Introduction
Osteosarcoma (OS) is the most common type of
malignant bone tumor in teenagers; a few patients are diagnosed at
terminal stage, which is usually accompanied by lung metastases
(1–3). The 5-year survival rate is <70%
with a high degree of malignancy and early metastasis (4). Despite accurate diagnoses, the
preoperative administration of chemotherapy, operative treatments
or even adjuvant chemotherapy after operation, 30–50% of patients
still develop distant metastasis. Fewer than 20% of patients
survive 5 years after disease onset. To improve early diagnosis
efficiency, novel therapeutic targets for implementing precision
treatment are needed and individualized treatments for OS hold
great promise. Therefore, investigation into the mechanisms of OS
cell occurrence, invasion and metastasis may lead to the
identification of potential molecular targets for OS treatment.
Annexin A3 (ANXA3) is a member of the Annexin
superfamily (5), which plays a
significant role in tumor occurrence, cell migration, immune
regulation and drug-fasting (6).
In mammalian cells, ANXA3 is usually localized to the cytoplasm,
maintaining a stable form that can affect the cytoskeleton or
proteins that mediate the cell-extracellular matrix, and are
involved in cell transduction, differentiation, migration and
apoptosis (7–8). In recent years, it has been shown
that the overexpression of ANXA3 is associated with ovarian, lung,
liver, prostatic, colorectal and pancreatic carcinoma (9–16),
and with drug resistance (16–18).
The abnormal expression of ANXA3 provides more direct methods for
diagnosis, determining pathological stage and in the treatment of
tumors, thus it has become a key area of research for treating
tumors (19). However, the
function and effect of ANXA3 on the regulation of OS cells has not
been investigated. Therefore, determining the expression and
significance of ANXA3 in OS cells might provide potential
therapeutic targets for treating OS. To the best of our knowledge,
the present study is the first to confirm that the overexpression
of ANXA3 is closely associated with OS cell proliferation and
apoptosis, as determined through a series of different
experiments.
The present study investigated the role of ANXA3 in
osteoblasts and the OS cell lines HOS and U2OS. First, it was
confirmed that ANXA3 was expressed in osteoblasts as well as in HOS
and U2OS cells. Secondly, ANXA3 was revealed to be significantly
upregulated in HOS and U2OS cells. Finally, it was demonstrated
that the inhibition of ANXA3 expression prevented tumor growth and
promoted OS cell apoptosis. These results suggest a significant
role for ANXA3 in the OS cell lines HOS and U2OS and highlight its
potential application in OS therapy.
Materials and methods
Materials
Formaldehyde and Triton X-100 were purchased from
Beijing Solarbio Science & Technology Co., Ltd. and diluted to
the indicated final concentrations. Anti-ANXA3 (1:1,000; cat. no.
ab33068) and mouse monoclonal anti-β-actin antibodies (1:2,000;
cat. no. ab8226) were obtained from Abcam. Horseradish peroxidase
(HRP)-conjugated anti-rabbit secondary antibodies (1:5,000; cat.
no. ZB2301) and HRP-conjugated anti-mouse secondary antibodies
(1:5,000; cat. no. ZB2305) were obtained from ZSGB-BIO; OriGene
Technologies, Inc. ProLong® Diamond Antifade Mountant
and 4′, 6-diamidino-2-phenylindole (DAPI) were obtained from Thermo
Fisher Scientific, Inc. and the Lipofectamine 2000™ transfection
reagent was obtained from Invitrogen; Thermo Fisher Scientific,
Inc. The RNAprep pure Cell kit and the Transcriptor First Strand
cDNA synthesis kit were purchased from Tiangen Biotech Co., Ltd.
The RNA expression profiles of ANXA3 were obtained from The Cancer
Genome Atlas (TCGA) database (www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga).
Annexin V-phycoerythrin (PE)/7-aminoactinomycin D (AAD) Apoptosis
Detection kit was obtained from BD Biosciences.
Cell culture
The human osteoblast cell line hFOB, and the human
osteosarcoma cell lines HOS and U2OS were obtained from the Cell
Collection of Chinese Academy of Sciences. HOS and U2OS cells were
cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2. While hFOB cells were cultured in DMEM: Nutrient
Mixture F-12 (DMEM/F12; Gibco; Thermo Fisher Scientific, Inc.) with
0.3 mg/ml G418 (Beijing Solarbio Science & Technology Co.,
Ltd.) at 33.5°C with 5% CO2. All media were supplemented
with 10% fetal bovine serum (Gemini Bio Products) and 100 µg/ml
Penicillin-Streptomycin Solution (Beijing Solarbio Science &
Technology Co., Ltd.).
Immunofluorescence assay
Cells were seeded in 6-well plates at a density of
1×106 cells/ml and fixed in formaldehyde (100%) at 37°C
for 5 min, after which they were allowed to attach for 2 h. Next,
0.1% Triton X-100 was added to the plates for 5 min and blocked for
1 h at 37°C with 1% BSA (Thermo Fisher Scientific, Inc.) They were
then washed with 0.01 mol/l phosphate buffer solution (PBS) and
incubated with anti-ANXA3 (1:1,000; cat. no. ab33068; Abcam)
overnight at 4°C on a shaking table. Incubation with Goat
Anti-Mouse Immunoglobulin G (cat. no. ab6708; Abcam) was performed
for 1 h at room temperature, and then cells were stained with DAPI
dihydrochloride for 3 min at room temperature. Then Antifade
Mounting Medium (Thermo Fisher Scientific, Inc.) was added to cells
at 4°C for 5 min in the dark following washing with
ddH2O and the staining results were observed by laser
confocal microscopy (Carl Zeiss AG; magnification, ×40).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Osteoblasts were planted in a culture bottle with
DMEM/F12 medium while HOS and U2OS cells were cultured with DMEM.
