Introduction
MicroRNAs (miRNAs/miRs) are small, noncoding RNAs
that negatively regulate gene expression at the
post-transcriptional level (1,2). As
previous studies have reported, miRNAs bind to the 3-untranslated
region (3′-UTR) of target mRNAs (3), resulting in degradation or inhibited
translation of the target mRNA (4–6).
miRNAs have been reported to serve important roles in tumorigenesis
(7,8).
Lung cancer has been a major health problem in
developed countries for several decades, and has emerged recently
as the leading cause of cancer death in many developing countries
(9). Squamous cell lung cancer
(SQCLC) is a common type of lung cancer, accounting for ~40,000
deaths in the USA in 2013 (10).
The 5-year survival of patients with SQCLC is only 16% (10). It has been reported that patients
with SQCLC tend to be older, typically displaying advanced stages
of the disease (11,12). SQCLC is closely associated with
smoking, with the majority of patients exhibiting centrally located
tumors (12) that are locally
aggressive, and which frequently lack actionable genetic
alterations. As a result, targeted agents developed for lung
adenocarcinoma are largely ineffective against SQCLC. Despite the
efforts that have been made in the study of lung cancer in recent
decades, the molecular mechanisms underlying SQCLC are yet to be
determined.
miR-195 is a member of the miR-15/16 family, which
comprises a group of miRNAs: miR-195, miR-15a, miR-15b, miR-16-1
and miR-16-2 (13). The functions
of miR-195 in non-small cell lung carcinoma (NSCLC) cells targeting
insulin-like growth factor 1 receptor (14) and checkpoint kinase 1 (CHEK1)
(15) have been previously
reported; additionally, it has been revealed to be a tumor
suppressor that inhibited tumor cell viability and migration
(15). miR-195 has been
demonstrated to suppress osteosarcoma cell metastasis by targeting
cyclin D1 (CCND1) (16), the role
of miR-195 in SQCLC remains unclear.
In the present study, miR-195 was revealed to serve
an important role in tumorigenesis. First, it was observed that the
levels of miR-195 were decreased in SQCLC cell lines, whereas it
was highly expressed in a control cell line. Second, as it was
previously reported that vascular endothelial growth factor (VEGF)
was a direct target of miR-195 (17), the present study revealed that
miR-195 regulated the expression of VEGF in SQCLC cells.
Collectively, the results of the present study suggested that
miR-195 functioned as a tumor suppressor in SQCLC.
Materials and methods
Cell lines
Two squamous carcinoma cell lines [H520 and SK-Mes-1
(Mes-1)] and a normal lung bronchus epithelial cell line (Beas-2B)
were obtained from the American Type Culture Collection and
cultured in RPMI 1640 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; PAN-Biotech GmbH),
100 U/ml penicillin, and 100 U/ml streptomycin at 37°C in an
atmosphere of 5% CO2. The human umbilical vein
endothelial cells (HUVECs) were isolated from umbilical cord vein
by collagenase treatment. The HUVECs were cultured in EGM2 (Lonza
Group, Ltd.). 293T cells obtained from the American Type Culture
Collection were maintained in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin/streptomycin (complete media) and incubated at 37C and
5% CO2.
Plasmids
miR-195 mimic (forward: 5′UAGCAGCACAGAAAUAUUGGC3′,
reverse: 3′AUCGUCGUGUCUUUAUAACCG5′) and inhibitor (forward:
5′GCCAAUAUUUCUGUGCUGCUA3′), and their respective negative controls
(NCs) (micrON™ mimic Negative Control #22:
5′UUUGUACUACACAAAAGUACUG3′, 3′AAACAUGAUGUGUUUUCAUGAC5′ and micrOFF™
inhibitor Negative Control #22: 5′CAGUACUUUUGUGUAGUACAAA3′) were
obtained from Guangzhou RiboBio Co., Ltd. The wild-type (WT) and
mutant (MUT) 3′-UTR of VEGF were inserted into the GV272
(http://www.genechem.com.cn/service/index.php?ac=gene&at=vector_search&keyword=gv272)
firefly luciferase plasmid by Shanghai GeneChem Co., Ltd.
Luciferase assay
Targets can version 7.2 (http://www.targetscan.org/) and miRanda (http://www.microrna.org/) were used for miRNA target
prediction and analysis. In order to confirm the association
between miR-195 and VEGF, the following experiment was designed:
The full-length WT VEGF 3′-UTR was cloned (Shanghai GeneChem Co.,
Ltd.) and inserted into a luciferase reporter plasmid (GV272),
downstream from the firefly luciferase gene. CV045 Renilla was
co-transfected as internal reference. A mutated VEGF 3′-UTR
(Shanghai GeneChem Co., Ltd.), cloned as control, was inserted into
the same plasmid backbone. Subsequently, miRNA mimic or inhibitor,
or their respective NC RNAs, with CV045 (http://www.genechem.com.cn/service/index.php?ac=gene&at=vector_search&keyword=CV045)
were co-transfected with the constructed plasmids into 293T cells.
