Introduction
Intravesical instillation of bacillus
Calmette-Guérin (BCG) has become the golden standard for the
treatment of superficial transitional cell carcinoma (TCC) of the
bladder, and has been successfully used for approximately four
decades, significantly reducing mortality rates (1). Although the mechanism of BCG remains
unclear, it is generally conceded that BCG instillation can induce
multiple immunoreactions for tumor management and prevention
(2). Cancer cells are first
infected by BCG, which then induces the expression of various types
of cytokines, such as tumor necrosis factor (TNF)-α, interleukin
(IL)-6 and IL-8, indicating an immunological response. Finally, the
antitumor effect of BCG is performed by cytotoxic T lymphocytes,
macrophages and natural killer cells. Neutrophils, which constitute
the major cell subset in the leukocyturia detected after BCG
instillation, or the TNF-related apoptosis-inducing ligand (TRAIL;
also known as Apo-2L) protein produced by neutrophils have also
been confirmed to be involved in the antitumor effect of BCG
(3).
Neutrophils are the most abundant type of white
blood cells, accounting for 40–70% of these cells. They are formed
from stem cells in the bone marrow and are a crucial part of the
innate immune system (4).
Neutrophils are also one of the first responders of inflammation,
particularly in bacterial infection, and are recruited within min.
It has been reported that neutrophils can synthesize and secrete a
small number of cytokines, including IL-1, IL-8, IL-12, TNF-α,
macrophage inflammatory protein 1 and transforming growth factor 1,
which may affect macrophage stimulation, neutrophil recruitment,
and lymphocyte activation, proliferation and differentiation
(5). Therefore, the function of
neutrophils can be clarified by analyzing the expression profile of
cytokines.
Although numerous studies have focused on BCG and
neutrophils (6–8), the mechanism of neutrophil-mediated
activation of tumor immunity remains unclear in the medical
community. In the present study, in order to elucidate the
antitumor effect of neutrophils stimulated by BCG, a tumor-bearing
model was constructed in BALB/c mice, which was then subjected to
BCG or normal saline (NS) intervention. The gross tumor volume
(GTV), survival time and amount of neutrophils in the mice were
examined.
Materials and methods
Ethical approval
The study was approved by the Ethics Committee of
China Medical University (Shenyang, China), and all experiments
were conducted according to the institutional guidelines for the
care and use of laboratory animals (http://www.cmu.edu.cn/sydwb/index.htm).
Reagents and cell lines
Phosphate-buffered saline (PBS; 0.01 M) was
sterilized by high-pressure steam for 15 min and stored at 4–8°C,
following preparation by dissolving 8 g NaCI, 0.2 g KCl, 1.15 g
Na2HPO4 and 0.2 g
KH2PO4 in 900 ml ddH2O with pH 7.4
and bringing the whole volume to 1,000 ml with ddH2O.
The BCG solution [10 mg/ml; 108 colony-forming units
(CFU)/ml] was obtained by dissolving 1 g BCG powder (Shanghai
Research Institute of Biological Products) in 100 ml NS, and was
then further attenuated to 107, 106 and
105 CFU/ml solutions. Oyster glycogen solution (2%) was
prepared by adding 2 g oyster glycogen (Sigma-Aldrich; Merck KGaA)
to 100 ml NS, followed by attenuation to 0.25, 0.5, 1 and 1.5%
solutions, filtration and sterilization. Fetal calf serum
FCS)-RPMI-1640 medium was prepared by dissolving 10.4 g RPMI-1640
powder in 200 ml ddH2O, and adding 2 g NaHCO3
and 100 ml FCS, and the medium was then stored at 4–8°C. Mouse
neutrophil separation solution was purchased from Tianjin Haoyang
Biological Manufacture Co., Ltd., and the ELISA kits were from
Thermo Fisher Scientific, Inc. The instruments used included a
centrifuge (Jouan CR411; Thermo Fisher Scientific, Inc.), optical
microscope (Olympus Corporation), −80°C freezer (Kelvinator;
Electrolux) and an analytical balance (L-1660 DTP; Shimadzu
Corporation).
MethA fibrosarcoma cells were obtained from the
Hirosaki University of Medicine (Hirosaki, Japan), while yeast
artificial chromosome-1 (YAC-1) cells were obtained from the China
Medical University (Shenyang, China). Cells were maintained in
RPMI-1640 culture medium at 37°C in 5% CO2 for 24–48 h
and modified to a concentration of 1×105/ml.
Animals
Female BALB/c mice (age, 6–8 weeks; weight, 18–22 g)
were obtained from the Chinese Academy of Sciences (Shanghai,
China). The mice were kept in plastic cages (23±2°C and 55.5±10%
relative humidity) with free access to water and food and exposed
to a 12 h fluorescent light/dark cycle for a week before
experiments.
Extraction of neutrophils
BALB/c mice were subcutaneously injected with 0.1 ml
BCG of 106 CFU/ml (BCG group) or with NS (NS group)
according to previously described experiments on detecting the
effect of BCG (6,7). A third group without any intervention
was selected as the control. At 4 h after intraperitoneal injection
with 1.5 ml oyster glycogen solution (1%), the mice were euthanized
and their enterocoelia were lavaged with 7–8 ml sterile PBS, and
then peritoneal cells were extracted by centrifugation at 1,000 × g
for 5 min at room temperature. The supernatant was discarded and
cells were resuspended with 1 ml RPMI-1640 medium. Next, the
resuspended cell solution was transferred to the top of the mouse
neutrophil separation liquid (2 ml) in a 15-ml tube, and
centrifugation was again performed at 1,000 × g at 23°C for 5 min.
The liquid at the top of the neutrophil cellular layer was
subsequently discarded, and 4 ml sterile PBS was added to the
remaining solution and further centrifuged (1,000 × g, 23°C, 5
min). Finally, the neutrophils were rinsed twice and resuspended
with 1 ml RPMI-1640 medium.