Total RNA was extracted with the RNAsimple total RNA kit (Tiangen
Biotech Co., Ltd.) from cells then reverse transcribed to cDNA
using a FastQuant RT kit (Tiangen Biotech Co., Ltd.) at 42°C for 15
min, followed by two incubation at 95°C for 3 min and at 4°C for 5
min. Assessment of gene expression was performed using a SYBR Green
PCR kit (Takara Bio, Inc.) using the iCycler (Bio-Rad Laboratories,
Inc.) according to the manufacturer's instructions. Briefly, RNase
Free ddH2O was added into 2 µl of 5X gDNA Eraser Buffer,
1 µl of gDNA Eraser, 1 µl of Total RNA up to 10 µl, and vortex
mixed at 42°C for 15 min, followed by storage at −20°C. Next, 1 µl
10X FastRT Buffer, 2 µl RT Primer Mix and 2 µl FQ-RT Primer Mix
were added as well as RNase Free ddH2O to a total volume
of 10 µl, followed by gentle mixing. PCR was performed by
activating the DNA polymerase at 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 45 sec, and a final
extension at 75°C for 10 min. The gene primer sequences were as
follows: For human ANXA3, forward 5′-CCCATCAGTGGATGCTGAAG-3′ and
reverse 5′-TCACTAGGGCCACCATGAGA-3′; and GAPDH (the internal
control), forward 5′-GGATATTGTTGCCATCAATGACCT-3′ and reverse
5′-AGCCTTCTCCATGGTGGTGAAGA-3′. Finally, gene expression data were
evaluated according to the 2−ΔΔCq method (20).
Western blot analysis
Proteins were extracted using RIPA lysis buffer
(Beyotime Institute of Biotechnology), then cells were centrifuged
at 12,000 × g for 20 min at 4°C. Supernatants containing 20–30 µg
protein were collected, and the protein content was quantified
using a BCA protein assay kit (Thermo Fisher Scientific, Inc.).
Samples (20 µg/lane) were separated by 12% SDS-PAGE and transferred
to PVDF membranes. The blots were blocked with 5% skim milk in TBST
for 2 h at room temperature, incubated with primary anti-ANXA3
(1:1,000) or anti-β-actin (1:2,000) primary antibodies at 4°C
overnight. After washing with TBST (0.05% Tween-20), the membranes
were incubated with HRP-conjugated anti-rabbit secondary antibodies
(1:5,000) or HRP-conjugated anti-mouse secondary antibodies
(1:5,000) at 27°C for 2 h. The bands were visualized using an
enhanced chemiluminescence detection kit (Tiangen Biotech Co.,
Ltd.) and quantified by Quantity One software (version 2.4; Bio-Rad
Laboratories, Inc.).
Cell transfection
Small interfering RNA (siRNA) ANXA3-651 (forward
5′-GAAAUCUUAACUACCAGGATT-3′, reverse 3′-UCCUGGUAGUUAAGAUUUCTT-5′),
siRNA ANXA3-732 (forward 5′-GAUGACAUUAGUUCCGAAATT-3′, reverse
3′-UUUCGGAACUAAUGUCAUCTT-5′), siRNA ANXA3-1094 (forward
5′-GAAGGGUAUUGGAACUGAUTT-3′, reverse 3′-AUCAGUUCCAAUACCCUUCTT-5′)
and negative control (NC) siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′) were
designed and synthesized by the Whitehead Institute for Biomedical
Research. GAPDH (forward 5′-UGACCUCAACUACAUGGUUTT-3′, reverse
3′-TTACUGGAGUUGAUGUACCAA−5′) was used as positive control, negative
control siRNA (forward 5′-UUCUCCGAACGUGUCACGUTT-3′, reverse
3′-TTAAGAGGCUUGCACAGUGCA-5′) was used as negative control, and a
no-transfection group was used as the blank control. In order to
inhibit the expression of ANXA3, cells at 50–60% confluence were
seeded overnight and transfected with 50 nM siRNAs targeting ANXA3
or control siRNAs using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) in OptiMEM (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
48 h of transfection, cells were collected for subsequent
research.
Flow cytometric analysis
After 48 h of transfection, cells were plated in
6-well plates at a density of 1×105 for 24 h, then
harvested and washed with ice-cold PBS (Beijing Solarbio Science
& Technology Co., Ltd.). All cells were digested with EDTA-free
typsin (Beijing Solarbio Science & Technology Co., Ltd.) and
stained with the Annexin V-PE/7-AAD apoptosis detection kit (BD
Biosciences), and incubated in the dark for 15 min at room
temperature. Apoptosis was analyzed using CellQuest software
(version 5.1; BD Biosciences) with a FACSCalibur flow cytometer (BD
Biosciences).