The 293T cells were cultured for 24 h, and subsequently harvested
for Renilla and firefly luciferase activity assays using the
Dual-Luciferase Report Assay system (cat. no. E1910; Promega
Corporation). The firefly luciferase activity to Renilla
luciferase activity.
miRNA transfection
miR-195 NC, miR-195 mimic, miR-195 inhibitor NC or
miR-195 inhibitor were purchased from Guangzhou RiboBio Co., Ltd.
Mes1 cells or HUVECs were seeded in 12-well plates
(8×104 cells/well) for 12 h. For transfection, cells
were transfected with 100 nM miR-195 NC, miR-195 mimic, miR-195
inhibitor NC or miR-195 inhibitor using Lipofectamine®
2000 (2 µl; 11668-019, Invitrogen; Thermo Fisher Scientific, Inc.).
After 24 h Samples were collected for quantification of miRNA or
protein expression.
Construction of Mes-1-miR-control
(Mes1-control), Mes-1-miR-195 mimic (Mes1-195) and
Mes-1-miR-195-inhibitor (Mes1-195-inhibitor) cell lines
LV3(H1/GFP&Puro) expressing plasmids encoding
miR-195 mimic, miR-195 inhibitor and miR-control, and polybrene
were purchased from Shanghai GenePharma Co., Ltd. 293T cells were
seeded in 10-cm plates (1×106 cells per well) and
incubated at 37°C overnight, and subsequently the cells were
transfected with the packaging plasmid (containing the vsv-g,
rev, and rre genes) and the target plasmids. The
packaging plasmid and the target plasmids were purchased from
Shanghai GenePharma Co., Ltd. Following culture for 72 h, the
lentiviral supernatant was harvested. Mes-1 cells were seeded into
6-cm plates (2×105 cells per well), incubated overnight,
and subsequently the cells were infected with lentiviral
supernatant s(1×107 TU/ml) containing miR-195-mimic,
miR-195-inhibitor or miR-control, and 5 µg/ml polybrene at 37°C in
an atmosphere of 5% CO2 for 12 h. The culture medium was
then removed and replaced with fresh medium containing 10% FBS; the
incubation was continued for a further 48 h. The infected cells
expressed GFP, and transformed cells were selected in the presence
of puromycin (10 µg/ml). Therefore, three stable cell lines,
Mes1-control, Mes1-195 and Mes1-195-inhibitor, were generated. The
transduction efficiency was evaluated via reverse
transcription-quantitative PCR (RT-qPCR) analysis to determine
miR-195 expression.
RNA isolation and RT-qPCR
Total cells were collected and homogenized in
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
and subsequently total RNA was extracted from the cultured cells
using TRIzol, according to the manufacturer's protocol. miRNAs was
extracted with an miRNA isolation kit was (Qiagen GmbH). miRNAs and
total RNA were reverse-transcribed into cDNA using a Reverse
Transcription kit (Takara Biotechnology Co., Ltd.). The reverse
transcription reaction was performed under the following
conditions: 37°C for 30 min; then 85°C for 5 sec; and holding at
4°C. For qPCR amplification of the cDNA, the following
thermocycling conditions were used: 30 sec at 95°C, followed by 40
cycles of 95°C for 10 sec and 60°C for 1 min. qPCR was performed
using a SYBR Premix Ex Taq II kit (Takara Biotechnology Co., Ltd.)
on a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories
Inc.). Sense and antisense primer sequences for VEGF and β-actin
were as follows: VEGF, forward 5′-ATCTTCAAGCCATCCTGTGTGC-3′,
reverse 5′-GCTCACCGCCTCGGCTTGT-3′; and β-actin, forward
5′-CACATCGCTCAGACACCA-3′, reverse 5′-ATGGCAACAATATCCACTTT-3′. The
miRNA primers (RT, and forward and reverse primers for miR-195- and
U6) were purchased from Guangzhou RiboBio Co., Ltd. Relative
expression of miR-195-5p was evaluated using the 2−∆∆Cq
method (18) with U6 small nuclear
RNA used for normalization; β-actin used for normalization of VEGF
expression.
Western blot analysis
The cells were lysed in RIPA buffer (Beijing
Solarbio Science & Technology Co., Ltd.) supplemented with
protease inhibitors (cat. no. 4693116001; Roche Diagnostics).
