Introduction
Osteoarthritis (OA) is a chronic progressive bone
and joint disease caused by articular cartilage degeneration and
bone hyperplasia (1). An
epidemiological study demonstrated that the incidence rates of OA
are 44–70 and 60–70% in patients >55 and >65 years old,
respectively (2). MicroRNAs
(miRNAs) are a class of non-coding small RNAs that have been found
to act as oncogenes or tumor suppressors during the development of
tumors (3), and a previous study
have shown that the expression of tumor-related miRNAs is
associated with tumor occurrence and prognosis (4). miRNAs can regulate the proliferation,
differentiation and apoptosis of tumor cells, affecting cell cycle
and terminal differentiation (5,6). The
occurrence of various types of tumors is associated with miRNA
dysregulation (5,7). Therefore, investigating the
regulatory mechanism of miRNAs is important for tumor pathogenesis
and medical diagnosis (8,9). Importantly, an increasing number of
studies have shown that miRNAs serve an important role in the
occurrence and development of OA (10–12).
miRNA-let-7a (Let-7a) is the second identified miRNA
(13). It has been reported that
Let-7 is significantly downregulated in various tumor cells, such
as ovarian cancer (14) and breast
cancer (15). However, to the best
of our knowledge, a limited number of studies examined the role of
Let-7 in OA.
OA is an inflammatory disease characterized by
articular cartilage degradation and joint inflammation (16–18),
and apoptosis of chondrocytes is one of the main pathological
features of OA (19,20). LPS-induced chondrocyte cell
inflammatory injury has been widely used as an in vitro
model to investigate OA (21–23).
The aim of the present study was to investigate the role of let-7
in an in vitro model of OA induced by LPS. Additionally, the
present study aimed to examine the effects of let-7 on chondrocyte
cell proliferation and apoptosis. The identification of the
mechanism associated with let-7 provided a theoretical basis for
the development of new strategies for the prevention and treatment
of OA.
Materials and methods
Cell culture
ATDC5 cells were purchased from the Cell Bank of
Shanghai Institute of Cell Biology. Cells were cultured in 75
cm2 flasks with DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin, (Nanjing Sunshine Biotechnology Co.,
Ltd.) and 100 µg/ml streptomycin (Nanjing Sunshine Biotechnology
Co., Ltd.). Cells were incubated at 37°C with 5% CO2.
LPS treatment was performed when cell confluency reached 75%. Cells
were treated for 5 h with LPS (Beyotime Institute of Biotechnology)
at various concentrations (0, 1, 5 and 10 µg/ml). Cell counting
Kit-8 (CCK-8; Sigma-Aldrich; Merck KGaA) was used to detect cell
viability.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from 1×106 ATDC5
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The concentration
of RNA was detected using a NanoDrop 2000 (Thermo Fisher
Scientific, Inc.). The RNA samples were stored at −80°C. Then, cDNA
was synthesized using a miScript Reverse Transcription kit (Qiagen
GmbH) according to the manufacturer's protocol. The QuantiFast SYBR
Green PCR kit (Qiagen GmbH) was used to perform quantitative
real-time polymerase chain reaction (RT-qPCR) under a CFX Connect
Real-Time System (Bio-Rad Laboratories, Inc.). GAPDH was used as
the internal control. The thermocycling conditions were as follows:
95°C for 10 min followed by 35 cycles of 95°C for 15 sec and 55°C
for 40 sec. The 2−ΔΔCq method (24) was used to quantify the relative
gene expression levels of the target genes. The following primers
were purchased from GenScript Corporation: Let-7a forward,
5′-TGAGGTAGTAGGTTGTATAGTTAAA-3′ and reverse,
5′-AACGAGACGACGACAGACTTT-3′; interleukin 6 receptor (IL-6R)
forward, 5′-TGGTGGATGTTCCCCCCGAG-3′ and reverse,
5′-TCCTGGGAATACTGGCACGG-3′; IL-1β forward,
5′-TGTGAAATGCCACCTTTTGA-3′ and reverse, 5′-TGAGTGATACTGCCTGCCTG-3′;
IL-6 forward, 5′-CCGGAGAGGAGACTTCACAG-3′ and reverse,
5′-CAGAATTGCCATTGCACA-3′; TNF-α forward, 5′-GAACTGGCAGAAGAGGCACT-3′
and reverse, 5′-GGTCTGGGCCATAGAACTGA-3′; IL-8 forward,
5′-AGTGAGCTCATTGGCTGGCTTATCTTC-3′ and reverse,
5′-AGTAAGCTTGTTTCTTCCTGGCTCTTG-3′; STAT3 forward,
5′-AAGAGGCGGCAACAGATT-3′ and reverse, 5′-CGGTCTTGATGACGAGGG-3′;
GAPDH forward, 5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse,
5′-GTAGAGGCAGGGATGATGTTCT-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The experiments were repeated three
times.
Cell transfection
ATDC5 cells were seeded into six-well plates
(1×106 cells/well) and cultured at 37°C for 24 h. Then,
the cells were transfected with 100 nM miR-NC inhibitor
(5′-UUCUCCGAACGUGUCACGU-3′; Guangzhou RiboBio Co., Ltd.), 100 nM
Let-7a inhibitor (5′-AACUAUACAACCUCCUACCUCA-3′; Guangzhou RiboBio
Co., Ltd.), 1 µg control-small interfering (si)RNA (cat. no.
EYK-BVIS00414; Xiamen Yanke Biotechnology Co., Ltd.), 1 µg
IL6R-siRNA (cat. no. XWCRH2387; Xiamen Yanke Biotechnology Co.,
Ltd.) or 100 nM Let-7a inhibitor + 1 µg IL6R-siRNA using
Lipofectamine 3000 reagent (Invitrogen Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The transfection
efficiency was detected after 48 h. The experiments were repeated
three times.
CCK-8
ATDC5 cells were treated with 5 µg/ml LPS for 5 h
after 48 h of transfection. In total, 100 µl cell suspension
(1×104 cells/ml) was added to each well of the 96-well
plates, and then the cells were cultured for 24 h. Subsequently, 10
µl CCK-8 reagent (Sigma-Aldrich; Merck KGaA) was added to each
well. The absorbance at 450 nm was measured by an automatic
enzyme-linked immune detector after 2 h of incubation. The CCK-8
experiments were repeated three times.