Statistical analysis
All quantitative assays were performed in triplicate
and statistical analysis was performed using SPSS 18.0 software
(IBM Corp.). Experimental data were expressed as the mean ±
standard deviation. Comparisons in datasets containing multiple
groups were conducted using one-way analysis of variance followed
by Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
ANXA3 expression in the OS cell lines
HOS and U2OS
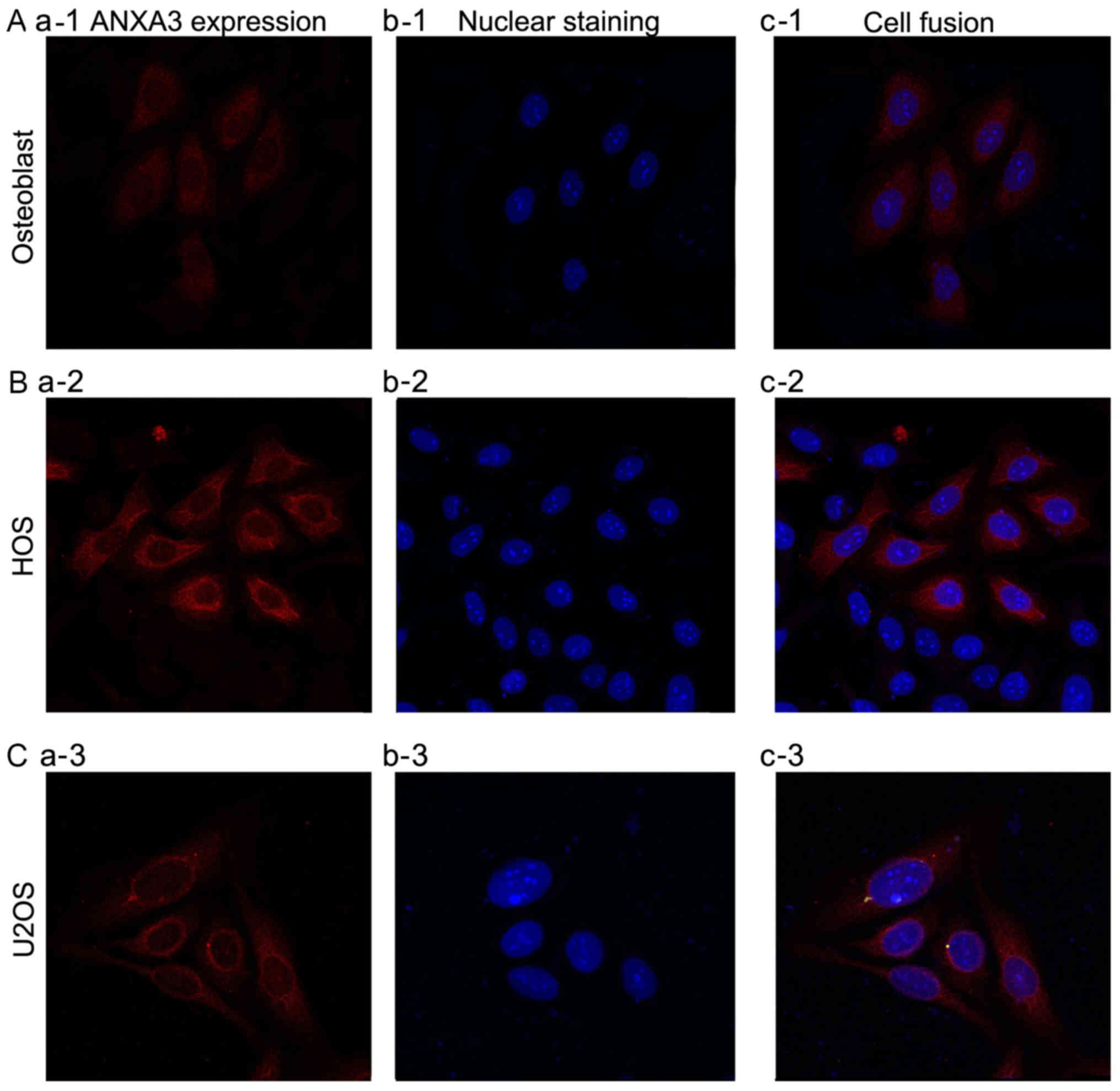
In order to determine whether ANXA3 was expressed in
osteoblasts, and the OS cell lines HOS or U2OS, an
immunofluorescence assay was performed and the results were
observed by laser confocal microscopy. The results showed that
ANXA3 was expressed in osteoblasts, and HOS and U2OS cells
(Fig. 1), and was markedly
expressed in both HOS and U2OS cells.
ANXA3 mRNA expression levels in
osteoblast, and the OS cell lines HOS and U2OS
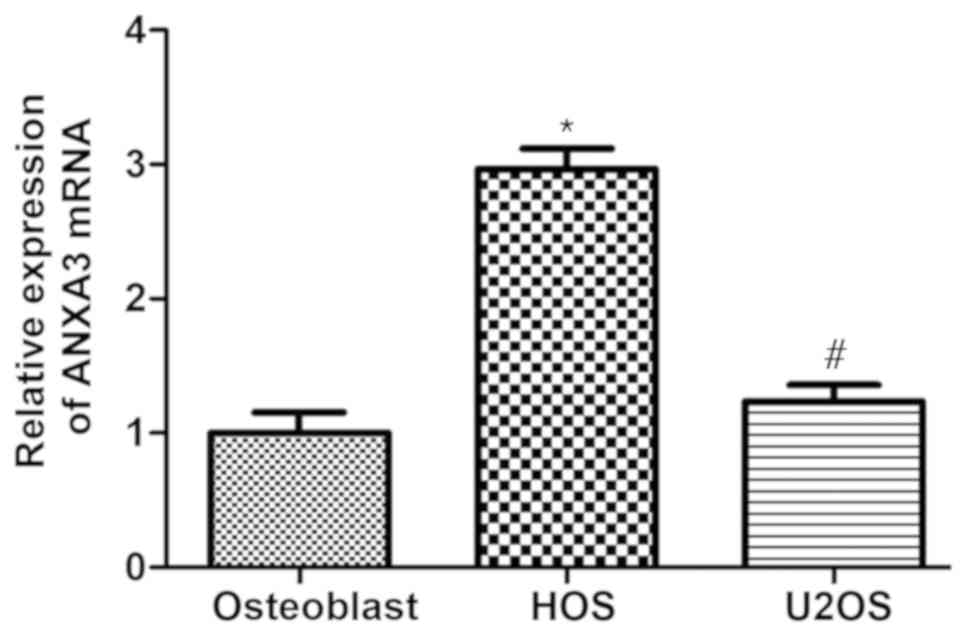
In order to investigate whether ANXA3 mRNA was
expressed in osteoblasts, and HOS and U2OS cells, the present study
performed RT-qPCR. The results revealed that ANXA3 mRNA was
expressed in the OS cell lines HOS and U2OS as well as in
osteoblasts (Fig. 2). The mRNA
levels were significantly upregulated in both HOS and U2OS cells
compared with the osteoblasts; ANXA3 expression in HOS cells was
increased by ~2.99-fold and in U2OS cells levels were also
increased by 1.25-fold when compared with osteoblasts (Fig. 2). Therefore, the subsequent
experiments were designed based on these results.
