to previous studies, the present report
indicated different EMT features in CRSsNP than those noted in
CRSwNP, which may be attributed to histological distinctions.
Edematous and fibrous types are common histological patterns of
nasal polyps (NPs) (22).
Edematous NPs exhibit a thin layer of epithelium and a lower number
of fibroblasts in ECM compared with those noted in fibrous NPs. In
the present study, edematous and fibrous types comprised 84.6 and
15.4% of the NPs, respectively (Fig.
S2). Since pathological classification was not conducted in the
previous studies, the corresponding comparisons with the present
study could not be performed. This suggests that further
investigations are required for a more accurate conclusion
(10–12).
Although EMT was observed in the nasal epithelial
cells (NECs), several studies conducted previously used cell lines
as models instead of primary NECs (11–13).
Moreover, certain studies have investigated NECs derived from NPs
(10), and reports on primary NECs
from CRSsNP remain limited (14–16).
The incidence of CRSsNP is higher than that of CRSwNP and the two
types of CRS may have distinct pathogenic mechanisms. Therefore, it
is of great significance to understand the pathogenesis of CRSsNP.
We investigated EMT-related features including morphology and
expression of the relevant markers in primary NECs. Following
TGF-β1 exposure, NECs acquired a mesenchymal shape. In addition,
NECs co-expressing α-SMA and cytokeratin were detected and TGF-β1
was able to induce α-SMA expression and inhibit E-cadherin
expression in NECs. The present study validated that NECs could
respond to TGF-β1 in a time dependent manner, which is consistent
with previous research (15).
Taken collectively, the data demonstrated that primary NECs from
CRS were able to undergo EMT in response to TGF-β1 treatment.
The TGF-β1 pathway plays a key role in EMT of many
pathological conditions (27).
TGF-β1 regulates EMT through canonical or non-canonical pathways.
The canonical pathway is the SMAD signaling pathway. When TGF-β1
binds to TGF-β receptor II (TGFβR II), the cascade signaling is
initiated. TGFβR II recruits and phosphorylates TGFβR I. TGFβR I
then phosphorylates R-Smad which is a complex formed with Smad2 and
Smad3. Phosphorylated R-Smad forms Co-Smad by binding to Smad4,
which then shuttles into the nucleus to regulate transcription via
binding to target genes (28). The
non-canonical pathways are triggered when the TβRI/II complex
activates certain signals such as MAPK, p38 or JNK pathways
(27). Currently, it has not been
determined whether canonical or non-canonical TGF-β1 pathways are
implicated in EMT of CRS, which warrants further investigation in
the future.
The association between EMT markers and clinical
parameters in CRS has been scarcely investigated. Hupin et
al reported that increased expression of vimentin was
associated with CT score (16).
Therefore, the investigation of the association of EMT markers with
the clinical features of CRS is helpful for the treatment of such
patients. In the present study, partial EMT was noted in the sinus
mucosa of patients with CRS, which exhibited a different expression
pattern of epithelial markers compared with that noted in the
typical EMT process. In sinonasal mucosa, fibroblast markers, such
as fibronectin and vimentin are expressed not only in fibroblasts,
but also in endothelial cells, while the α-SMA protein is
specifically expressed in myofibroblasts (29). Therefore, we assessed the
correlation between α-SMA levels and clinical characteristics of
patients with CRS. In the present study, no association was noted
between α-SMA levels and total CT score. It is interesting to note
that a negative correlation was found between α-SMA levels and CT
score of the anterior ethmoids, suggesting that CRS involving
anterior ethmoid was less likely to undergo EMT. This may be
related to the anatomical differences of the tissues, since natural
drainage of the anterior ethmoid is usually better than that of the
other paranasal sinuses. Furthermore, this is the first study to
evaluate the prognostic value of the EMT marker α-SMA in the
surgical outcomes of CRS subjects. Although α-SMA was not
associated with preoperative symptoms, it was negatively correlated
with postoperative symptoms including hyposmia, hearing loss,
fatigue and reduced productivity. These were systemic symptoms
rather than nasal symptoms, suggesting that the use of α-SMA as a
marker was more relevant to the overall QOL of CRS following
surgery. With regard to the endoscopic parameters, α-SMA levels
were negatively correlated with preoperative and postoperative
scores, and with postoperative signs including polyps, discharge
and crusting. These unexpected results provided the first
preliminary evidence showing that EMT could predict the surgical
outcome of CRS subjects. This hypothesis requires verification
using larger sample sizes. According to our clinical experience,
the prognosis of CRSsNP is better than that of CRSwNP as a whole,
which might be an explanation for the degree of EMT noted in CRSsNP
compared with that of CRSwNP.