Construction of a tumor-bearing mouse
model
The abdominal cavity of BALB/c mouse was injected
with 1×106/ml MethA cells and lavaged with 2–3 ml
sterile PBS after 5 days. Next, ascitic fluid was collected and
centrifuged (1,000 × g, room temperature, 5 min). The supernatant
was discarded, and cells were resuspended with 5 ml sterile PBS and
centrifuged (1,000 × g, room temperature, 5 min). Subsequently, the
supernatant was once more discarded, and 1 ml NS was added to
suspend the cells, which were then modified to a concentration of
1×107/ml. Finally, 0.1 ml MethA suspended cells were
hypodermically injected into the right posterior limb of the mice.
After 1 week, a mass could be observed, indicating the
accomplishment of tumor-bearing mouse models, which were divided
into group A and group B.
Next, BCG (0.1 ml; 106 CFU/ml) or NS was
injected into group A tumor-bearing mice once every 2 days for 6
days. Neutrophils were extracted from these mice on the second day
of the third injection. Subsequent to rinsed for two times,
neutrophils were resuspended with 1 ml NS and then modified to a
concentration of 1×107/ml. Then, group B tumor bearing
mice (n=15) were subcutaneously injected with these neutrophils
extracted from group A tumor-bearing mice treated by BCG or NS,
which were titled as BCGT and NST groups. The rest mice injected
with the neutrophils from untreated group A tumor-bearing mice were
assigned to CT group.
Assessment of cytokine activity
Cytokine activity was assessed by lactate
dehydrogenase (LDH) Cytotoxicity Assay Kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol.
Neutrophils from the three groups (namely BCG, NS and control
groups) were modified to obtain a concentration of
5×106/ml using RPMI-1640 medium supplemented with 10%
FCS (Biochrom). Next, 0.1 ml MethA and YAC-1 cells were
individually placed in a 96-well plate with a 50:1 effector target
ratio. Each group was plated in triplicate. Subsequent to culturing
at 37°C for 3 h, 0.1 ml supernatant taken from each well was
reacted with 0.1 ml LDH substrate, and 30 µl 1 mol/l citric acid
solution was added to terminate the enzymatic reaction after 20
min. Absorbance of each samples was measured at 490 nm test
wavelength and at 630 nm reference wavelength by a microplate
reader (Bio-Rad Laboratories, Inc.). The percentage of cell death
was calculated using the following formula: cytotoxicity (%) =
(experimental value-low control)/(high control-low control) ×
100%.
In addition, neutrophils extracted from BCG-treated
and NS-treated mice were modified to obtain a concentration of
2×106/ml using RPMI-1640 medium supplemented with 10%
FCS, and cultured for 6 h. Next, the supernatant was collected
following centrifugation at 1,000 × g for 10 min at room
temperature. According to the ELISA (Thermo Fisher Scientific,
Inc.) protocol, the densities (pg/ml) of the cytokines TNF-α
(BMS607-3), IL-1β (BMS6002), IL-6 (BMS603-2) and TRAIL/Apo-2L
(EMTNFSF10) were calculated.
Furthermore, neutrophils extracted from untreated
mice were modified to a concentration of 2×106/ml, and
treated with NS or BCG at different concentrations (0.01, 0.1 and 1
mg/ml BCG in groups 1, 2 and 3, respectively). Cells were cultured
for 6 h, and then the densities (pg/ml) of TNF-α, IL-1β, IL-6 and
TRAIL/Apo-2L were assessed.
Survival rate and GTV of tumor-bearing
mice
Subsequent to neutrophil injection, the mortality of
mice was recorded every day for a total of 18 days, and the
survival rate was calculated using the Graph Pad Prism 5 software
(GraphPad Software, Inc.). In addition, the long and short radius
(mm) of the tumor was assessed every 2 days, and the GTV was
calculated according to the following formula: GTV = (Long radius ×
short radius2)/2.
Pathological examination of MethA
tumor
At 24 h after neutrophil injection, the mice were
sacrificed and stabilized in the prone position. Tumors were
extracted, fixed in 6% buffered formalin, routinely processed with
paraffin, stained by hematoxylin and eosin and finally mounted with
neutral resins. An optical microscope was employed to calculate the
amount of neutrophils in 10 fields-of-view.
Statistical analysis
Statistical analysis was performed by SPSS software,
version 11.5 (SPSS, Inc.). Analysis of variance and Dunnett's
multiple comparisons test were used to test for significant
differences between the mean values. Differences were considered to
be statistically significant at P≤0.05.
