Introduction
Atherosclerosis is a chronic inflammatory disease of
the arterial wall that can cause cardiovascular disease, such as
stroke or myocardial infarction, and peripheral artery disease
(1,2). The pathogenesis of atherosclerotic
lesion formation is a multistage process. Endothelial injury and
lipid-loaded macrophages participate in the development of
atherosclerotic plaques (3).
Macrophages serve an important role in the development and
progression of atherosclerosis. Macrophage apoptosis and apoptotic
body scavenging failure result in atherosclerosis inflammation
(4). Inflammation serves a crucial
role in all stages of the development of atherosclerosis (5,6). At
present, atherosclerosis is a global clinical problem that faces
enormous challenges. Therefore, the molecular mechanism of
atherosclerosis needs to be studied in an effort to seek new and
effective therapeutic targets.
MicroRNAs (miRNAs) are a group of small endogenous
non-coding RNAs, ~22 nucleotides long, that post-transcriptionally
regulate gene expression by binding to the 3′-untranslated region
(UTR) of target mRNAs (7–9). miRNAs serve critical roles in the
regulation of a number of biological processes, including cell
proliferation, differentiation and apoptosis (10–12).
Studies have demonstrated that miRNAs serve a key role in the
regulation of normal physiology and disease (13), including atherosclerosis (14). miRNA (miR)-217 has been studied in
various types of cancer including liver cancer, gastric cancer,
lung adenocarcinoma and acute myeloid leukemia (15–18).
Previous studies have revealed upregulation of miR-217 in
atherosclerosis (19,20). However, whether miR-217 is involved
in the development of atherosclerosis through the regulation of
macrophages is still unclear.
Sirtuin 1 (SIRT1), an NAD+-dependent
deacetylase, plays an important role in the regulation of apoptosis
and inflammatory responses (21–25).
A recent report indicated that the inhibition of SIRT1 may promote
atherosclerosis (26). However,
the relationship between miR-217 and SIRT1 in atherosclerosis
remains unclear.
Lipid-loaded macrophages participate in the
development of atherosclerotic plaques (27). Oxidized low-density lipoprotein
(ox-LDL) triggers intracellular events that enhance
pro-inflammatory cytokines expression, resulting in the apoptosis
of macrophages (28). Macrophage
apoptosis and failure to clear apoptotic bodies may lead to
atherosclerosis and inflammation (4). ox-LDL treated macrophages have been
widely used to study atherosclerosis in vitro (29,30).
The aim of the present study was to investigate the
role of miR-217 in atherosclerosis using an in vitro
cellular model induced by ox-LDL.
Materials and methods
Clinical samples
A total of 60 peripheral blood samples (2 ml per
individual) from 60 patients with atherosclerosis (age range,
47–78; male:female, 2:1), as well as 60 peripheral blood samples
from 60 healthy volunteers (age range, 45–79; male:female, 2:1)
were collected at Wuhan Central Hospital (Wuhan, China) between
April 2016 and April 2017. Clinicopathological characteristics of
patients were shown in Table I.
The protocols were approved by the Ethics Committee of Wuhan
Central Hospital and informed consent was obtained from each
patient.
 | Table I.Clinical characteristic of
atherosclerotic patients and healthy controls. |
Table I.
Clinical characteristic of
atherosclerotic patients and healthy controls.
| Variables | Patients
(n=60) | Controls
(n=60) |
|---|
| Age (range,
years) | 47–78 | 45–79 |
| Sex |
|
Male | 40 | 40 |
|
Female | 20 | 20 |
| Stable CAD for ≥4
months | 60 | 0 |
| Hypertension | 45 | 0 |
| Diabetes
mellitus | 32 | 0 |
| Active smoker | 28 | 11 |
| Obesity (BMI>25
kg/m2) | 13 | 2 |
| Total cholesterol
(mmol/l) | 5.32±0.81 | 4.61±0.45 |
| LDL cholesterol
(mmol/l) | 3.79±0.39 | 2.80±0.45 |
| HDL cholesterol
(mmol/l) | 1.19±0.52 | 1.20±0.30 |
| Triglycerides
(mmol/l) | 1.83±0.42 | 1.37±0.64 |
Cell culture and atherosclerotic cell
model
The human acute monocytic leukemia cell line THP-1
was purchased from the American Type Culture Collection (cat. no.
TIB-202). Cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin mixed solution.
Cells were incubated at 37°C with 5% CO2.
To differentiate THP-1 monocytes into macrophages,
THP-1 monocytes were treated with 10 nM phorbol 12-myristate
13-acetate (Sigma-Aldrich; Merck KGaA) for 48 h (31). Atherosclerotic cell model of
macrophages was established as previously described by treating
differentiated THP-1 cells with 25 µg/ml ox-LDL for 24 h (31); the significant formation of THP-1
foam cells suggested that the model was successfully
established.
Cell transfection
miR-217 inhibitor (5′-UACUGCAUCAGGAACUGAUUGGA-3′;
Shanghai GenePharma Co., Ltd.), inhibitor control
(5′-GCCUCCGGCUUCGCACCUCU-3′; Shanghai GenePharma Co., Ltd.),
control-siRNA (cat. no. sc-36869; Santa Cruz Biotechnology, Inc.),
SIRT1-siRNA (cat. no. sc-40986; Santa Cruz Biotechnology, Inc.) or
miR-217 inhibitor + SIRT1-siRNA was transfected into THP-1
macrophages (ox-LDL untreated) using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol at 37°C for 48 h. Transfection efficiency
was measured at 48 h post-transfection using reverse
transcription-quantitative PCR (RT-qPCR).