Flow cytometry analysis
ATDC5 cells were treated with 5 µg/ml LPS for 5 h
after 48 h of transfection. Then, cells were digested using 0.25%
trypsin, followed by washing with PBS. Cells were subsequently
fixed with 70% ethanol overnight at 4°C. Apoptosis was detected
using the Annexin V-FITC- propidium iodide kit (cat. no.
70-AP101-100; Multisciences Lianke Biotech Co., Ltd.) according to
the manufacturer's protocol. Cell apoptosis rate was measured using
a FACSCalibur flow cytometer (BD Biosciences) with Cell Quest
software version 5.1 (BD Biosciences). The experiment was performed
in triplicate.
ELISA
ATDC5 cells were treated with 5 µg/ml LPS for 5 h
after 48 h of transfection. Then, the expression levels of TNF-α
(cat. no. PT512; Beyotime Institute of Biotechnology), IL-1β (cat.
no. PI301; Beyotime Institute of Biotechnology), IL-6 (cat. no.
PI326; Beyotime Institute of Biotechnology) and IL-8 (cat. no.
GD-QX2854; Shanghai Guduo Biological Technology Co., Ltd.) in the
cell culture supernatant were detected using ELISA kits according
to the manufacturer's protocol. Samples were diluted and cytokine
standards were added to ELISA plates. Detection antibodies were
added to the samples and incubated at room temperature for 1 h.
Following incubation with streptavidin-horseradish peroxidase (HRP)
conjugated secondary antibodies at room temperature for 20 min,
plates were read at 450 nm. The experiment was repeated three
times.
Western blot assay
ATDC5 cells were treated with 5 µg/ml LPS for 5 h
after 48 h of transfection. Cells were washed with ice-cold PBS and
then lysed with RIPA (Beyotime Institute of Biotechnology) buffer
with 1% PMSF at 4°C for 1 h. Protein samples were collected by
centrifugation for 5 min at 4°C at 12,000 × g. Protein
concentration was determined using a bicinchoninic acid protein
assay kit. Proteins (30 µg/lane) were separated by SDS-PAGE on 10%
gels, electroblotted onto PVDF membranes and then blocked in 5%
non-fat milk at room temperature for 2 h. Membranes were then
incubated with primary antibodies: IL-6R (cat. no. ab83053;
1:1,000; Abcam), STAT3 (cat. no. ab119352; 1:1,000; Abcam),
phosphorylated STAT3 (cat. no. ab76315; 1:1,000; Abcam) and GAPDH
(cat. no. ab181602; 1:1,000; Abcam), overnight at 4°C and washed
with PBS-0.1% Tween-20 (PBST) four times. Subsequently, the
membranes were incubated with the HRP-conjugated anti-rabbit
secondary antibody (cat. no. 7074; 1:2,000; Cell Signaling
Technology, Inc.) for 2 h at room temperature, the membranes were
then washed with PBST four times. Finally, ECL reagent (EMD
Millipore) was used to visualize the protein bands using a
FluorChem FC3 system (ProteinSimple). AlphaView 3.4.0 software
(ProteinSimple) was used for the quantification of the protein
bands. The experiments were repeated three times.
Dual-luciferase reporter assay
TargetScan version 7.2 (http://www.targetscan.org/vert_72/) was used to
predict the potential targets of Let-7a, and an interaction between
IL-6R and Let-7 was identified. To verify this prediction, the
wild-type (WT) and mutant 3′-untranslated regions (UTRs) of IL-6R
were cloned into a pmiR-RB-Report dual luciferase reporter gene
plasmid vector (Guangzhou RiboBio Co., Ltd.). Cells were
transfected with the reporter constructs and Let-7a mimic
(5′-UGAGGUAGUAGGUUGUAUAGUU-3′; Guangzhou RiboBio Co., Ltd.) or
miRNA-negative control (NC) mimic (5′-UUCUCCGAACGUGUCACGU-3′;
Guangzhou RiboBio Co., Ltd.) using Lipofectamine 2000 (Life
Technologies; Thermo Fisher Scientific, Inc.). Luciferase activity
was assessed after 48 h using the Dual-Luciferase Reporter Assay
system (Promega Corporation) according to the manufacturer's
protocol. Luciferase activity was normalized to the Renilla
luciferase activity. The experiment was repeated three times.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5 (GraphPad Software, Inc.). Data are presented as the mean ±
SD. Comparisons between two groups were analyzed using Student's
t-test. One-way ANOVA followed by Tukey's test was performed to
compare multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
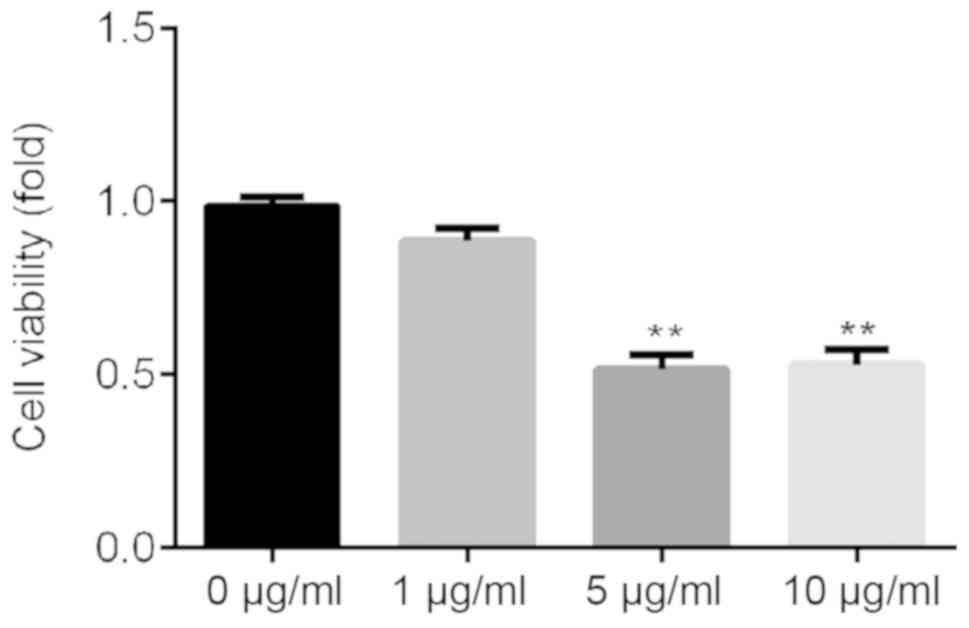
LPS inhibits ATDC5 cell viability
ATDC5 cells were treated with various concentrations
of LPS (0, 1, 5 and 10 µg/ml) for 24 h, and CCK-8 assay was used to
detect cell viability. The present results suggested that 5 and 10
µg/ml LPS could significantly inhibit ATDC5 cell viability
(Fig. 1). Then, 5 µg/ml LPS was
selected for further experiments.