Results
Cytotoxic activity of BCG-treated
neutrophils
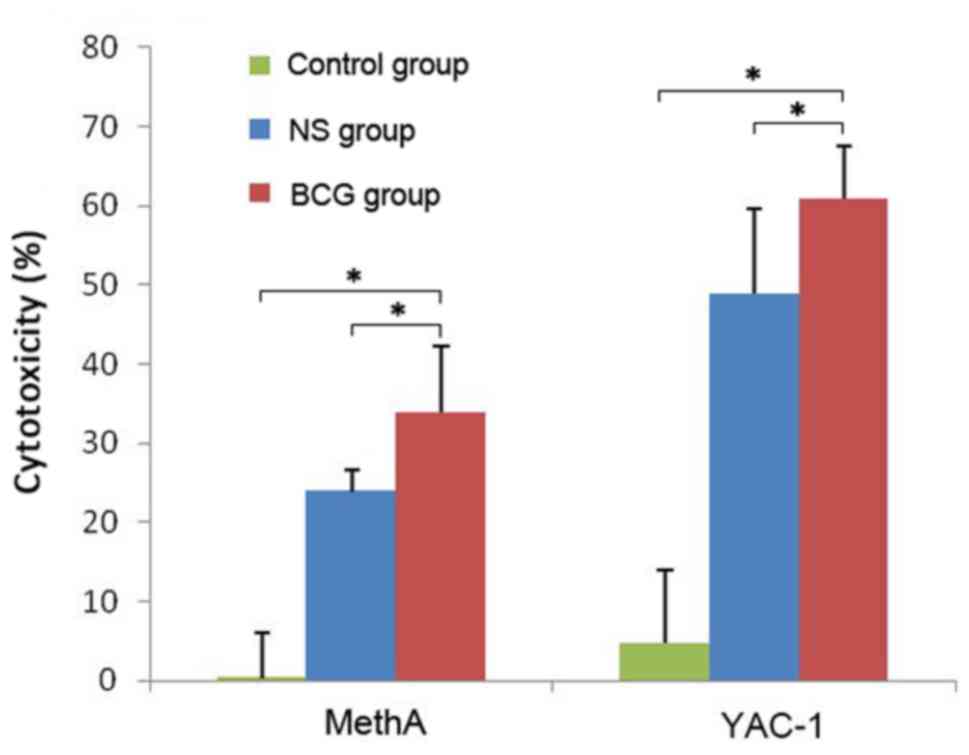
As shown in Fig. 1,
BCG-treated (0.1 mg/ml, 106 CFU/ml) neutrophils
presented higher percentage of cytotoxicity in both MethA and YAC-1
tumor cells compared with the NS group (P<0.05) and the control
group without any intervention (P<0.05). This indicated that BCG
can enhance the cytotoxic activity of neutrophils in defeating
tumor cells.
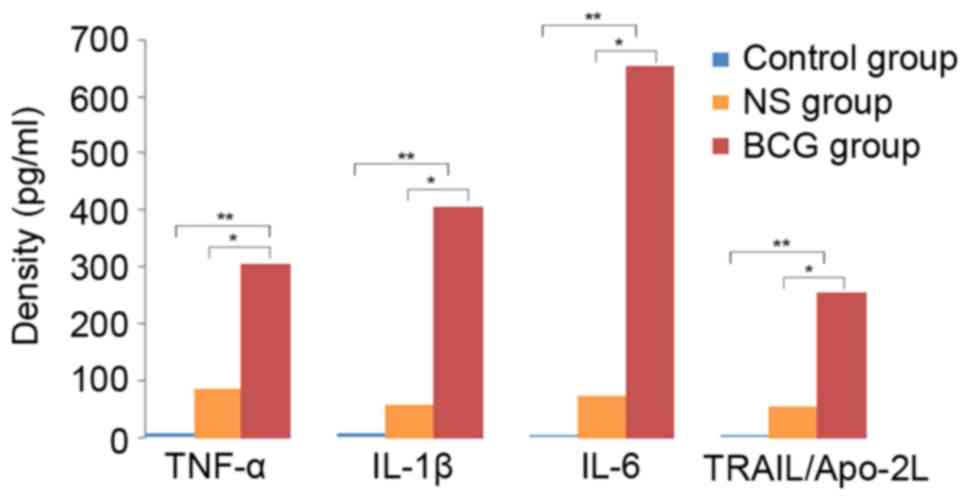
Concentration of cytokines in
BCG-treated neutrophils
In order to clarify the possible mechanism involved
in the effect of BCG on neutrophils, the concentration of cytokines
in neutrophil supernatant extracted from treated mice was detected.
It was observed that the concentration of the cytokines TNF-α,
IL-1β, IL-6 and TRAIL/Apo-2L was significantly higher in the BCG
group (0.1 mg/ml, 106 CFU/ml) as compared with the
control (P<0.01) and NS groups (P<0.05). This indicated that
BCG-treated neutrophils can induce cytokines to kill tumor cells
(Fig. 2).
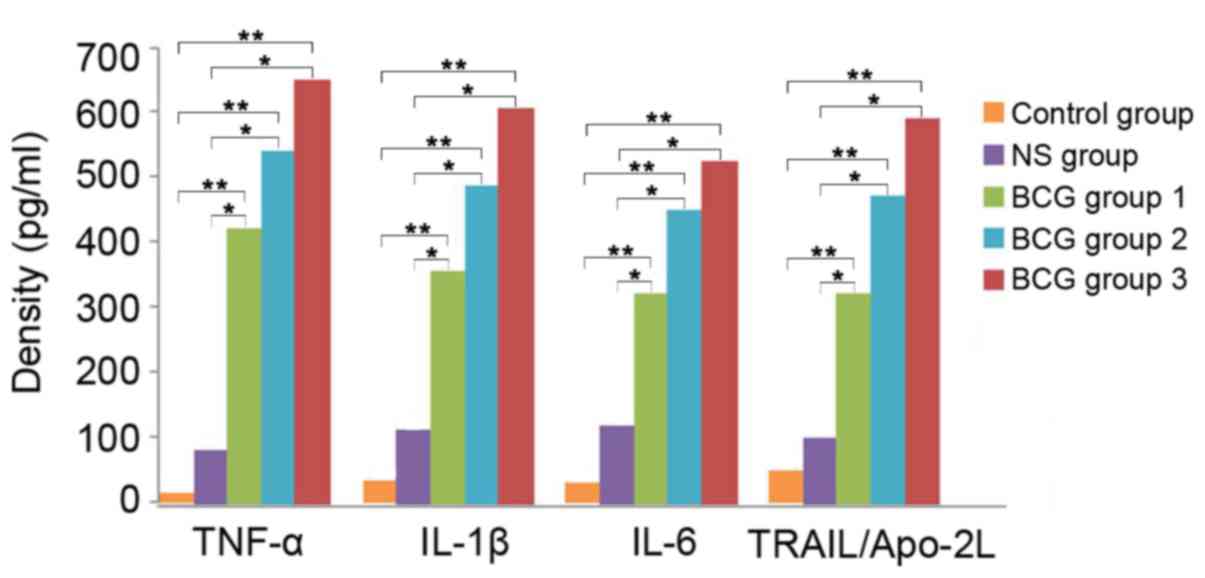
Concentration of cytokines in
neutrophils treated with different doses of BCG
Further experiments were conducted to determine
whether different concentrations of BCG could affect the results.