Following cell lysis, the lysates were centrifuged at 12,000 × g at
4°C for 20 min. The protein concentration was measured using the
BCA method. The proteins (40 µg per lane) were separated via 12%
SDS-PAGE and subsequently transferred on to PVDF membranes (Roche
Diagnostics). Membranes were blocked with 5% non-fat milk powder in
TBS-T buffer (20 mM Tris-HCl, pH 7.4, 137 mM NaCl, and 0.1% Tween)
for 1 h at room temperature. The samples were incubated with
primary antibodies at 4°C overnight, followed by subsequent
incubation with horseradish peroxidase-conjugated secondary
antibodies. The films were developed using an enhanced
chemiluminescence system (EMD Millipore). ImageJ version 1.48
(National Institutes of Health) was used to quantify protein
expression. Polyclonal anti-VEGF (1:1,000; cat. no. sc-152; Santa
Cruz Biotechnology, Inc.) and anti-β-actin (1:1,000; cat. no. 8457;
Cell Signaling Technology, Inc.) primary antibodies, and mouse
anti-rabbit secondary antibodies (1:10,000; cat. no. sc-2357; Santa
Cruz Biotechnology, Inc.) were used during the study.
Cell migration assays
Cell migration assays were performed using Transwell
chambers. The cells were suspended in serum-free F12 medium (Gibco;
Thermo Fisher Scientific, Inc.) and seeded in the upper chambers at
a total density of 0.8×105 cells/well; 500 µl F12 medium
with 10% FBS was added to the lower chambers, and the Transwell
plates were incubated at 37°C for 12 h. Cells that remained on the
upper surfaces of the membranes were removed using cotton swabs,
and the migrated cells on the lower surface were fixed with 4%
paraformaldehyde for 10 min, followed by staining with 0.1% crystal
violet (Beyotime Institute of Biotechnology) for 15 min at room
temperature. Images of migrated cells were captured using a light
microscope (BX61; Olympus Corporation). Digital images
(magnification, ×10) of the underside of the inserts were acquired
with the microscope. A total of 5 random fields were captured from
each membrane.
Cell viability
The MTT assay was used to examine the viability of
Mes-1 cells that were stably transfected as aforementioned. A total
of 2,000 cells/well were plated in 96-well plates and incubated at
37°C. Cell viability was measured by MTT reagent, Cells was
measured after cultured 1, 2, 3 and 4 days. They were gently washed
with PBS, and 20 µl MTT (5 mg/ml) were added in the cell culture.
After 4 h incubation, the media were discarded, and 150 µl DMSO was
added in each well to dissolve the precipitates and the absorbance
at 560 nm was measured using a microplate reader (Promega
Corporation).
In vitro angiogenesis assay
Human umbilical vein endothelial cells (HUVECs) were
plated in 24-well plates (1×105 cells/well), and
cultured with EGM2 (Lonza Group Ltd.) overnight at 37°C in an
atmosphere containing 5% CO2. Cells was transfected with
miR-195 NC, miR-195 mimic, miR-195 inhibitor NC or miR-195
inhibitor. Following incubation for 6 h, the HUVECs were digested
and seeded in 48-well plates(0.3×105 cells/well)
containing 50 µl solidified Matrigel™, and incubated at 37°C for 9
h. The cells were stained with 3 µM calcein-acetoxymethyl
(Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at 37°C in
the presence of 5% CO2. Formation of capillary-tubule
structures was observed, and digital images were captured under a
light microscope (magnification, ×100). The branch points of the
formed tubes were quantified using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc.) from at least five fields.
Statistical analysis
At least three repeats were conducted for each
experiment. Data were analyzed with GraphPad Prism 7.0 software
(GraphPad Software, Inc.). Data were analyzed using Student's
t-test or one-way analysis of variance followed by Dunnett's post
hoc test for multiple comparisons. Data are presented as the mean ±
standard deviation of three independent experiments. P≤0.05 was
considered to indicate a statistically significant difference.
Results
VEGF is upregulated in SQCLCs
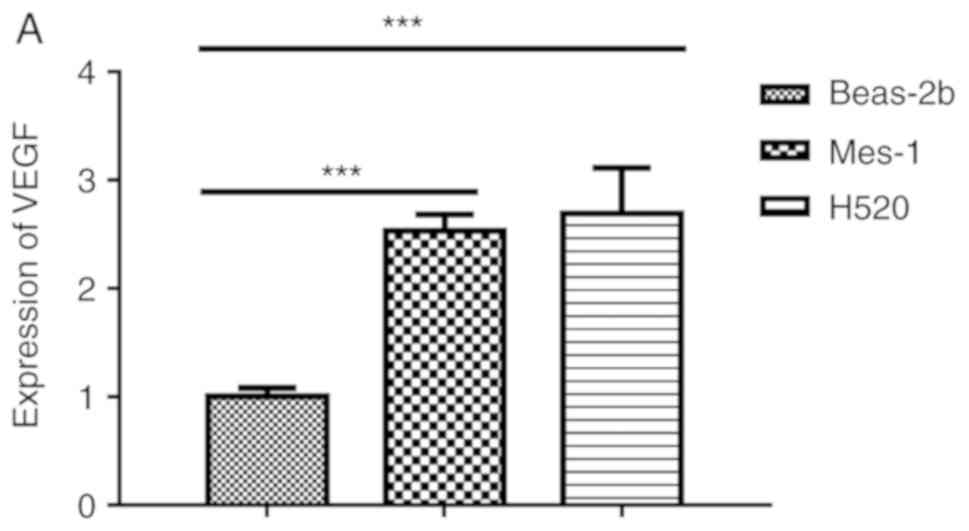
RT-qPCR analysis revealed that VEGF was upregulated
in the SQCLC cell lines, H520 and Mes-1, compared with in the
normal lung bronchus epithelial cell line, Beas-2B (Fig. 1A). Furthermore, the expression of
miR-195 in the two SQCLC cell lines was significantly downregulated
(P<0.001; Fig. 1B).