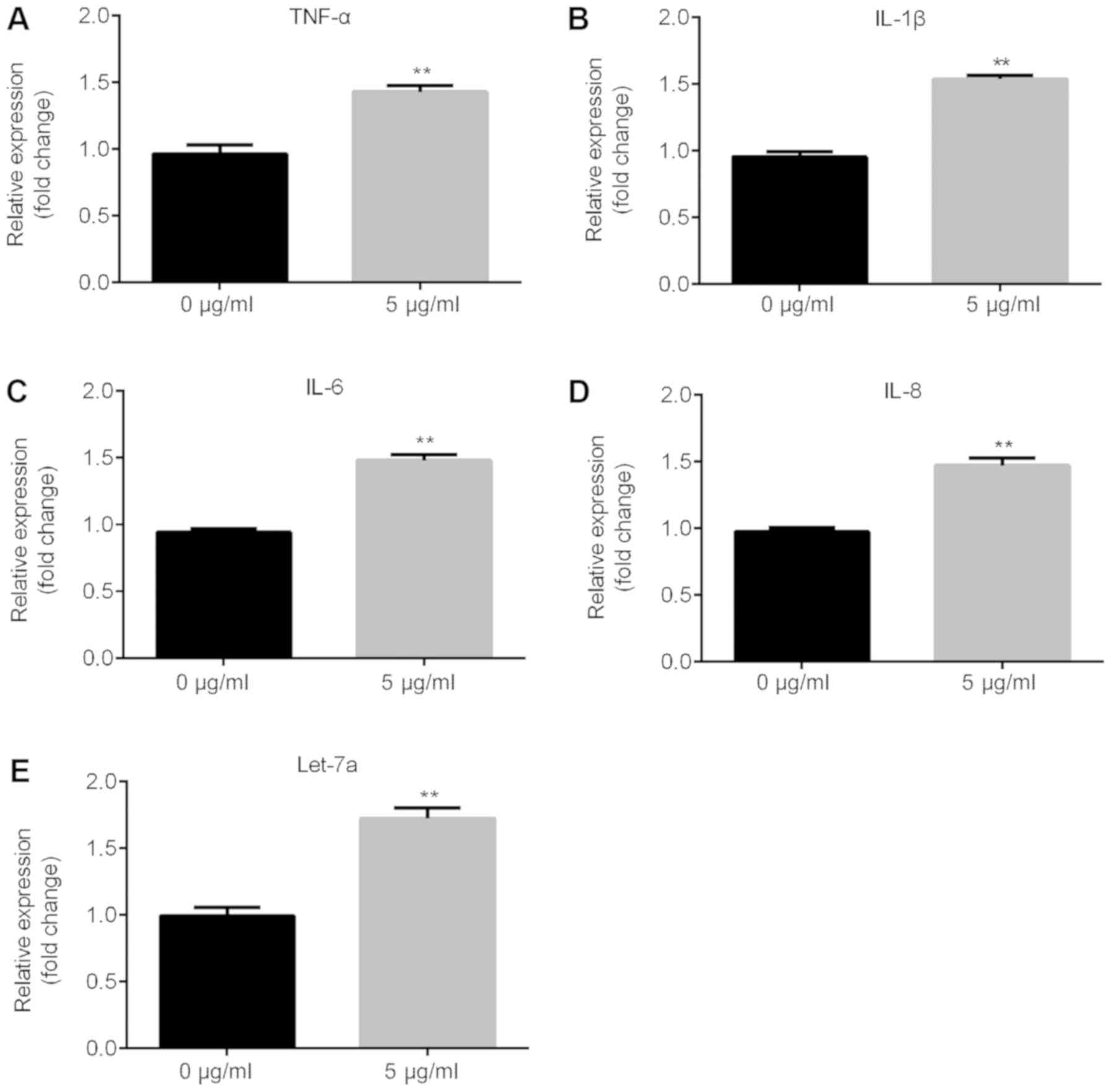
LPS significantly induces inflammatory
response and increases the expression level of Let-7a
The present RT-qPCR results suggested that the mRNA
expression levels of inflammatory factors, such as TNF-α, IL-1β,
IL-6 and IL-8, significantly increased following treatment with 5
µg/ml LPS in ATDC5 cells compared with the control group (Fig. 2A-D) and Let-7a expression level was
significantly increased (Fig.
2E).