As shown in Fig. 3, the
concentration of abovementioned cytokines remained high in all
three BCG groups tested (0.01, 0.1 and 1 mg/ml, 106
CFU/ml) compared with the control and NS groups (P<0.01). Higher
doses of BCG led to increased concentration of cytokines,
suggesting that BCG at higher doses may lead to an improved
antitumor effect.
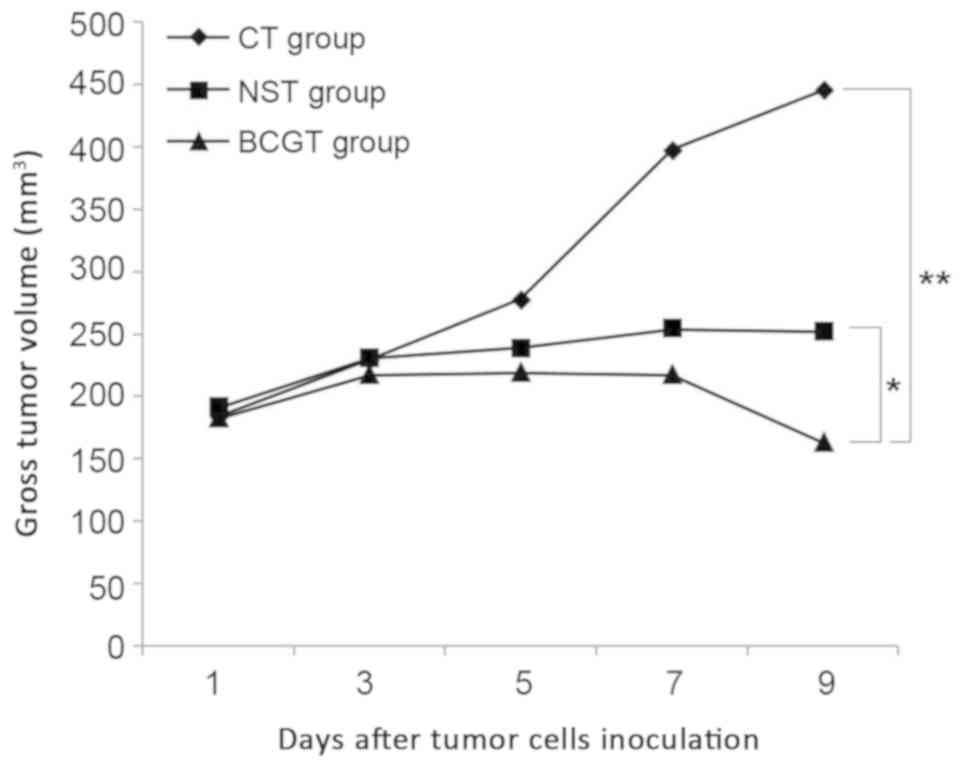
Survival rate of tumor-bearing
mice
In order to examine the anticancer effectiveness of
BCG-treated neutrophils, group A tumor-bearing model was initially
constructed and treated with BCG or NS. Next, neutrophils were
extracted from these tumor-bearing mice and injected into group B
tumor-bearing mice. Finally, the results obtained in the CT, NST
and BCGT groups were compared. At 1 week after MethA cell
inoculation, a phyma was observed, indicating the successful
establishment of the tumor-bearing model. As shown in Fig. 4, a decline in the survival rate was
observed on day 12 in the BCGT group, on day 9 in the NST group and
on day 10 in the CT group. On day 18, the survival rate in the
three groups was 80, 20 and 0%, respectively. The survival rate in
the BCGT group was significantly higher compared with the NST and
CT groups (both P<0.05; Fig.
4).