VEGF is a target of miR-195
The position of the miR-195 target site in VEGF, and
the mutated site that was designed in the clone, are presented in
Fig. 2A. 293T cells were
transfected with miR-195 mimic, miR-195 NC, miR-195 inhibitor or
miR-195 inhibitor NC; the transfection efficiency was presented in
Fig. 2B. The results of the
luciferase activity experiments revealed that the levels of
luciferase activity were significantly reduced in the miR-195 mimic
group cells compared with the miR-195 NC group (Fig. 2B). Conversely, the levels of
luciferase activity in the miR-195 inhibitor group cell were not
significantly different to those in the miR-195 inhibitor NC group
(Fig. 2B). Of note, the inhibitory
effects of miR-195 mimic, as determined from the luciferase assay,
were lost when the predicted binding site was mutated (Fig. 2B). Subsequently, the Mes-1 cell
line was used to generate stable cells with altered miR-195
expression, Mes1-195, Mes1-195-inhibitor and Mes1-control, via
lentiviral transduction. The expression levels of miR-195 and VEGF
were evaluated in these cell lines. The results demonstrated that,
compared with Mes1-control, miR-195 was significantly upregulated
in the Mes1-195 cell line, and downregulated in the
Mes1-195-inhibitor cell line (Fig.
2C). Subsequently, western blot analysis was conducted to
investigate the protein expression levels of VEGF for the different
experimental groups. VEGF was significantly downregulated in the
Mes1-195 cell line, whereas it was upregulated in the
Mes1-195-inhibitor cell line compared with the Mes1-control cell
line (Fig. 2D). Collectively,
these data indicated that miR-195 directly interacted with the VEGF
3′-UTR, inhibiting VEGF expression.
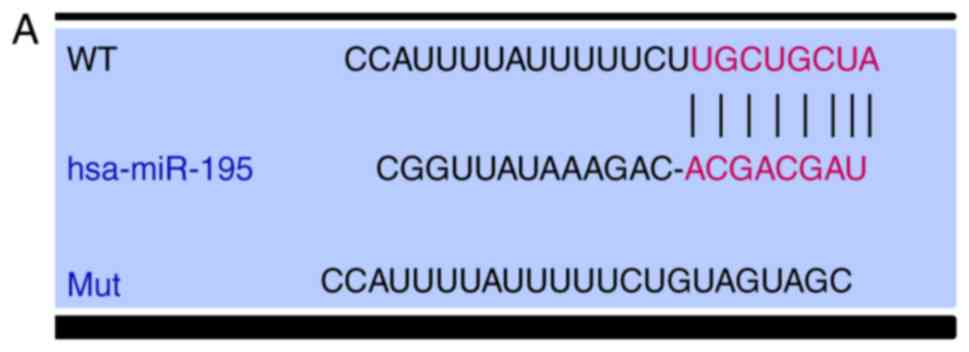 | Figure 2.VEGF is a target of miR-195 in SQCLC
cells. (A) Position of the miR-195 binding site in VEGF, and the
Mut sequence. (B) 293T cells were co-transfected with a firefly