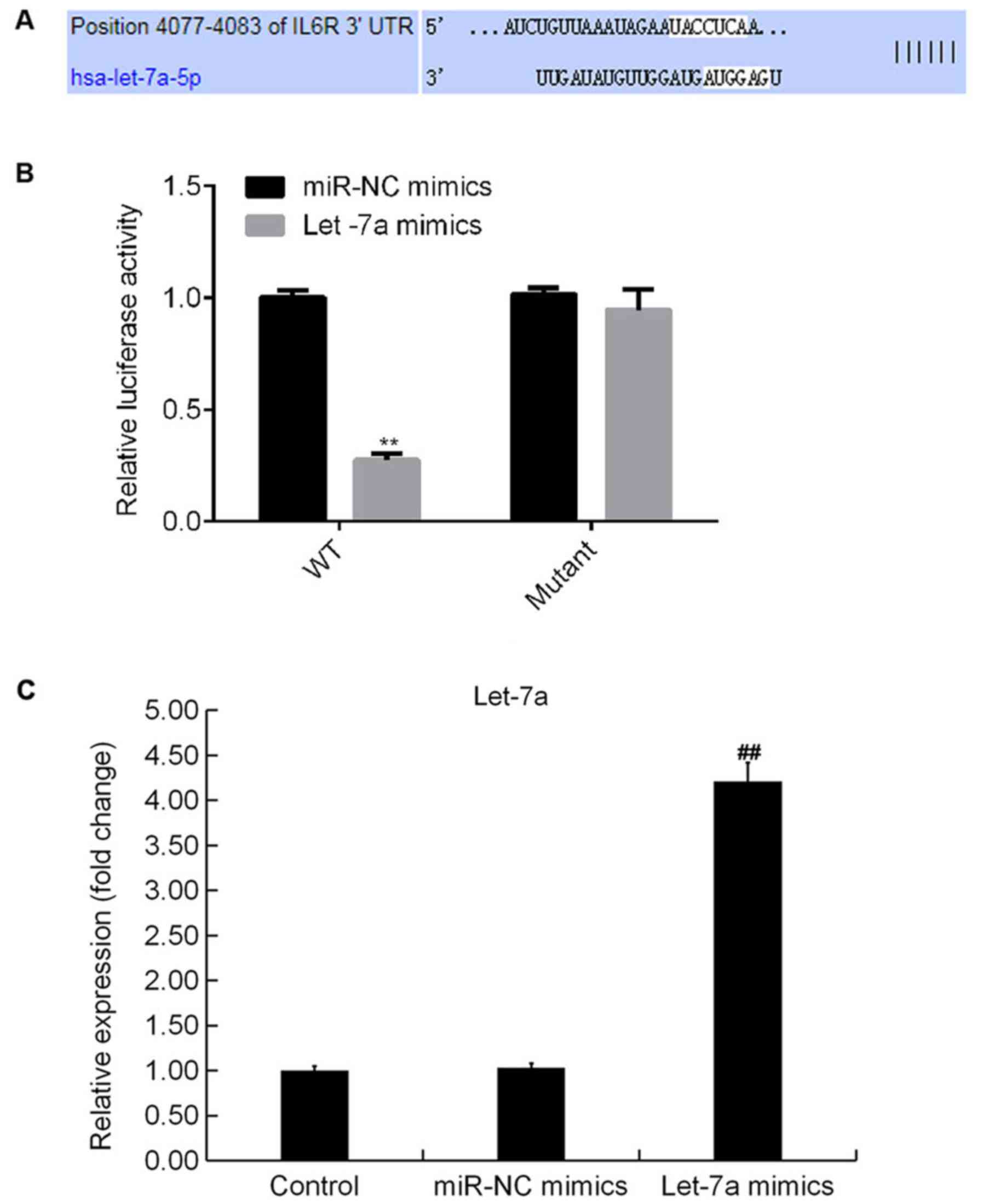
IL-6R is a target of Let-7a
TargetScan analysis identified potential binding
sites between Let-7a and IL-6R (Fig.
3A). The dual-luciferase reporter gene assay results suggested
that compared with cotransfection of WT-3′UTR-IL-6R plasmid and
mimic control, luciferase activity was significantly decreased
following cotransfection with WT-3′UTR-IL-6R and Let-7a mimics. The
present results suggested that IL-6R may be a direct target of
Let-7a (Fig. 3B). In addition,
Let-7a mimics significantly increased the expression level of
Let-7a in ATDC5 cells (Fig.
3C).
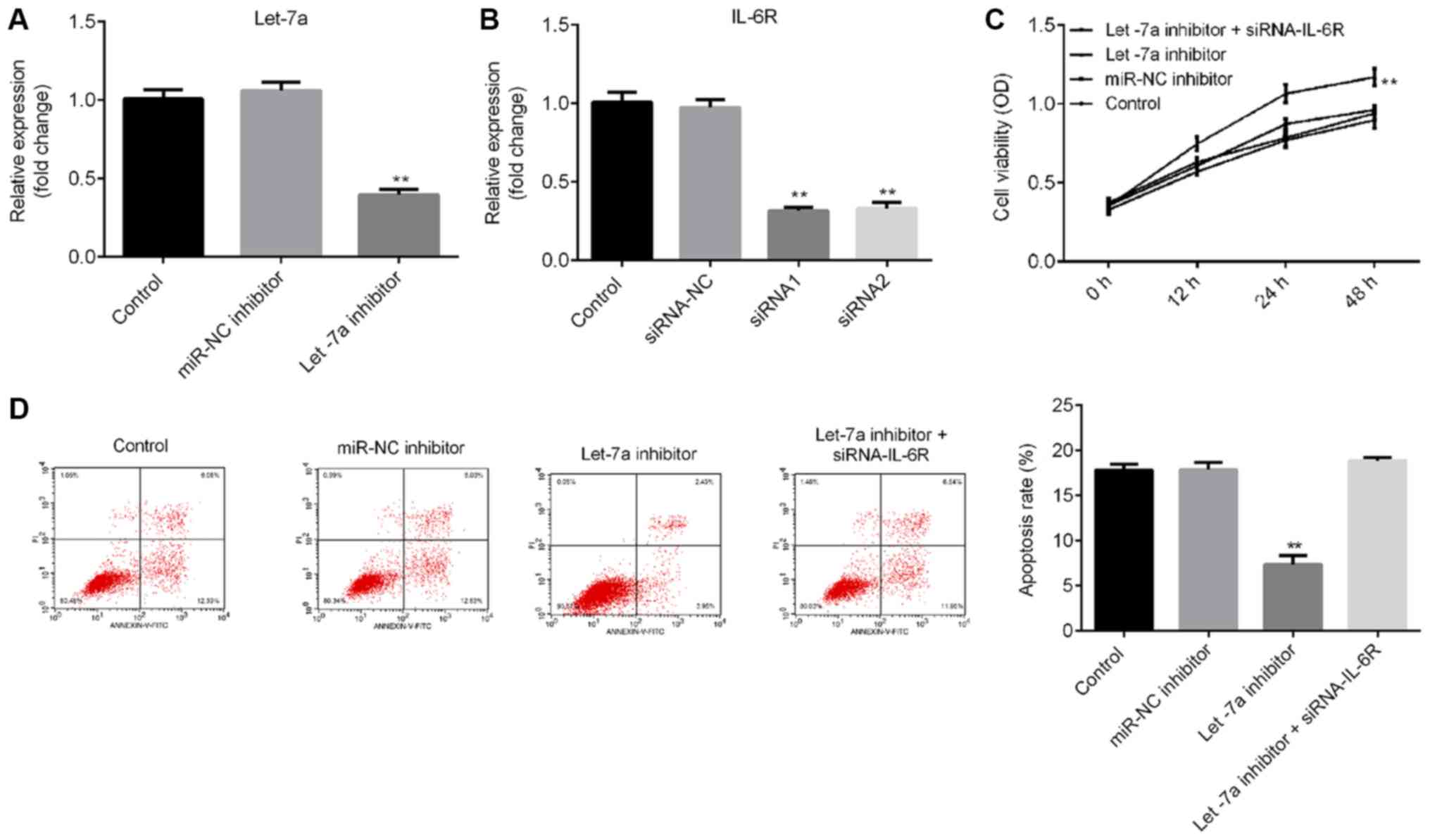
Let-7a inhibitor significantly
promotes cell viability and reduces apoptosis
To investigate the effects of Let-7a on LPS-treated
ATDC5 cells, ATDC5 cells were transfected with Let-7a inhibitor,
miRNA-NC inhibitor or Let-7a inhibitor + IL-6R-siRNA for 48 h, and
then the cells were treated with 5 µg/ml LPS for 5 h. RT-qPCR