In conclusion, it was demonstrated that partial EMT
occurred in CRS in vivo and that primary NECs responded to
TGF-β1 induction in vitro, indicating that EMT is involved
in the pathogenesis of CRS. In addition, we demonstrated that α-SMA
could be a predictor for improved postoperative endoscopic and
symptomatic outcomes in CRS. These findings require further
validation using a large cohort study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 8187040939 and
81600783).
Availability of data and materials
All the data generated and analyzed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
HL, XCS and DHW conceived and designed the
experiments. HL, HW and QL performed the experiments. LH, HPY and
QL analyzed the data and conducted the statistical analysis. HL and
HW wrote the manuscript. All the authors read and approved the
final version of the manuscript and agree to be accounTable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of AEENTH and written informed consent was obtained from
every patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bhattacharyya N and Gilani S: Prevalence
of potential adult chronic rhinosinusitis symptoms in the United
States. Otolaryngol Head Neck Surg. 159:522–525. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi JB, Fu QL, Zhang H, Cheng L, Wang YJ,
Zhu DD, Lv W, Liu SX, Li PZ, Ou CQ and Xu G: Epidemiology of
chronic rhinosinusitis: Results from a cross-sectional survey in
seven Chinese cities. Allergy. 70:533–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stone RC, Pastar I, Ojeh N, Chen V, Liu S,
Garzon KI and Tomic-Canic M: Epithelial-mesenchymal transition in
tissue repair and fibrosis. Cell Tissue Res. 365:495–506. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang B, Guan Y, Shen HJ, Zhang LH, Jiang
JX, Dong XW, Shen HH and Xie QM: Akt/PKB signaling regulates
cigarette smoke-induced pulmonary epithelial-mesenchymal
transition. Lung Cancer. 122:44–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stasikowska-Kanicka O,
Wagrowska-Danilewicz M and Danilewicz M: Immunohistochemical study
EMT-related proteins in HPV-, and EBV-negative patients with
sinonasal tumours. Pathol Oncol Res. 22:781–788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lai T, Li Y, Chen M, Pan G, Wen X, Mai Z,
Yuan Y, Lv Y, Lv Q, Cen R, et al: Heparin-binding epidermal growth
factor contributes to COPD disease severity by modulating airway
fibrosis and pulmonary epithelial-mesenchymal transition. Lab
Invest. 98:1159–1169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun J, Gu X, Wu N, Zhang P, Liu Y and
Jiang S: Human antigen R enhances the epithelial-mesenchymal
transition via regulation of ZEB-1 in the human airway epithelium.
Respir Res. 19:1092018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shin HW, Cho K, Kim DW, Han DH,
Khalmuratova R, Kim SW, Jeon SY, Min YG, Lee CH, Rhee CS and Park
JW: Hypoxia-inducible factor 1 mediates nasal polypogenesis by
inducing epithelial-to-mesenchymal transition. Am J Respir Crit
Care Med. 185:944–954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee M, Kim DW, Yoon H, So D, Khalmuratova
R, Rhee CS, Park JW and Shin HW: Sirtuin 1 attenuates nasal
polypogenesis by suppressing epithelial-to-mesenchymal transition.