luciferase reporter containing the WT or Mut VEGF 3′-UTR, and
miR-195 mimic, miR-195 inhibitor or the corresponding NCs. Data are
presented as the mean ± standard deviation of three independent
experiments. **P<0.01, ***P<0.001, ****P<0.0001. (C)
Relative levels of miR-195 in Mes-1 cells infected with lentivirus
encoding miR-control, miR-195 mimic or miR-195 inhibitor plasmids,
determined by reverse transcription-quantitative PCR. (D) Western
blot analysis of changes in VEGF protein expression levels in Mes-1
cells infected with the aforementioned lentiviruses. Data are
presented as the mean ± standard deviation of three independent
experiments. **P<0.01; ***P<0.001. miR-, microRNA; VEGF,
vascular endothelial growth factor; SQCLC, squamous cell lung
cancer; Mes-1/Mes1, SK-Mes-1; WT, wild-type; Mut, mutant; NC,
negative control; Mes1-control, Mes-1 cells transfected with
miR-control; Mes1-195, Mes-1 cells transfected with miR-195 mimic;
Mes1-195-inhibitor, Mes-1 cells transfected with miR-195
inhibitor. |
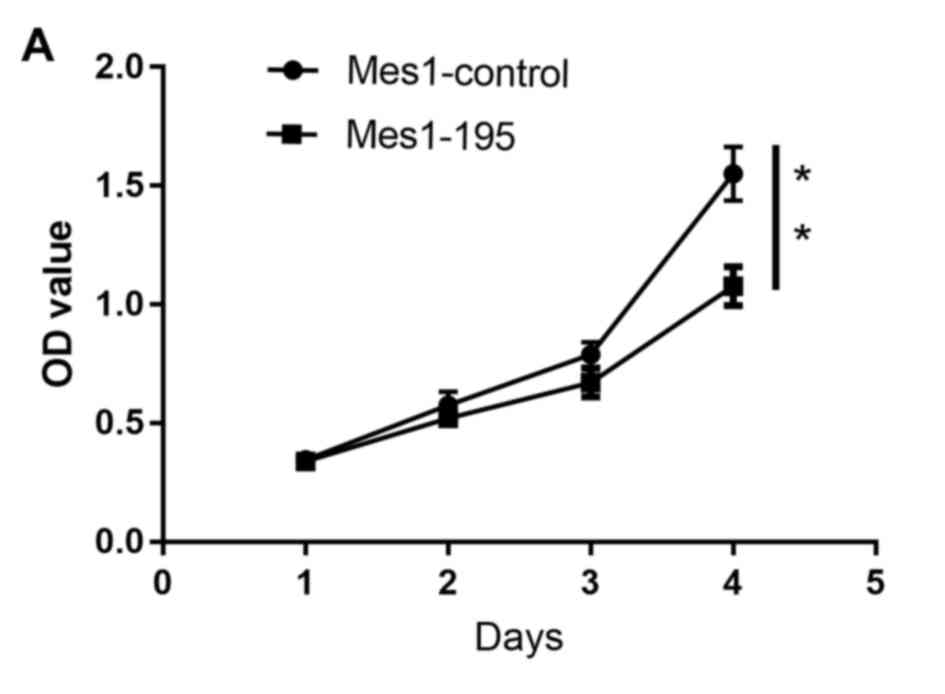
miR-195 inhibits cell viability
Following generation of the Mes1-control, Mes1-195
and Mes1-195-inhibitor cell lines, they were subsequently used to
measure cell viability, which was determined using an MTT assay.
The results revealed that the number of viable cells was
significantly reduced in the Mes1-195 cell line compared with the
Mes1-control cell line (Fig. 3A).
Conversely, the number of viable cells in the Mes1-195-inhibitor
group was significantly increased compared with the Mes1-control
group (Fig. 3B).
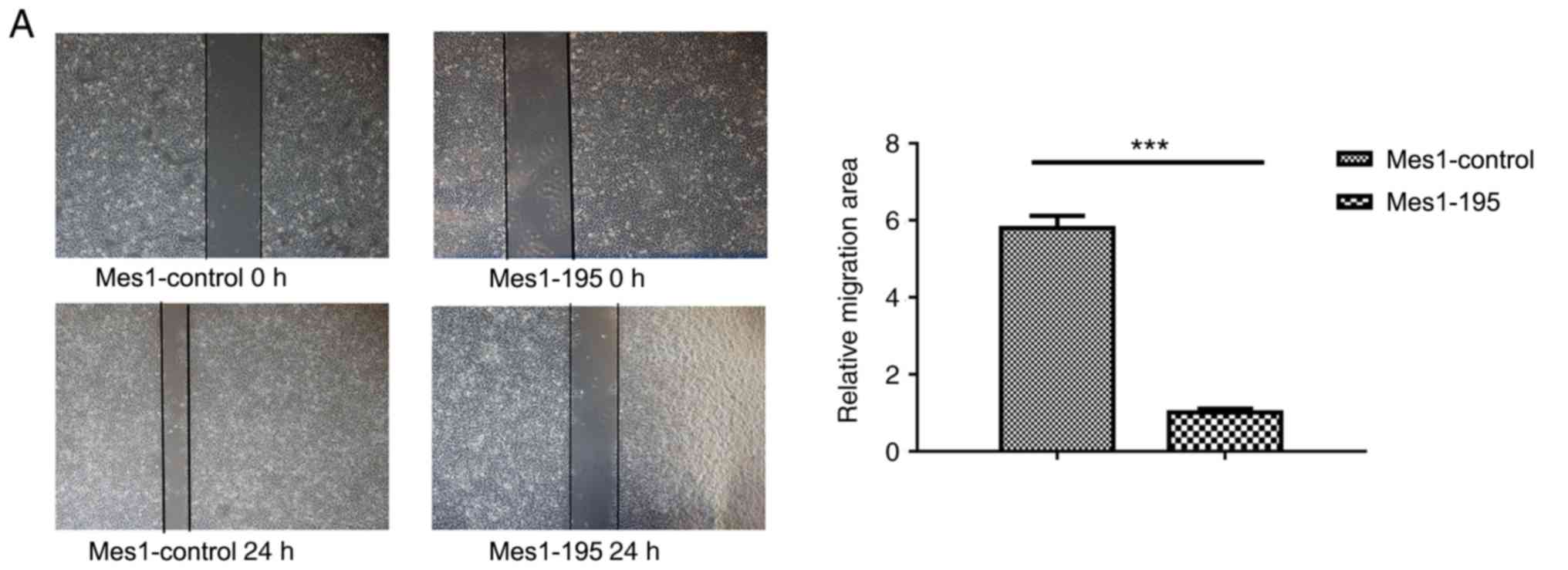
Expression of miR-195 affects cell
migration Subsequently, wound healing and Transwell assays were
performed using the cell lines
A wound healing assay revealed that miR-195
overexpression significantly inhibited cell migration by ~6-fold in
the Mes-1 cell line (Fig. 4A).