results indicated that Let-7a inhibitor significantly decreased
Let-7a expression levels in ATDC5 cells (Fig. 4A), and the mRNA level of IL-6R in
ATDC5 cells was significantly decreased following treatment with
IL-6R-siRNA (Fig. 4B). The CCK-8
results and flow cytometry analysis suggested that Let-7a inhibitor
significantly promoted cell viability (Fig. 4C) and decreased cell apoptosis
(Fig. 4D) in LPS-treated ATDC5
cells. These effects were reversed by IL-6R knockdown.
Let-7a inhibitor significantly reduces
the protein expression levels of multiple inflammatory factors
ELISA results suggested that Let-7a inhibitor
significantly reduced the expression levels of TNF-α, IL-1β, IL-6
and IL-8 in LPS-treated ATDC5 cells, and these effects were
reversed by IL-6R knockdown (Fig.
5).
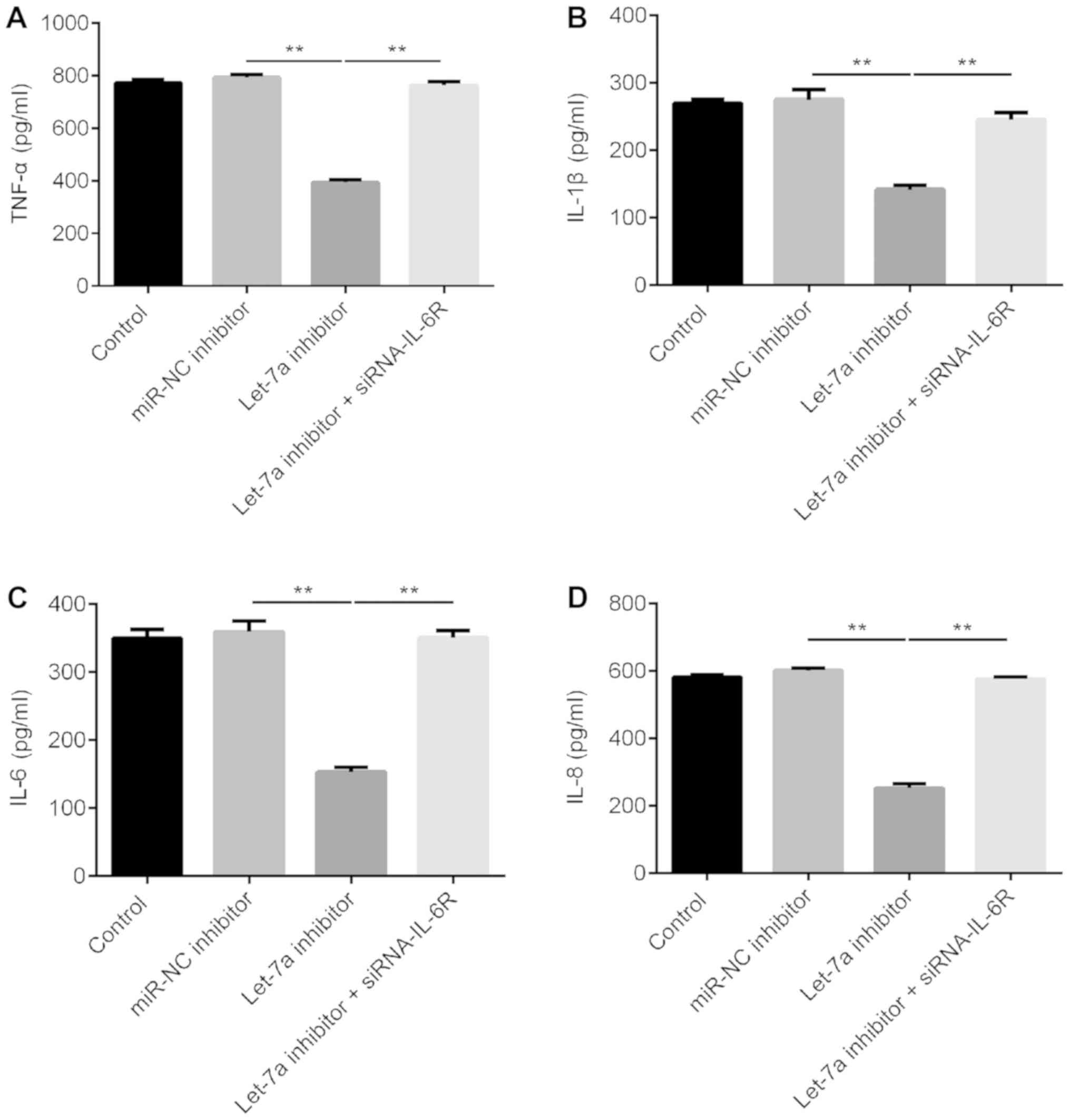 | Figure 5.Effects of Let-7a inhibitor on the
expression of inflammatory factors in LPS-treated ATDC5 cells.