J Allergy Clin Immunol. 137:87–98.e7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Razali RA, Nik Ahmad Eid NAH, Jayaraman T,
Amir Hassan MA, Azlan NQ, Ismail NF, Sainik N, Yazid MD, Lokanathan
Y, Saim AB and Hj Idrus RB: The potential of Olea europaea extracts
to prevent TGFβ1-induced epithelial to mesenchymal transition in
human nasal respiratory epithelial cells. BMC Complement Altern
Med. 18:1972018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang HW, Lee SA, Shin JM, Park IH and Lee
HM: Glucocorticoids ameliorate TGF-β1-mediated
epithelial-to-mesenchymal transition of airway epithelium through
MAPK and Snail/Slug signaling pathways. Sci Rep. 7:34862017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park IH, Kang JH, Shin JM and Lee HM:
Trichostatin A inhibits epithelial mesenchymal transition induced
by TGF-β1 in Airway Epithelium. PLoS One. 11:e01620582016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Konnecke M, Burmeister M, Pries R, Boscke
R, Bruchhage KL, Ungefroren H, Klimek L and Wollenberg B:
Epithelial-mesenchymal transition in chronic rhinosinusitis:
Differences revealed between epithelial cells from nasal polyps and
inferior turbinates. Arch Immunol Ther Exp (Warsz). 65:157–173.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hupin C, Gohy S, Bouzin C, Lecocq M,
Polette M and Pilette C: Features of mesenchymal transition in the
airway epithelium from chronic rhinosinusitis. Allergy.
69:1540–1549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fokkens W, Lund V and Mullol J; European
Position Paper on Rhinosinusitis and Nasal Polyps Group, : European
position paper on rhinosinusitis and nasal polyps 2007. Rhinol
Suppl. 20:1–136. 2007.PubMed/NCBI
|
|
18
|
Zhang L and Zhang LH: Comparison of
different endoscopic scoring systems in patients with chronic
rhinosinusitis: Reliability, validity, responsiveness and
correlation. Rhinology. 55:363–368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greguric T, Trkulja V, Baudoin T, Grgic M,
Smigovec I and Kalogjera L: Differences in the sino-nasal outcome
test 22 and visual analog scale symptom scores in chronic
rhinosinusitis with and without nasal polyps. Am J Rhinol Allergy.
30:107–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dietz de Loos DA, Hopkins C and Fokkens
WJ: Symptoms in chronic rhinosinusitis with and without nasal
polyps. Laryngoscope. 123:57–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hellquist HB: Nasal polyps update.
Histopathology. Allergy Asthma Proc. 17:237–242. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sedaghat AR: Chronic Rhinosinusitis. Am
Fam Physician. 96:500–506. 2017.PubMed/NCBI
|
|
24
|
Campbell K: Contribution of
epithelial-mesenchymal transitions to organogenesis and cancer
metastasis. Curr Opin Cell Biol. 55:30–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lovisa S, LeBleu VS, Tampe B, Sugimoto H,
Vadnagara K, Carstens JL, Wu CC, Hagos Y, Burckhardt BC,
Pentcheva-Hoang T, et al: Epithelial-to-mesenchymal transition
induces cell cycle arrest and parenchymal damage in renal fibrosis.
Nat Med. 21:998–1009. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sader F, Denis JF, Laref H and Roy S:
Epithelial to mesenchymal transition is mediated by both TGF-β
canonical and non-canonical signaling during axolotl limb
regeneration. Sci Rep. 9:11442019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weiss A and Attisano L: The TGFbeta
superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol.
2:47–63. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Darby IA, Zakuan N, Billet F and
Desmoulière A: The myofibroblast, a key cell in normal and
pathological tissue repair. Cell Mol Life Sci. 73:1145–1157. 2016.
View Article : Google Scholar : PubMed/NCBI
|