ANXA3 protein expression levels in
osteoblasts, and the OS cell lines HOS and U2OS
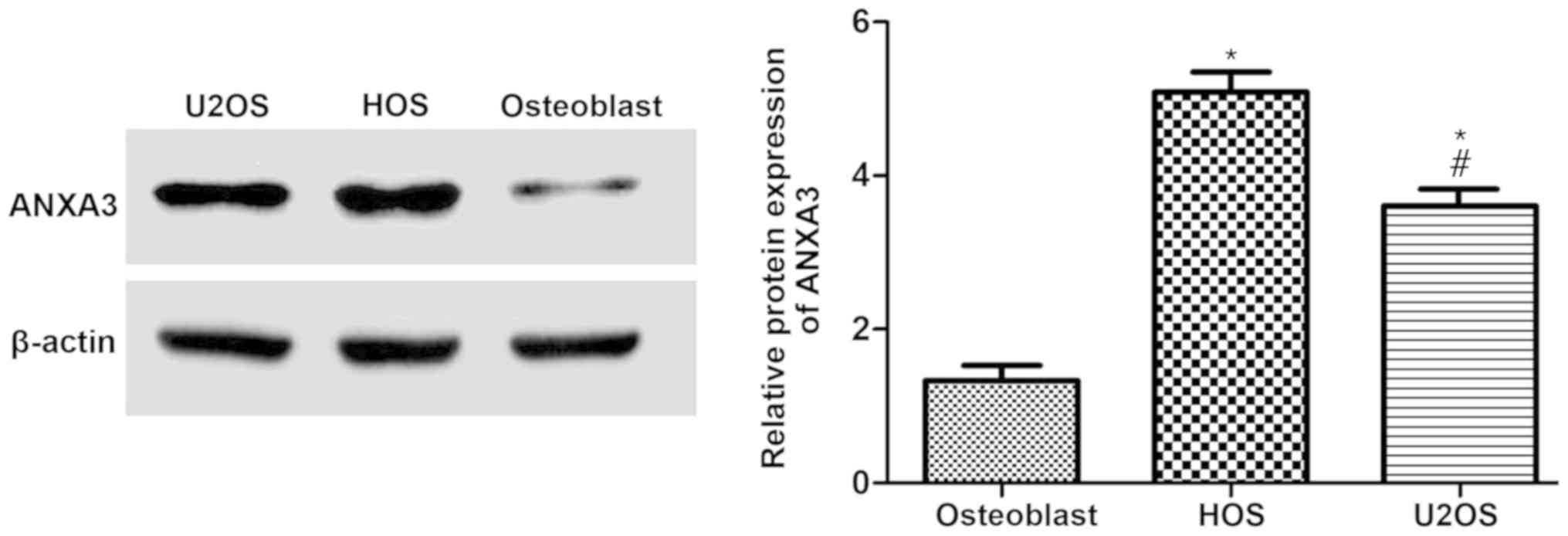
In order to determine whether ANXA3 protein was
involved in osteoblasts, and HOS and U2OS cells, the extracted
proteins were examined and quantified by western blotting. The
results demonstrated that ANXA3 was significantly increased in HOS
and U2OS cells when compared with osteoblasts (Fig. 3A). Furthermore, the protein
expression of ANXA3 in HOS cells increased by 3.25-fold, and
increased by ~2.33-fold in U2OS cells (Fig. 3B).