ATDC5 cells were treated with 5 µg/ml LPS for 5 h after 48 h of
transfection with miR-NC inhibitor, Let-7a inhibitor or Let-7a
inhibitor + IL-6R-siRNA. ELISA was used to detect the expression of
inflammatory factors, including (A) TNF-α, (B) IL-1β, (C) IL-6 and
(D) IL-8. Data are presented as the mean ± SD. **P<0.01. Let-7a,
microRNA-let7a; LPS, lipopolysaccharide; IL-6R, interleukin 6
receptor; siRNA, small interfering RNA; NC, negative control;
TNF-α, tumor necrosis factor α; IL-, interleukin; miR,
microRNA. |
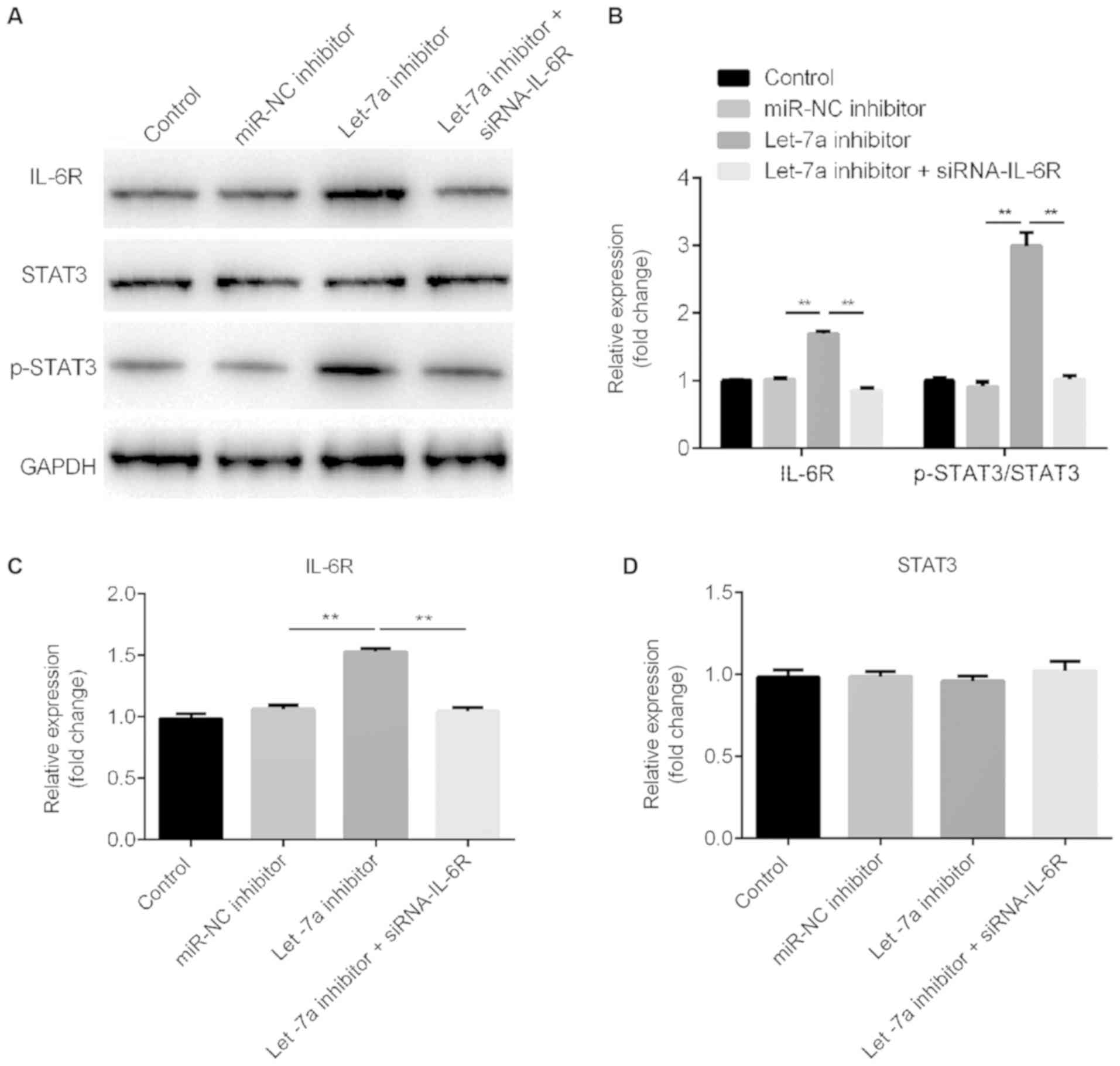
Let-7a inhibitor activates the STAT3
signaling pathway
Western blot assay ad RT-qPCR results suggested that
Let-7a inhibitor increased IL-6R protein and mRNA expression
levels, respectively. These effects were reversed by IL-6R-siRNA
(Fig. 6A-C). Let-7a inhibitor
increased the protein expression level of phosphorylated STAT3 and
this effect was reversed by IL-6R knockdown (Fig. 6A and B). Notably, Let-7a inhibitor
and IL-6R-siRNA did not exhibit effects on the protein and mRNA
expression levels of STAT3 (Fig. 6A
and D).
Discussion
The deterioration of the main joint structure,
cartilage, bone and synovium are a major feature of OA (25,26),
which is the most common joint disease. Joint pain, stiffness and
impaired movements are the major clinical symptoms of OA. The
prevalence of OA increases with age. Notably, OA can cause chronic
pain and reduce the quality of life of elderly patients (27).
LPS is one of the main components of the cell wall
of Gram-negative bacteria (28).