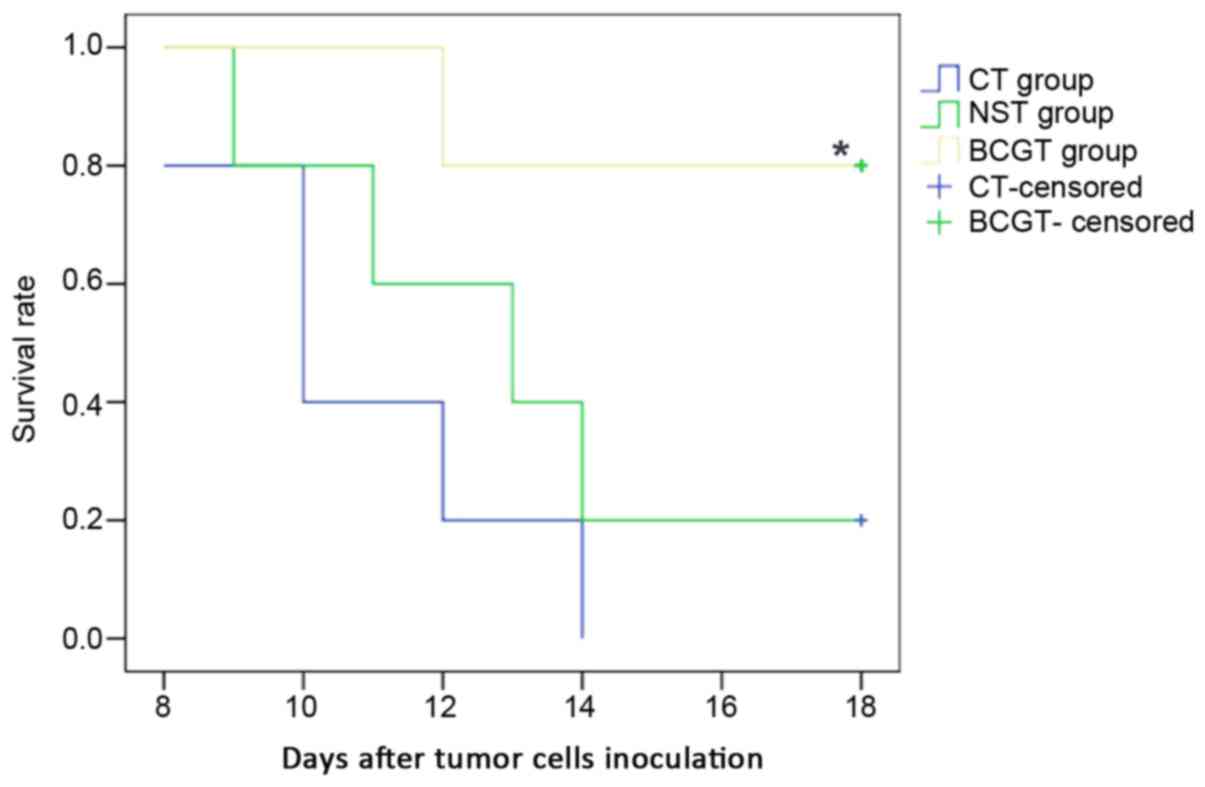 | Figure 4.Survival rate of tumor-bearing mice. A
decline in the survival rate began on day 12 in the BCGT group
(106 CFU/ml), on day 9 in the NST group, and on day 10
in the CT group. On day 18, the survival rates in the three groups
were 80, 20 and 0%, respectively. The survival rate in the BCGT
group was higher as compared with that in the NST and CT groups.
*P<0.05 vs. CT and NST groups. A cross signifies the final day
of observation. BCG, bacillus Calmette-Guérin; NS, normal saline;
BCGT, intervention with BCG-treated neutrophils from tumor-bearing
mice; NST, intervention with NS-treated neutrophils from
tumor-bearing mice; CT, intervention with untreated neutrophils
from tumor-bearing mice. |
GTV
At 7 days after neutrophil injection, the GTV in the
BCGT group was significantly lower in comparison with that in the
NST and CT groups (both P<0.05). Furthermore, the GTV exhibited
a tendency to decrease only in the BCGT group on day 9, indicating
that BCG enhanced the effect of neutrophils and further decreased
the tumor volume (Fig. 5).
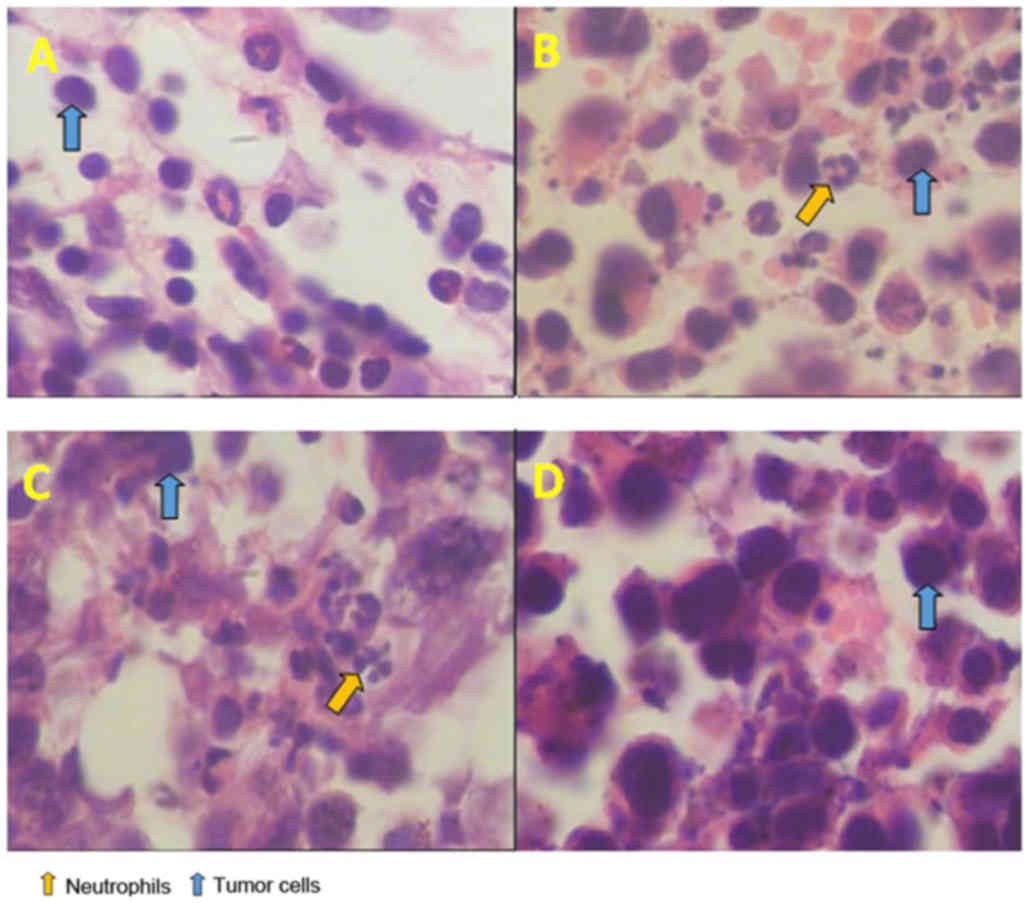
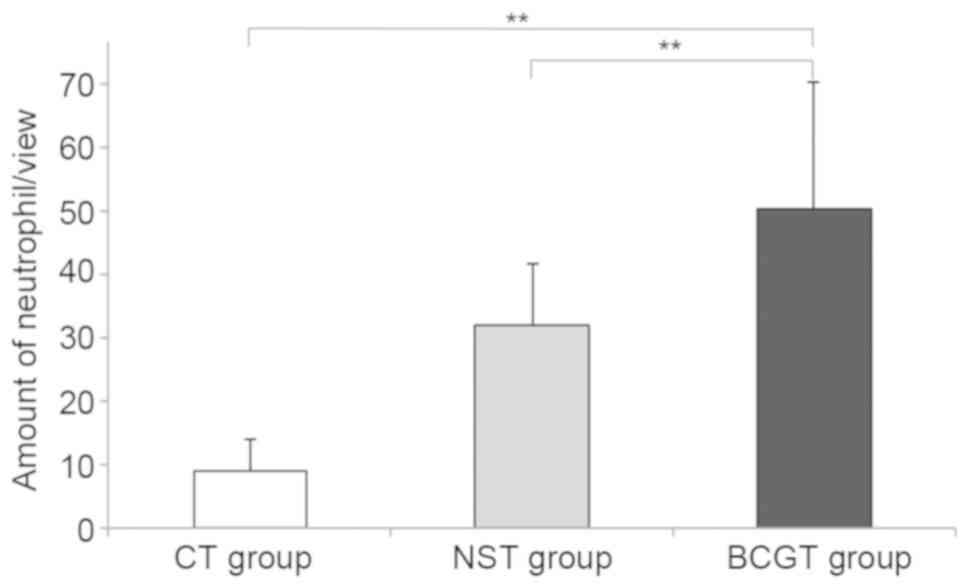
Pathological examination of MethA
tumor in mice
The tumor-bearing mice were sacrificed and
stabilized in the prone position subsequent to neutrophil injection
for 24 h. After evaluation under an optical microscope, enhanced
neutrophil infiltration was observed in the BCGT group compared
with the NST and CT groups (both P<0.01; Figs. 6 and 7). In addition, a consistent amount of
neutrophils was detected in 10 fields-of-view.
Discussion
In the present study, two experiments were
performed: Initially, the cytokine activity of BCG-treated
neutrophils was evaluated, and it was observed that BCG enhanced
the antitumor effect of neutrophils by inducing cytokine
expression. Subsequently, a tumor-bearing mouse model was
constructed, and the results suggested that the group injected with
BCG-treated neutrophils extracted from tumor-bearing mice exhibited
increased neutrophil infiltration with significantly decreased
tumor GTV percentage and elevated survival rate. Overall, the
results demonstrated that BCG-treated neutrophils were effective in
reducing tumor growth and extending the life span of mice due to
their enhanced cytotoxic activity.
In recent years, neutrophils have received attention
since they are believed to serve an important role in BCG therapy.
Neutrophils are the most abundant among all white blood cells in
the circulatory system of humans, and these cells can recognize and
induce tumor cell death through cytotoxicity. SR/CR mice are known
to be capable of killing tumor cells through their innate immune
system and neutrophils serve an important role in this process.
Based on cell phenotype detection, it is observed that the
cytotoxicity of neutrophils on tumor cells was 38%, compared with
35 and 26% in natural killer cells and macrophages respectively