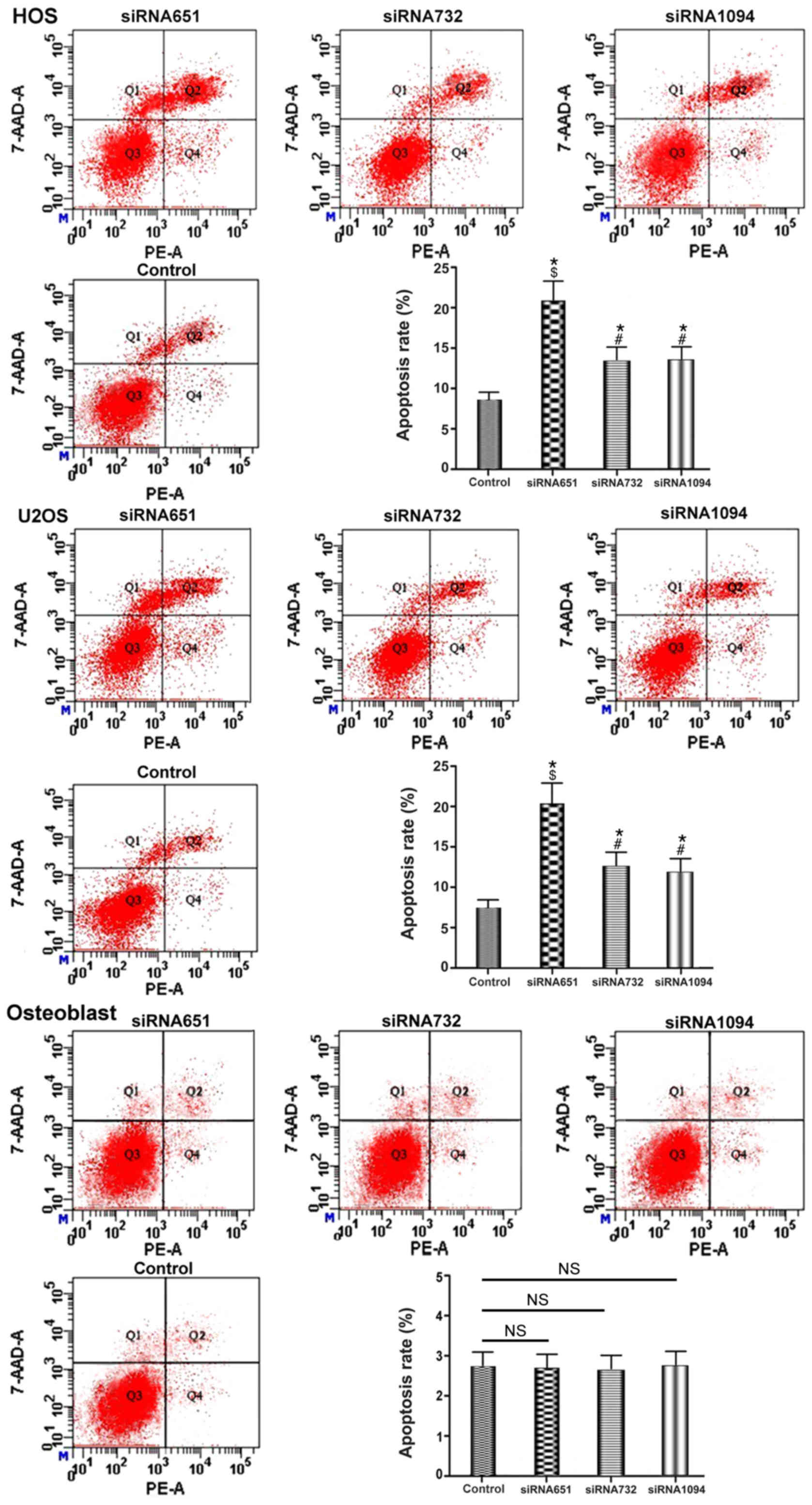
ANXA3 decreases apoptosis in the HOS
and U2OS OS cell line
On the basis of the aforementioned results, it was
concluded that the expression of ANXA3 in the OS cell lines HOS and
U2OS was significantly different when compared with the osteoblast
group. In addition, ANXA3 in HOS cells was significantly increased
when compared with U2OS cells as determined by RT-qPCR and western
blot analyses. To investigate whether the expression levels of
ANXA3 were associated with the apoptotic rate, the present study
downregulated ANXA3 via siRNA transfection for 48 h and the
collected cells were examined via western blotting, RT-qPCR and
flow cytometry.
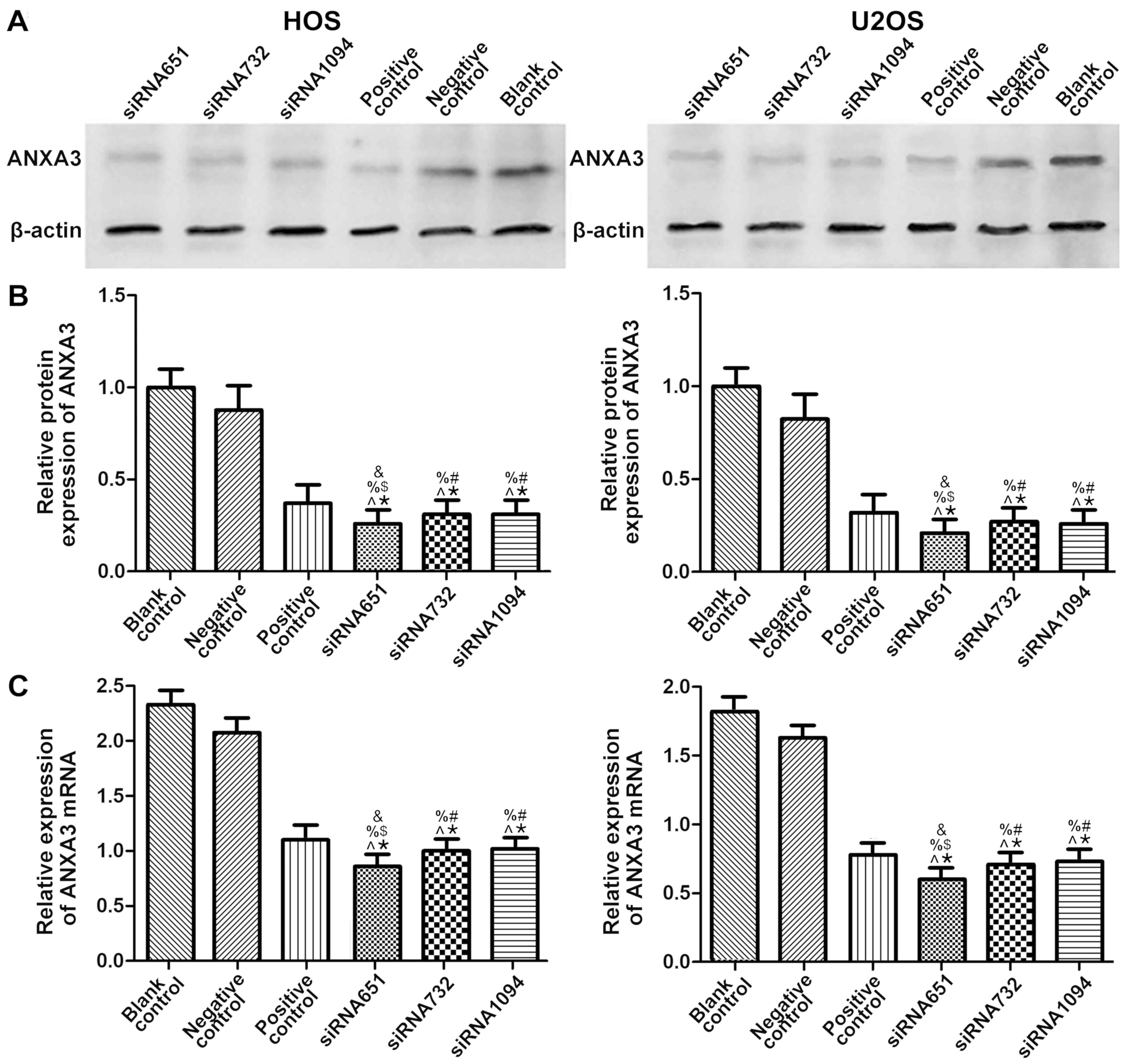
The present results indicated that ANXA3 was
significantly decreased in both HOS and U2OS cells (Fig. 4A). Compared with the blank control
group, in HOS cells the protein expression of the siRNA651 group
decreased to 0.26-fold, whereas both the siRNA732 and siRNA1094
groups decreased to 0.31-fold. In addition, the positive control
group decreased to 0.37-fold and the NC group exhibited a 0.87-fold
decrease (P<0.05; Fig. 4B). In
U2OS cells, compared with the blank control group, the protein
expression of ANXA3 in the siRNA651 group decreased to 0.21-fold,
the siRNA732 group decreased to 0.27-fold and the siRNA1094 group
decreased to 0.26-fold. In addition, the positive control group
decreased to 0.32-fold, and the NC group caused a 0.81-fold
decrease (P<0.05; Fig. 4B).
The mRNA expression levels of ANXA3 in HOS cells,
compared with the blank control group, in the siRNA651 group was
decreased by ~0.37-fold, the siRNA732 group was approximately
decreased to 0.43-fold, and the siRNA1094 group was approximately
decreased to 0.44-fold. The positive control group was decreased to
~0.47-fold, and the NC group was decreased to ~0.91-fold
(P<0.05; Fig. 4C). The mRNA
expression levels of ANXA3 in the U2OS cells, when compared with
the blank control group, in the siRNA651 group were decreased to
~0.33-fold, the siRNA732 group was decreased to ~0.39-fold, and the
siRNA1094 group was decreased ~0.44-fold. The positive control
group was decreased to ~0.43-fold, and the negative control group
was decreased to ~0.89-fold (P<0.05; Fig. 4C).
The apoptotic rate in the HOS cells of the control
group was 8.1%, whereas in the siRNA651 group it was 21.3%, for the
siRNA732 group it was 13.55%, and in the siRNA1094 group it was