LPS can activate macrophages, triggering the inflammatory response
and activating the innate immunity (28). In the present study, LPS-treated
ATDC5 cells were used to establish an in vitro a model of
chondrocyte inflammatory injury. The present results suggested that
LPS was able to induce ATDC5 cells to synthesize a large amount of
inflammatory cytokines, including TNF-α, IL-1β, IL-6 and IL-8, in
line with previous studies (29,30).
The Let-7 family consists of miRNAs that are highly
expressed in adult tissues (13).
In the present paper, IL-6R was a identified as a direct target of
Let-7a using a dual-luciferase reporter assay, and IL-6R was found
to be negatively regulated by Let-7a. Consistent with a previous
study, the present results suggested that Let-7a was involved in
cell proliferation and apoptosis (31). In the present study, Let-7a
inhibitor promoted cell viability and inhibited apoptosis in
LPS-treated ATDC5 cells. Accumulating evidence demonstrated that
pro-inflammatory cytokines, such as TNF-α and IL-1β, serve key
roles in the pathogenesis of OA (32,33).
TNF-α is a type of TNF released by macrophages, and acts as a
trigger for the inflammatory response (34,35).
IL-1β and IL-1α are the two types of IL-1, and these two cytokines
can be synthesized by various cell types, such as monocyte
macrophages and vascular endothelial cells (36). Notably, IL-1β is an important
inflammatory mediator of the inflammatory process (37). IL-1β can not only induce the
release of inflammatory mediators, including nitric oxide,
prostaglandin E2 and matrix metalloproteinases, but also promote
chondrocyte apoptosis, leading to articular cartilage damage
(38,39). IL-6 is a cytokine produced by
various cell types and it belongs to the interleukin family. IL-6
was identified to be able to transduce signals, activating and
regulating immune cells, and mediating T and B cell activation,
proliferation and differentiation, serving an important role in the
inflammatory response (40,41).
The inflammatory response involves lipid peroxidation and
activation of multiple receptors, stimulating macrophages and other
cells to secrete pro-inflammatory factors, such as IL-1, IL-2 and
IL-8, activating a signaling cascade (34,35).
In the present study, ELISA was used to detect the expression level
of inflammatory factors in LPS-treated ATDC5 cells in various
conditions. The present results suggested that Let-7a inhibitor
significantly reduced the expression levels of TNF-α, IL-1β, IL-6
and IL-8 in LPS-treated ATDC5 cells, and these effects were
reversed by IL-6R knockdown.
The IL-6/STAT3 signaling pathway is a key signal
transduction pathway for the development and progression of
malignant tumors (42–44). In addition, IL-6-mediated STAT3
activation is involved in cell proliferation and apoptosis
(42–44). In the present study, the effects of
Let-7a inhibitor on the STAT3 signaling pathway were investigated.
Western blotting results suggested that Let-7a inhibitor
significantly increased the protein expression level of
phosphorylated STAT3 and this effect was reversed by IL-6R
knockdown. The present results suggested that Let-7a inhibitor
could activate the STAT3 signaling pathway.
Collectively, the present results suggested that
Let-7a inhibitor could enhance cell proliferation, reduce apoptosis
and inhibit inflammatory response in LPS-treated ATDC5 cells. The
present study provided a novel potential therapeutic target and may
facilitate the development of new approaches to improve the
prevention and treatment of OA. However, the present study is a
preliminary study, and further experiments are required to validate
the role of Let-7a in OA. Importantly, it is necessary to
investigate the expression level of Let-7a in patients with OA. In
addition, the role of Let-7a in OA should be investigated using
in vivo models. Moreover, the association between the
expression level of Let-7a and the clinical features of patients
with OA requires further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by Youth Culture
Program of the First Affiliated Hospital of Anhui Medical
University (grant no. 2017kj06).
Availability of data and materials
All data sets used and/or generated during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
CS and LZ contributed to the design of the study,
data collection, statistical analysis and data interpretation. YH
contributed to data collection, manuscript preparation and the
literature search. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Allen KD and Golightly YM: State of the
evidence. Curr Opin Rheumatol. 27:276–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heijink A, Vanhees M, van den Ende K, van
den Bekerom MP, van Riet RP, Van Dijk CN and Eygendaal D:
Biomechanical considerations in the pathogenesis of osteoarthritis
of the elbow. Knee Surg Sports Traumatol Arthrosc. 24:2313–2318.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cummins JM, He Y, Leary RJ, Pagliarini R,
Diaz LA Jr, Sjoblom T, Barad O, Bentwich Z, Szafranska AE,
Labourier E, et al: The colorectal microRNAome. Proc Natl Acad Sci
USA. 103:3687–3692. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2016. View Article : Google Scholar
|
|
5
|
Hydbring P, Wang Y, Fassl A, Li X, Matia
V, Otto T, Choi YJ, Sweeney KE, Suski JM, Yin H, et al:
Cell-cycle-targeting micrornas as therapeutic tools against
refractory cancers. Cancer Cell. 31:576–590.e8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drusco A and Croce CM: MicroRNAs and
cancer: A long story for short RNAs. Adv Cancer Res. 135:1–24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee H, Han S, Kwon CS and Lee D:
Biogenesis and regulation of the let-7, miRNAs and their functional
implications. Protein Cell. 7:100–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beyer C, Zampetaki A, Lin NY, Kleyer A,
Perricone C, Iagnocco A, Distler A, Langley SR, Gelse K, Sesselmann
S, et al: Signature of circulating microRNAs in osteoarthritis. Ann
Rheum Dis. 74:e182015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xue H, Tu Y, Ma T, Wen T, Yang T, Xue L,
Cai M, Wang F and Guan M: miR-93-5p attenuates IL-1β-induced
chondrocyte apoptosis and cartilage degradation in osteoarthritis
partially by targeting TCF4. Bone. 123:129–136. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malemud CJ: MicroRNAs and osteoarthritis.