(9,10). A previous study suggested that,
along with the increasing expression levels of various cytokines, a
great number of neutrophils and monocytes were infiltrated in the
bladder wall (11). Furthermore,
Suttmann et al (12)
reported that tumor-bearing mice intervened with BCG exhibited an
increasing neutrophil infiltration. The study also indicated that
the therapeutic effect of BCG would disappear and the survival rate
would decrease to the level of untreated control mice if
neutrophils were depleted, indicating that BCG treatment would
become invalid in mice with a lack of neutrophils. It is also
reported that certain cytokines were detected following the first
BCG instillation, demonstrating that neutrophils can induce
cytokines to inhibit tumor cells (13,14).
In the present study, we attempted to identify the
mechanism of BCG-treated neutrophils in defeating tumors by
detecting the activities of cytokines TNF-α, IL-1β, IL-6 and
TRAIL/Apo-2L in BCG-treated neutrophil supernatant. In the
experiments, MethA and YAC-1 cell lines were adopted, since the
former cells are frequently used for the evaluation of the
antitumor activity of different agents (15,16),
and the later cells are typically used as target cells for the
determination of the cytotoxic activity of natural killer cells in
mice (17). Besides, MethA and
YAC-1 cells are commonly used in our lab to detect the antitumor
effect of various agents.
TNF-α is postulated to be a cell signaling protein
with high killing effect on tumor cells, which can be induced by
activated neutrophils (18). In a
recent study, Bisiaux et al (19) reported the secretion of TNF-α
following BCG stimulation. IL-1β is an important mediator of
inflammatory response produced by activated macrophages, which is
also involved in a variety of cellular activities, including cell
proliferation, differentiation and apoptosis (20). IL-6, a cytokine with multiple
functions, is regarded as a pro-inflammatory cytokine and
anti-inflammatory myokine, and is secreted by T cells and
macrophages. In addition, TRAIL/Apo-2L, a type 2 membrane protein
and a member of the TNF superfamily, is produced by activated T
cells (21,22), B cells (23), natural killer cells (24), dendritic cells (25) and monocytes (26). It is reported that TRAIL/Apo-2L
released from neutrophils is a crucial step in anticancer therapy
using BCG (27). It has also been
reported that patients treated with BCG had higher TRAIL/Apo-2L
urinary levels, which can induce apoptosis in bladder tumor cells
(28). Therefore, the
abovementioned cytokines can serve as the endpoints for detecting
the antitumor effect of neutrophils stimulated by BCG. In the
current study, BCG-treated neutrophils were found to be effective
in killing MethA and YAC-1 cells, as well as inducing a
significantly larger number of cytokines compared with those in the
control and NS groups, which is consistent with the findings of the
aforementioned studies.