13.82%, respectively. In addition, in U2OS cells, the apoptotic
rate for the control group was 7.4%, whereas in the siRNA651 group
it was 21.08%, in the siRNA732 group it was 13.63%, and in the
siRNA1094 group it was 12.52% (P<0.05; Fig. 5). In the osteoblast group, the
apoptotic rate in the control group was 2.76%, while in the
siRNA651 group it was 2.75%, in the siRNA732 group it was 2.75% and
in the siRNA1094 group it was 2.78% (P>0.05; Fig. 5). The results suggested that ANXA3
downregulation may increase apoptotic rates of the OS cell lines
HOS and U2OS but it had no significant effect on osteoblasts.
Discussion
The results of the present study demonstrated that
ANXA3 was expressed in the OS cell lines HOS and U2OS as well as
osteoblasts. Notably, the ANXA3 expression levels in HOS and U2OS
were significantly increased when compared with osteoblasts. In
addition, the present study further confirmed that downregulation
of AXNA3 induced apoptosis. Thus, the present findings suggest that
the expression of ANXA3 may be critically related to OS cells, and
inhibiting ANXA3 may be an effective method for OS therapy.
A previous study reported that the abnormal
expression of ANXA3 has been observed in various types of tumors
and is associated with cancer progression making it a novel
therapeutic target (21). For
example, several studies have reported that ANXA3 activated the JNK
signaling pathway of stress-activated proteins in hepatocellular
carcinoma (22–24). Furthermore, previous studies have
demonstrated that the JNK signaling pathway activated
CD133+ glioblastoma stem cell self-renewal to promote
hepatocellular carcinoma differentiation (25–27).
The JNK signaling pathway plays a significant role in
ANXA3-mediated cancer stem cells. It has been reported that
blocking JNK pathway reduced the expression of ANXA3 as determined
by RT-qPCR; this result suggested that the expression levels of
ANXA3 in hepatocellular carcinoma cells may be positively
correlated with the JNK pathway (12). Further study confirmed that
transfection of dendritic cells with ANXA3 activated autologous
CD4+ T cells and CD8+ T cells to kill
CD133+ hepatocellular carcinoma cells (28). Other previous studies have
suggested that increased ANXA3 expression may be associated with
the drug resistance of ovarian cancer (16,29),
poor prognosis of breast cancer (29,30)
and increased lymphatic metastasis (31).