Cells. 7:E922018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
caenorhabditis elegans. Nature. 403:901–906. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu L, Katsaros D, Risch HA, Canuto EM,
Biglia N and Yu H: MicroRNA let-7a modifies the effect of
self-renewal gene HIWI on patient survival of epithelial ovarian
cancer. Mol Carcinog. 55:357–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu K, Zhang C, Li T, Ding Y, Tu T, Zhou
F, Qi W, Chen H and Sun X: Let-7a inhibits growth and migration of
breast cancer cells by targeting HMGA1. Int J Oncol. 46:2526–2534.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson VL and Hunter DJ: The epidemiology
of osteoarthritis. Best Pract Res Clin Rheumatol. 28:5–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheumatism. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burr DB and Gallant MA: Bone remodelling
in osteoarthritis. Nat Rev Rheumatol. 8:665–673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HA, Lee YJ, Seong SC, Choe KW and Song
YW: Apoptotic chondrocyte death in human osteoarthritis. J
Rheumatol. 27:455–462. 2000.PubMed/NCBI
|
|
20
|
Yan S, Wang M, Zhao J, Zhang H, Zhou C,
Jin L, Zhang Y, Qiu X, Ma B and Fan Q: MicroRNA-34a affects
chondrocyte apoptosis and proliferation by targeting the SIRT1/p53
signaling pathway during the pathogenesis of osteoarthritis. Int J
Mol Med. 38:201–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shu Y, Long J, Guo W and Ye W:
MicroRNA-195-5p inhibitor prevents the development of
osteoarthritis by targeting REGγ. Mol Med Rep. 19:4561–4568.
2019.PubMed/NCBI
|
|
22
|
Hu Y, Li S and Zou Y: Knockdown of LncRNA
H19 relieves LPS-induced damage by modulating miR-130a in
osteoarthritis. Yonsei Med J. 60:381–388. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ying H, Wang Y, Gao Z and Zhang Q: Long
non-coding RNA activated by transforming growth factor beta
alleviates lipopolysaccharide-induced inflammatory injury via
regulating microRNA-223 in ATDC5 cells. Int Immunopharmacol.
69:313–320. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldring MB and Goldring SR: Articular
cartilage and subchondral bone in the pathogenesis of
osteoarthritis. Ann NY Acad Sci. 1192:230–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis).
Osteoarthritis Cartilage. 21:16–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malfait AM: Osteoarthritis year in review
2015: Biology. Osteoarthritis Cartilage. 24:21–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan Q, Zhang D, Liu C, Zhang C and Yuan
D: Chikusetsusaponin V Inhibits LPS-activated inflammatory
responses via SIRT1/NF-κB signaling pathway in RAW264.7 cells.
Inflammation. 41:2149–2159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng Y, Yang C, Luo D, Li X, Le XC and
Rong J: N-Propargyl caffeamide skews macrophages towards a
resolving M2-Like phenotype against myocardial ischemic injury via
activating Nrf2/HO-1 pathway and inhibiting NF-κB pathway. Cell
Physiol Biochem. 47:2544–2557. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
De Santis R, Liepelt A, Mossanen JC, Dueck
A, Simons N, Mohs A, Trautwein C, Meister G, Marx G,
Ostareck-Lederer A and Ostareck DH: miR-155 targets caspase-3 mRNA
in activated macrophages. RNA Biol. 13:43–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toledano H: The role of the heterochronic
microRNA let-7 in the progression of aging. Exp Gerontol.
48:667–670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Amin AR: Regulation of tumor necrosis
factor-α and tumor necrosis factor converting enzyme in human
osteoarthritis. Osteoarthritis Cartilage. 7:392–394. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Calzascia T, Pellegrini M, Hall H, Sabbagh
L, Ono N, Elford AR, Mak TW and Ohashi PS: TNF-alpha is critical
for antitumor but not antiviral T cell immunity in mice. J Clin
Invest. 117:3833–3845. 2007.PubMed/NCBI
|
|
35
|
Liu M, Gu M, Xu D, Lv Q, Zhang W and Wu Y:
Protective effects of toll-like receptor 4 inhibitor eritoran on
renal ischemia-reperfusion injury. Transplant Proc. 42:1540–1544.
2010. View Article : Google Scholar
|
|
36
|
Striz I: Cytokines of the IL-1 family:
Recognized targets in chronic inflammation underrated in organ
transplantations. Clin Sci (Lond). 131:2241–2256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Logan RM, Stringer AM, Bowen JM, Yeoh AS,
Gibson RJ, Sonis ST and Keefe DM: The role of pro-inflammatory
cytokines in cancer treatment-induced alimentary tract mucositis:
Pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev.
33:448–460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Montaseri A, Busch F, Mobasheri A,
Buhrmann C, Aldinger C, Rad JS and Shakibaei M: IGF-1 and PDGF-bb
suppress IL-1β-induced cartilage degradation through
down-regulation of NF-κB signaling: Involvement of Src/PI-3K/AKT
pathway. PLoS One. 6:e286632011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ying X, Peng L, Chen H, Shen Y, Yu K and
Cheng S: Cordycepin prevented IL-β-induced expression of
inflammatory mediators in human osteoarthritis chondrocytes. Int
Orthop. 38:1519–1526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lv SY, Wu Q, Liu JP, Shao J, Wen LL, Xue
J, Zhang XS, Zhang QR and Zhang X: Levels of interleukin-1β,
interleukin-18, and tumor necrosis factor-α in cerebrospinal fluid
of aneurysmal subarachnoid hemorrhage patients may be predictors of
early brain injury and clinical prognosis. World Neurosurg.
111:e362–e373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ataie-Kachoie P, Pourgholami MH,
Richardson DR and Morris DL: Gene of the month: Interleukin 6
(IL-6). J Clin Pathol. 67:932–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bournazou E and Bromberg J: Targeting the
tumor microenvironment: JAK-STAT3 signaling. JAKSTAT.
2:e238282013.PubMed/NCBI
|
|
43
|
Rokavec M, Wu W and Luo JL: IL6-Mediated
suppression of miR-200c directs constitutive activation of
inflammatory signaling circuit driving transformation and
tumorigenesis. Mol Cell. 45:777–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Al Zaid Siddiquee K and Turkson J: STAT3
as a target for inducing apoptosis in solid and hematological
tumors. Cell Res. 18:254–267. 2008. View Article : Google Scholar : PubMed/NCBI
|