Furthermore, the experiments of the present study
revealed that the efficacy of neutrophils was largely dependent on
the dose of BCG. Previous bladder cancer research conceded that, on
account of the innate defense mechanism of the bladder, higher
doses may lead to better outcomes, since the glycosaminoglycans on
the surface of the urothelium are negatively charged to protect the
bladder urothelium bacteria, such as BCG, whose surfaces are highly
negatively charged; thus, a high dose of BCG is required for
intravesical therapy (2).
Additionally, it was observed that the neutrophils
from BCG-treated tumor-bearing mice presented a surprising
effectiveness compared with those in the NS group, with the
difference reaching statistical significance. However, there has
been no study, to the best of the authors' knowledge, which
analyzes the antitumor effect of neutrophils from the BCG-treated
tumor-bearing mice, which is considered to be one of the most
important findings of the present study.
Since first adopted by Morales in 1976 (29), BCG has become a standard treatment
for non-muscle-invasive bladder cancer, and has been demonstrated
to be effective and superior to chemotherapy. Bladder cancer is
also one of the immunogenic types of cancer, capable to escape from
immune-mediated elimination even in the presence of
antigen-specific immune cell infiltration (30). Therefore, BCG is suggested to
function by inducing inflammatory response to activate the immune
reaction. Nevertheless, there is no consensus on the mechanism
underlying the anticancer effect of BCG. Kawai et al
(2) suggested an immunotherapy
model for BCG in a previous review. These authors suggested that
instillation of BCG into the bladder leads to the attachment of BCG
to tumor cells through fibronectin mediation, which is regarded as
the first step of immune response stimulated by BCG. Next, BCG
internalization induces an immunological response characterized by
cytokine accumulation, and macrophage and lymphocyte recruitment
into the bladder wall to defeat tumors.
However, since we were unable to access bladder
tumor cells, this study only evaluated the antitumor effect of
BCG-treated neutrophils, which may be the possible mechanism of
BCG-treated neutrophils in bladder cancer. Besides, neutrophils
have conflicting functions: on one hand, they can defeat tumor
cells; on the other, they can also lead to redundant inflammation
and induce factors for malignant cells and matrix-degrading enzyme
secretion (31), which prevent the
development of long-lasting immunity (7) and promote cancer under long-term
influence (12). Therefore, future
research focused on bladder cancer is required.
In conclusion, ex vivo and in vivo
experiments on mice were performed in the present study to
demonstrate that BCG-treated neutrophils were effective in killing
tumor cells and extending the life span of mice by stimulating
cytotoxic activity.
Acknowledgements
Not applicable.
Funding
This study was financially supported by grants from
the National Natural Science Foundation of China (no. 31270972) and
the Shenyang Science and Technology Project ‘Anti-tumor effect of
neutrophils induced by BCG’ in 2009 (no. 1091175-1-02).
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
HW developed concept and design of the study; DW,
YF, HW and CL performed the experiments; JZ collected and analyzed
data; HW and YF interpreted data; CL drafted and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
China Medical University (Shenyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have are no competing
interests.
References
|
1
|
Spencer BA, McBride RB, Hershman DL, Buono
D, Herr HW, Benson MC, Guptamohile S and Neugut AI: Adjuvant
intravesical bacillus calmette-guérin therapy and survival among
elderly patients with non-muscle-invasive bladder cancer. J Oncol
Pract. 9:92–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawai K, Miyazaki J, Joraku A, Nishiyama H
and Akaza H: Bacillus Calmette-Guerin (BCG) immunotherapy for
bladder cancer: Current understanding and perspectives on
engineered BCG vaccine. Cancer Sci. 104:22–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saint F, Patard JJ, Irani J, Salomon L,
Hoznek A, Legrand P, Debois H, Abbou CC and Chopin DK: Leukocyturia
as a predictor of tolerance and efficacy of intravesical BCG
maintenance therapy for superficial bladder cancer. Urology.
57:617–622. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ermert D, Niemiec MJ, Röhm M, Glenthøj A,
Borregaard N and Urban CF: Candida albicans escapes from mouse
neutrophils. J Leukoc Biol. 94:223–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koga Y, Matsuzaki A, Suminoe A, Hattori H
and Hara T: Neutrophil-derived TNF-related apoptosis-inducing
ligand (TRAIL): A novel mechanism of antitumor effect by
neutrophils. Cancer Res. 64:1037–1043. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antony VB, Sahn SA, Antony AC and Repine
JE: Bacillus Calmette-Guérin-stimulated neutrophils release
chemotaxins for monocytes in rabbit pleural spaces and in vitro. J
Clin Invest. 76:1514–1521. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moliva JI, Turner J and Torrelles JB:
Immune responses to bacillus Calmette-Guérin vaccination: Why do
they fail to protect against Mycobacterium tuberculosis? Front
Immunol. 8:4072017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosevear HM, Lightfoot AJ, O'Donnell MA
and Griffith TS: The role of neutrophils and TNF-related
apoptosis-inducing ligand (TRAIL) in bacillus Calmette-Guérin (BCG)
immunotherapy for urothelial carcinoma of the bladder. Cancer
Metastasis Rev. 28:345–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hicks AM, Riedlinger G, Willingham MC,
Alexander-Miller MA, Von Kap-Herr C, Pettenati MJ, Sanders AM, Weir
HM, Du W, Kim J, et al: Transterable anticancer innate immunity in
spontaneous regression/complete resistance mice. Proc Natl Acad Sci
USA. 103:7753–7758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui Z, Willingham MC, Hicks AM,
Alexandermiller MA, Howard TD, Hawkins GA, Miller MS, Weir HM, Du W
and DeLong CJ: Spontaneous regression of advanced cancer:
Identification of a unique genetically determined, age-dependent
trait in mice. Proc Natl Acad Sci USA. 100:6682–6687. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Souto JC, Vila L and Brú A:
Polymorphonuclear neutrophils and cancer: Intense and sustained
neutrophilia as a treatment against solid tumors. Med Res Rev.