Conversely, when miR-195 expression was inhibited, cell migration
was significantly promoted by ~2-fold compared with Mes1-control
cells (Fig. 4B).
In the Transwell assay, it was observed that an
increase in miR-195 led to a significant reduction in cell
invasion, whereas a decrease in miR-195 promoted cell invasion
(Fig. 4C).
miR-195 inhibits angiogenesis in
vitro
To investigate the effects of miR-195 on
angiogenesis, HUVECs were transfected with miR-195 mimic (Fig. 5). It was revealed that miR-195
overexpression significantly inhibited the ability of the HUVECs to
form capillary-like tubules on a Matrigel coating (Fig. 5A). Conversely, when miR-195
inhibitor was overexpressed in HUVECs, it was revealed that the
cells exhibited a significantly enhanced capacity for tubule
formation (Fig. 5A). The protein
expression levels of VEGF in the transfected HUVECs were also
detected via western blot analysis (Fig. 5B). It was observed that the
presence of the miR-195 mimic led to a significant reduction in the
expression levels of VEGF compared with the control, whereas the
presence of the miR-195 inhibitor led to an increase in the VEGF
levels compared with the control. The transfection efficiency of
the transfected HUVECs was presented in Fig. 5C.
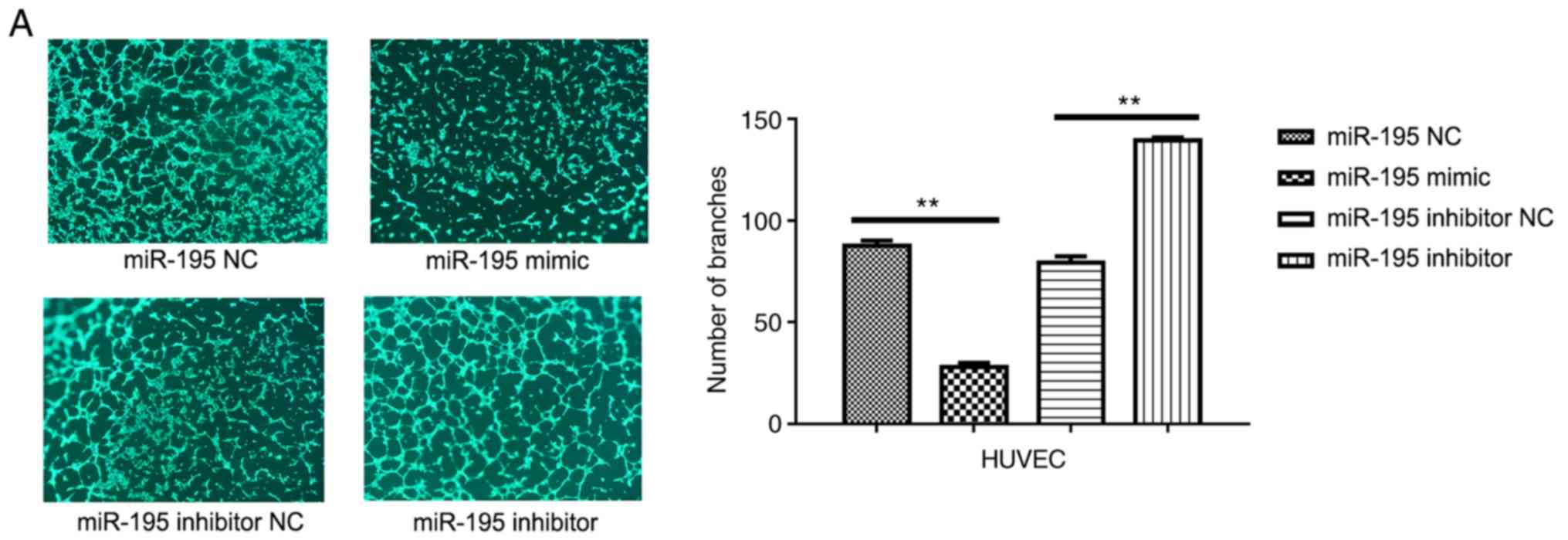 | Figure 5.miR-195 suppresses angiogenesis in the
tumor microenvironment. (A) Representative images of HUVECs in
Matrigel. Quantitative analysis of the number of branches is
presented on the right. Data are presented as the mean ± standard
deviation. of three independent experiments. **P<0.01. (B)
Western blot analysis of the protein expression levels of VEGF in
transfected cells; quantitative analysis of expression is presented
on the right. Data are presented as the mean ± standard deviation
of three independent experiments. ***P<0.001. VEGF, vascular
endothelial growth factor. (C) Relative levels of miR-195 in HUVECs
transfected with miR-195 NC, miR-195 mimic, miR-195 inhibitor, or
miR-195 inhibitor NC, as determined via reverse
transcription-quantitative PCR analysis. **P<0.01,
****P<0.0001. Data are presented as the mean ± standard
deviation of three independent experiments. HUVECs, human umbilical
vein endothelial cells; miR-195, microRNA-195; NC, negative
control; VEGF, vascular endothelial growth factor. |
Discussion
At present, there are no effective targeted drug
therapies for SQCLC. Angiogenesis is critical for tumor
progression, whereas metastasis is the major cause of tumor
recurrence and patient mortality (17). Therefore, miRNAs that induce
anti-angiogenic or anti-metastatic effects may provide novel
targets for anticancer therapies. The function of miR-195 has been
reported in prostate cancer (19),
hepatocellular carcinoma (HCC) (20), osteosarcoma (21) and breast cancer (22). Accumulating evidence has indicated
an important role for miRNAs in the progression of NSCLC (23,24);
however, the role of miR-195 has not been determined in SQCLC. A
role for miR-195 was first identified in HCC by Wang et al