Previous studies have also confirmed that ANXA3 is
highly correlated with many types of tumors indicating that the
activation of ANXA3 may increase proliferative activity in various
types of cancers (32–35). A new therapeutic method that could
inhibit ANXA3 could also decrease the activation of relevant types
of cancers. The present data suggested that the expression of ANXA3
may be positively correlated with OS and demonstrated the
previously mentioned hypothesis. Therefore, ANXA3 is expected to
become a new target for OS therapy.
The traditional Chinese medicine celastrol is a
natural pentacyclic triterpene, extracted from the Thunder God Vine
and has an anti-cancer effect in many malignant tumors. However,
its potential anti-cancer mechanism in OS requires further
investigation. Our previous studies have shown that γδ T cell
immunotherapy has a positive effect on OS (4,36–39),
and the results also indicated that celastrol increased OS cell
lysis by γδ T cells via the upregulation of death receptor
(40). This result indicated that
celastrol may be able to induce primary OS cell death in
vivo for γδ T cell lysis. It is hypothesized that ANXA3
downregulation via treatment with celastrol may increase the
killing effect of γδ T cells on OS cells. The mechanism of
celastrol in ANXA3 will be further examined in the future.
Taken together, the present results indicated that
ANXA3 was significantly overexpressed in the OS cell lines HOS and
U2OS, and downregulated AXNA3 expression increased the apoptotic
ability of HOS and U2OS cells. The present results suggested that
anti-ANXA3 has a potential function as an inhibitor of OS.
Therefore, ANXA3 could be used in the early diagnosis of and
intervention of bone tumors as a novel target for OS therapy.
However, further investigation into the underlying mechanisms is
required.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81360400).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZL conceived and designed the study. XZ performed
the experiments and was the primary contributor in writing the
manuscript. SW conducted the data analyses. PG and HW collected the
data and produced all of the figures. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Michaelis J: Osteosarcoma. Lancet.
1:11741988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translation biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gianferante DM, Mirabello L and Savage SA:
Germline and somatic genetics of osteosarcoma-connecting aetiology,
biology and therapy. Nat Rev Endocrinol. 13:480–491. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li ZX, Wang RY and Tang JC: Sodium
valproate enhance γδ T cells killing osteosarcoma. Chin J Exp Surg.
33:154–157. 2016.
|
|
5
|
Perron B, Lewit-Bentley A, Geny B and
Russo-Marie F: Can enzymatic activity, or otherwise, be inferred
from structural studies of annexin III? J Biol Chem.
272:11321–11326. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gerke V, Creutz CE and Moss SE: Annexins:
Linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell
Biol. 6:449–461. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moss SE and Morgan RO: The annexins.
Genome Biol. 5:2192004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerke V and Moss SE: Annexins: From
structure to function. Physiol Rev. 82:331–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamelin-Peyron C, Vlaeminck-Guillem V,
Haidous H, Schwall GP, Poznanovic S, Gorius-Gallet E, Michles S,
Larue A, Guillotte M, Ruffion A, et al: Prostate cancer biomarker
annexin A3 detected in urines obtained following digital rectal
examination presents antigenic variablility. Clin Biochem.
47:901–908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu N, Liu S, Guo C, Hou Z and Sun MZ: The
role of annexin A3 playing in cancers. Clin Transl Oncol.
15:106–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeidan B, Jackson TR, Larkin SE, Cutress
RI, Coulton GR, Ashton-Key M, Murray N, Packham G, Gorgoulis V,
Garbis SD and Townsend PA: Annexin A3 is a mammary marker and
potential neoplastic breast cell therapeutic target. Oncotarget.
6:21421–21427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong M, Fung TM, Luk ST, Ng KY, Lee TK,
Lin CH, Yam JW, Chan KW, Ng F, Zheng BJ, et al: ANXA3/JNK signaling
promotes self-renewal and tumor growth, and its blockade provides a
therapeutic target for hepatocellular carcinoma. Stem Cell Reports.
5:45–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YF, Xiao ZQ, Li MX, Zhang PF, Li C, Li
F, Chen YH, Yi H, Yao HX and Chen ZC: Quantitative promote analysis
reveals annexin A3 as a novel biomarker in lung adenocarcinoma. J
Pathol. 217:54–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu YF, Chen YH, Li MY, Zhang PF, Li GQ,
Xiao ZQ and Chen ZC: Quantitative proteomic analysis identifying
three annexins as lymph node metastasis-related proteins in lung
adenocarcinoma. Med Oncol. 29:174–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan QZ, Pan K, Weng DS, Zhao JJ, Zhang XF,
Wang DD, Lv L, Jiang SS, Zheng HX and Xia JC: Annexin A3 promotes
tumorigenesis and resistance to chemotherapy in hepatocellular
carcinoma. Mol Carcinog. 54:598–607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan X, Yin J, Yao H, Mao N, Yang Y and Pan
L: Increased expression of annexin A3 is a mechanism of platinum
resistance in ovarian cancer. Cancer Res. 70:1616–1624. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tong SW, Yang YX, Hu HD, An X, Ye F, Hu P,
Ren H, Li SL and Zhang DZ: Proteomic investigation of
5-flourouracil resistance in a human hepatocellular carcinoma cell
line. J cell Biochem. 113:1671–1680. 2012.PubMed/NCBI
|
|
18
|
Pénzváltó Z, Tegze B, Szász AM,
Sztupinszki Z, Likó I, Szendrői A, Schäfer R and Győrffy B:
Identifying resistance mechanisms against five tyrosine kinase
inhibitors targeting the ERBB/RAS pathway in 45 cancer cell lines.