31:311–363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suttmann H, Riemensberger J, Bentien G,
Schmaltz D, Stöckle M, Jocham D, Böhle A and Brandau S: Neutrophil
granulocytes are required for effective bacillus Calmette-Guérin
immunotherapy of bladder cancer and orchestrate local immune
responses. Cancer Res. 66:8250–8257. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Boer EC, De Jong WH, Steerenberg PA,
Aarden LA, Tetteroo E, De Groot ER, Van der Meijden AP, Vegt PD,
Debruyne FM and Ruitenberg EJ: Induction of urinary interleukin-1
(IL-1), IL-2, IL-6 and tumour necrosis factor during intravesical
immunotherapy with bacillus Calmette-Guérin in superficial bladder
cancer. Cancer Immunol Immunother. 34:306–312. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Boer EC, Somogyi L, de Ruiter GJ, de
Reijke TM, Kurth KH and Schamhart DH: Role of interleukin-8 in
onset of the immune response in intravesical BCG therapy for
superficial bladder cancer. Urol Res. 25:31–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Monzavi-Karbassi B, Pashov A, Jousheghany
F, Artaud C and Kieber-Emmons T: Evaluating strategies to enhance
the anti-tumor immune response to a carbohydrate mimetic peptide
vaccine. Int J Mol Med. 17:1045–1052. 2006.PubMed/NCBI
|
|
16
|
Yoshie O, Aso H, Nanjo M, Tamura K, Ebina
T and Ishida N: Antitumor effect of recombinant human interferon
alpha A/D on Meth-A sarcoma in mice. Jpn J Cancer Res. 77:413–418.
1986.PubMed/NCBI
|
|
17
|
Huntington ND, Vosshenrich CA and Di Santo
JP: Developmental pathways that generate natural-killer-cell
diversity in mice and humans. Nat Rev Immunol. 7:703–714. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JM, Kim JS, Jung HC, Song IS and Kim
CY: Apoptosis of human gastric epithelial cells via caspase-3
activation in response to Helicobacter pylori infection: Possible
involvement of neutrophils through tumor necrosis factor alpha and
soluble Fas ligands. Scand J Gastroenterol. 35:40–48. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bisiaux A, Boussier J, Duffy D,
Quintana-Murci L, Fontes M and Albert ML; Milieu Intérieur
Consortium, : Deconvolution of the response to bacillus
Calmette-Guérin reveals NF-κB-induced cytokines as autocrine
mediators of innate immunity. Front Immunol. 8:7962017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Queiroz DM, Guerra JB, Rocha GA, Rocha AM,
Santos A, De Oliveira AG, Cabral MM, Nogueira AM and De Oliveira
CA: IL1B and IL1RN polymorphic genes and Helicobacter pylori cagA
strains decrease the risk of reflux esophagitis. Gastroenterology.
127:73–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kayagaki N, Yamaguchi N, Nakayama M,
Kawasaki A, Akiba H, Okumura K and Yagita H: Involvement of
TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated
cytotoxicity. J Immunol. 162:2639–2647. 1999.PubMed/NCBI
|
|
22
|
Kayagaki N, Yamaguchi N, Nakayama M, Eto
H, Okumura K and Yagita H: Type I interferons (IFNs) regulate tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL)
expression on human t cells: A novel mechanism for the antitumor
effects of type I IFNs. J Exp Med. 189:1451–1460. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mariani SM and Krammer PH: Surface
expression of TRAIL/Apo-2 ligand in activated mouse T and B cells.
Eur J Immunol. 28:1492–1498. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kashii Y, Giorda R, Herberman RB,
Whiteside TL and Vujanovic NL: Constitutive expression and role of
the TNF family ligands in apoptotic killing of tumor cells by human
NK cells. J Immunol. 163:5358–5366. 1999.PubMed/NCBI
|
|
25
|
Fanger NA, Maliszewski CR, Schooley K and
Griffith TS: Human dendritic cells mediate cellular apoptosis via
tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J
Exp Med. 190:1155–1164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Griffith TS, Wiley SR, Kubin MZ, Sedger
LM, Maliszewski CR and Fanger NA: Monocyte-mediated tumoricidal
activity via the tumor necrosis factor-related cytokine, TRAIL. J
Exp Med. 189:1343–1354. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Renshaw SA, Parmar JS, Singleton V, Rowe
SJ, Dockrell DH, Dower SK, Bingle CD, Chilvers ER and Whyte MK:
Acceleration of human neutrophil apoptosis by TRAIL. J Immunol.
170:1027–1033. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ludwig AT, Moore JM, Luo Y, Chen X,
Saltsgaver NA, O'Donnell MA and Griffith TS: Tumor necrosis
factor-related apoptosis-inducing ligand: A novel mechanism for
Bacillus Calmette-Guerin-induced antitumor activity. Cancer Res.
64:3386–3390. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morales A, Eidinger D and Bruce AW:
Intracavitary bacillus Calmette-Guerin in the treatment of
superficial bladder tumors. J Urol. 116:180–183. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kandoth C, McLellan MD, Vandin F, Ye K,
Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al:
Mutational landscape and significance across 12 major cancer types.
Nature. 502:333–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kusumanto YH, Dam WA, Hospers GA, Meijer C
and Mulder NH: Platelets and granulocytes, in particular the
neutrophils, form important compartments for circulating vascular
endothelial growth factor. Angiogenesis. 6:283–287. 2003.
View Article : Google Scholar : PubMed/NCBI
|