(17). The effects of miR-195 on
the expression of CHEK1 (15),
CCND1 (16), ribosomal protein S6
kinase b1 (19) and PHD finger
protein 19 (20) have also
previously been reported.
The present study was a preliminary investigation
into the function of miR-195 in SQCLC. In this study, the
tumor-suppressive function of miR-195 in SQCLC development and
progression in vitro was revealed. Downregulated expression
of miR-195 was observed in SQCLC cell lines compared with the
control. Upon overexpression of miR-195, suppression of Mes-1 cell
viability, migration, invasion and angiogenesis in HUVECs was
reported. Conversely, when the expression of miR-195 was
downregulated, opposing effects were observed. In addition, VEGF
was identified as a target of miR-195 in SQCLC cells. Collectively,
these data suggested that miR-195 is a potential therapeutic target
for SQCLC.
In conclusion, the results of the present study have
revealed that miR-195 was significantly decreased in SQCLC cell
lines, and that miR-195 overexpression suppressed the viability,
migration and invasion of Mes-1 cells, and angiogenesis of HUVECs,
potentially by targeting VEGF. miR-195 has been reported to promote
apoptosis or inhibit proliferation in various types of cancer
(15–17,19).
The present study suggested that miR-195 may be potentially
involved in the pathophysiology of SQCLC.
Acknowledgements
Not applicable.
Funding
This study was supported by a Cancer Translational
Medicine Seed Fund (grant no. 1606), and the Science &
Technology Development Fund of the Tianjin Education commission for
Higher Education (grant no. 2017ZD11).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HLL conducted the experimental work and designed the
study, reviewed the literature and drafted the manuscript. YLC, YL
and CGL contributed to the design and coordination of experimental
work, and acquisition of data. TTQ, MB, ZFZ and RJ participated in
the study design, data collection, analysis of data and preparation
of the manuscript. HLL, CLW and YJS analyzed the data and drafted
the manuscript. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gandellini P, Doldi V and Zaffaroni N:
microRNAs as players and signals in the metastatic cascade:
Implications for the development of novel anti-metastatic
therapies. Semin Cancer Biol. 44:132–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou W, Wang S, Ying Y, Zhou R and Mao P:
miR-196b/miR-1290 participate in the antitumor effect of
resveratrol via regulation of IGFBP3 expression in acute
lymphoblastic leukemia. Oncol Rep. 37:1075–1083. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gartel AL and Kandel ES: miRNAs: Little
known mediators of oncogenesis. Semin Cancer Biol. 18:103–110.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kai K, Dittmar RL and Sen S: Secretory
microRNAs as biomarkers of cancer. Semin Cell Dev Biol. 78:22–36.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nambara S and Mimori K: MicroRNA in
various aspects of cancer development. Gan To Kagaku Ryoho.