PLoS One. 8:e595032013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li ZX: Potential of human γδ T cells for
immunotherapy of osteosarcoma. Mol Biol Rep. 40:427–437. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mussunoor S and Murray GI: The role of
annexins in tumor development and progression. J Pathol.
216:131–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shao P, Qu WK, Wang CY, Tian Y, Ye ML, Sun
DG, Sui JD, Wang LM, Fan R and Gao ZM: MicroRNA-205-5p regulates
the chemotherapeutic resistance of hepatocellular carcinoma cells
by targeting PTEN/JNK/ANXA3 pathway. Am J Transl Res. 9:4300–4307.
2017.PubMed/NCBI
|
|
23
|
Yang YM, Kim SY and Seki E: Inflammation
and liver cancer: Molecular mechanisms and therapeutic targets.
Semin Liver Dis. 39:26–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Z, Zhang L, Gao M, Han M, Liu K, Zhang
Z, Gong Z, Xing L, Shi X, Lu K and Gao H: Endoplasmic reticulum
stress triggers xanthoangelol-induced protective autophagy via
activation of JNK/c-Jun Axis in hepatocellular carcinoma. J Exp
Clin Cancer Res. 38:82019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JB, Lee S, Kim HR, Park SY, Lee M,
Yoon JH and Kim YJ: Transforming growth factor-β decreases side
population cells in hepatocellular carcinoma in vitro. Oncol
Lett. 15:8723–8728. 2018.PubMed/NCBI
|
|
26
|
Jin Y, Mao J, Wang H, Hou Z, Ma W, Zhang
J, Wang B, Huang Y, Zang S, Tang J and Li L: Enhanced tumorigenesis
and lymphatic metastasis of CD133+ hepatocarcinoma ascites
syngeneic cell lines mediated by JNK signaling pathway in vitro and
in vivo. Biomed Pharmacother. 67:337–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hagiwara S, Kudo M, Nagai T, Inoue T,
Ueshima K, Nishida N, Watanabe T and Sakurai T: Activation of JNK
and high expression level of CD133 predict a poor response to
sorafenib in hepatocellular carcinoma. Br J Cancer. 106:1997–2003.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan Q, Pan K, Wang QJ, Weng DS, Zhao JJ,
Zheng HX, Zhang XF, Jiang SS, Lv L, Tang Y, et al: Annexin A3 as a
potential target for immunotherapy of liver cancer stem-like cells.
Stem Cells. 33:354–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou T, Li Y, Yang L, Yang L, Tang T,
Zhang L and Shi J: Annexin A3 as a prognostic biomarker for breast
cancer: A retrospective study. Biomed Res Int. 2017:26036852017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeng C, Ke Z, Song Y, Yao Y, Hu X, Zhang
M, Li H and Yin J: Annexin A3 is associated with a poor prognosis
in breast cancer and participates in the modulation of apoptosis in
vitro by affecting the Bcl-2/Bax balance. Exp Mol Pathol. 95:23–31.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu YF, Xiao ZQ, Li MX, Li MY, Zhang PF,
Li C, Li F, Chen YH, Yi H, Yao HX and Chen ZC: Quantitative
proteome analysis reveals annexin A3 as a novel biomarker in lung
adenocarcinoma. J Pathol. 217:54–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Zhou T, Liu L, Ju YC, Chen YT, Tan
ZR and Wang J: The regulatory role of Annexin 3 in nude mouse
bearing a subcutaneous xenograft of MDA-MB-231 human breast
carcinoma. Pathol Res Pract. 214:1719–1725. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeun M, Park S, Kim Y, Choi J, Song SH,
Jeong IG, Kim CS and Lee KH: Self-Normalized detection of ANXA3
from untreated urine of prostate cancer patients without digital
rectal examination. Adv Healthc Mater. 62017.
|
|
34
|
Liu YF, Liu QQ, Zhang YH and Qiu JH:
Annexin A3 knockdown suppresses lung adenocarcinoma. Anal Cell
Pathol (Amst). 2016:41314032016.PubMed/NCBI
|
|
35
|
Zhou T, Li Y, Yang L, Liu L, Ju Y and Li
C: Silencing of ANXA3 expression by RNA interference inhibits the
proliferation and invasion of breast cancer cells. Oncol Rep.
37:388–398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li ZX, Wang RY and Tang JC: Type I
IFN-mediated enhancement of anti-osteosarcoma cytotoxicity of human
γδ T cells. Chin J Immun. 11:1533–1535, 1542. 2014.
|
|
37
|
Li Z, Zhang J, Tang J and Wang R:
Celastrol increases osteosarcoma cell lysis by γδ T cells through
up-regulation of death receptors. Oncotarget. 7:84388–84397.
2016.PubMed/NCBI
|
|
38
|
Li Z, Xu Q, Peng H, Cheng R, Sun Z and Ye
Z: IFN-γ enhances HOS and U2OS cell lines susceptibility to γδ T
cell-mediated killing through the Fas/Fas ligand pathway. Int
Immunopharmacol. 11:496–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Z, Peng H, Xu Q and Ye Z: Sensitization
of human osteosarcoma cells to Vγ9Vδ2 T-cell-mediated cytotoxicity
by zoledronate. J Orthop Res. 30:824–830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li ZX, Zhang JZ, Wang ST, Wang RY and Tang
JC: Celastrol increases osteosarcoma cells line HOS lysis by γδ T
cells through TRAIL way. Chin J Immun. 32:1777–1780. 2016.
|