44:362–366. 2017.(In Japanese). PubMed/NCBI
|
|
7
|
Fang YX and Gao WQ: Roles of microRNAs
during prostatic tumorigenesis and tumor progression. Oncogene.
33:135–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su SF, Chang YW, Andreu-Vieyra C, Fang JY,
Yang Z, Han B, Lee AS and Liang G: miR-30d, miR-181a and
miR-199a-5p cooperatively suppress the endoplasmic reticulum
chaperone and signaling regulator GRP78 in cancer. Oncogene.
32:4694–4701. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J, Zhu J, Zhang YH, Chen YS, Ding LL,
Kensler TW and Chen JG: Lung cancer in a rural area of China: Rapid
rise in incidence and poor improvement in survival. Asian Pac J
Cancer Prev. 16:7295–7302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamano S, Gi M, Tago Y, Doi K, Okada S,
Hirayama Y, Tachibana H, Ishii N, Fujioka M, Tatsumi K and
Wanibuchi H: Role of deltaNp63posCD44vpos cells in the development
of N-nitroso-tris-chloroethylurea-induced peripheral-type mouse
lung squamous cell carcinomas. Cancer Sci. 107:123–132. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang YC, Zhou Q and Wu YL: Emerging
challenges of advanced squamous cell lung cancer. ESMO Open.
1:e0001292016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Funai K, Yokose T, Ishii G, Araki K,
Yoshida J, Nishimura M, Nagai K, Nishiwaki Y and Ochiai A:
Clinicopathologic characteristics of peripheral squamous cell
carcinoma of the lung. Am J Surg Pathol. 27:978–984. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Griffiths-Jones S, Saini HK, Van Dongen S
and Enright AJ: Enrigh: Tools for microRNA genomics. Nucleic Acids
Res. 36:D154–D158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Wang Y, Lan H and Li J: MiR-195
inhibits the growth and metastasis of NSCLC cells by targeting
IGF1R. Tumour Biol. 35:8765–8770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu B, Qu J, Xu F, Guo Y, Wang Y, Yu H and
Qian B: MiR-195 suppresses non-small cell lung cancer by targeting
CHEK1. Oncotarget. 6:9445–9456. 2015.PubMed/NCBI
|
|
16
|
Han K, Chen X, Bian N, Ma B, Yang T, Cai
C, Fan Q, Zhou Y and Zhao TB: MicroRNA profiling identifies MiR-195
suppresses osteosarcoma cell metastasis by targeting CCND1.
Oncotarget. 6:8875–8889. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang R, Zhao N, Li S, Fang JH, Chen MX,
Yang J, Jia WH, Yuan Y and Zhuang SM: MicroRNA-195 suppresses
angiogenesis and metastasis of hepatocellular carcinoma by
inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology.
58:642–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai C, Chen QB, Han ZD, Zhang YQ, He HC,
Chen JH, Chen YR, Yang SB, Wu YD, Zeng YR, et al: miR-195 inhibits
tumor progression by targeting RPS6KB1 in human prostate cancer.
Clin Cancer Res. 21:4922–4934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu H, Hu YW, Zhao JY, Hu XM, Li SF, Wang
YC, Gao JJ, Sha YH, Kang CM, Lin L, et al: MicroRNA-195-5p acts as
an anti-oncogene by targeting PHF19 in hepatocellular carcinoma.
Oncol Rep. 34:175–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai H, Zhao H, Tang J and Wu H: Serum
miR-195 is a diagnostic and prognostic marker for osteosarcoma. J
Surg Res. 194:505–510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Igglezou M, Vareli K, Georgiou GK, Sainis
I and Briasoulis E: Kinetics of circulating levels of miR-195,
miR-155 and miR-21 in patients with breast cancer undergoing
mastectomy. Anticancer Res. 34:7443–7447. 2014.PubMed/NCBI
|
|
23
|
Lin L, Huang Y and Gong W: Inhibition of
miR-92b suppresses nonsmall cell lung cancer cells growth and
motility by targeting RECK. Mol Cell Biochem. 387:171–176. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishikawa R, Goto Y, Kojima S, Enokida H,
Chiyomaru T, Kinoshita T, Sakamoto S, Fuse M, Nakagawa M, Naya Y,
et al: Tumor-suppressive microRNA-29s inhibit cancer cell migration
and invasion via targeting LAMC1 in prostate cancer. Int J Oncol.
45:401–410. 2014. View Article : Google Scholar : PubMed/NCBI
|