Dual luciferase reporter assay
TargetScan bioinformatics software (www.targetscan.org/vert_71) was used to predict
the potential targets of miR-217, and results identified binding
sites between miR-217 and 3′UTR of sirtuin 1 (SIRT1). Dual
luciferase reporter assay was performed to determine whether
miR-217 directly binds to SIRT1. Wild-type (WT-SIRT1) and mutant
(MUT-SIRT1) 3′UTR of SIRT1 were cloned into pmiR-RB-Report™ dual
luciferase reporter gene plasmid vectors (Guangzhou RiboBio Co.,
Ltd.) following the manufacturer's instructions. THP-1 cells were
co-transfected with WT-SIRT1 or MUT-SIRT1 and miR-217 mimic or
mimic control using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C for 48 h. At 48 h
post-transfection, luciferase activity was assessed by the
Dual-luciferase Assay System (Promega Corporation) and normalized
to Renilla luciferase activity.
Triglyceride (TG) and total
cholesterol (TC) assays
The levels of TG and TC in the cell lysates of THP-1
macrophages were determined using the TG Assay kit (cat. no.
ab65336; Abcam) and Cholesterol Assay kit (cat. no. ab133116;
Abcam) following the manufacturer's instructions.
Apoptosis assay
Transfected and ox-LDL-treated THP-1 cells were used
in the apoptosis assay. The Annexin V-FITC/propidium iodide (PI)
apoptosis detection kit (cat. no. 70-AP101-100; Hangzhou
MultiSciences Biotech Co., Ltd.) was used to analyze apoptotic
rates. Briefly, cells (1×106 cells) were dyed with 5 µl
Annexin V-FITC and 5 µl PI for 30 min at room temperature in the
dark. Apoptotic rates were analyzed using a flow cytometer (BD
Biosciences) with FlowJo software (version 7.6.1; FlowJo LLC).
ELISA
The levels of the tumor necrosis factor α (TNF-α)
(cat no. PT518), interleukin (IL)-6 (cat no. PT330) and IL-1β (cat
no. PT305) in the supernatant (100 µl) of transfected and
ox-LDL-treated THP-1 cells were determined using sandwich ELISA
kits from Beyotime Institute of Biotechnology following the
manufacturer's protocol of each kit. Cell supernatant was collected
through centrifugation (500 g, 5 min).
RT-qPCR
Total RNA from blood samples (1 ml) and cells
(1×106 cells) was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. For cDNA generation, reverse
transcription was performed using TaqMan MicroRNA Reverse
Transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). cDNAs were analyzed by qPCR assay with SYBR Premix Ex
Taq™ II (TliRNaseH Plus) kit (Takara Bio, Inc.). U6 for miRNA
and GAPDH for mRNA were used as internal controls. Primer sequences
for qPCR were as follows: miR-217, forward
5′-TACTGCATCAGGAACTGACTGGA-3′, reverse 5′-GTGCAGGGTCCGAGGT-3′;
SIRT1, forward 5′-AATCCAGTCATTAAAGGTCTACAA-3′, reverse
5′-TAGGACCATTACTGCCAGAGG-3′; U6, forward
5′-GCTTCGGCAGCACATATACTAAAAT-3′, reverse
5′-CGCTTCACGAATTTGCGTGTCAT-3′; GAPDH, forward
5′-CTTTGGTATCGTGGAAGGACTC-3′, reverse 5′-GTAGAGGCAGGGATGATGTTCT-3′.
Expression levels were normalized to the respective internal
controls and calculated using the 2−ΔΔCq method
(32).
Western blot assay
Proteins from blood samples (1 ml) and cells
(1×106 cells) were extracted by using RIPA lysis buffer
(cat. no. P0013E; Beyotime Institute of Biotechnology) following
the manufacturer's instructions. Bicinchoninic acid assay kit
(Pierce; Thermo Fisher Scientific; Inc.) was used to quantify the
protein samples. Equal amounts of protein samples (30 µg/lane) were
separated on 12% SDS-PAGE and transferred onto PVDF membranes
(Merck KGaA). Following blocking in 5% skimmed milk at room
temperature for 1.5 h, the membranes were incubated with primary
antibodies against SIRT1 (120 kDa; 1:1,000; cat. no. 9475; Cell
Signaling Technology, Inc.), phosphorylated (p)-AMP-activated
protein kinase α (AMPK-α; 62 kDa; 1:1,000; cat. no. 50081; Cell
Signaling Technology, Inc.), AMPK-α (62 kDa; 1:1,000; cat. no.
5831; Cell Signaling Technology, Inc.), p-NF-κB p65 (65 kDa;
1:1,000; cat. no. 3033; Cell Signaling Technology, Inc.), p65 (65
kDa; 1:1,000; cat. no. 8242; Cell Signaling Technology, Inc.) and
β-actin (45 kDa; 1:1,000; cat. no. 4970; Cell Signaling Technology,
Inc.) overnight at 4°C. Subsequently, the membranes were incubated
with the horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (1:2,000; cat. no. 7074; Cell
Signaling Technology, Inc.) at room temperature for 2 h. Protein
bands were visualized using chemiluminescent ECL reagent (EMD
Millipore) and quantified by densitometry (QuantityOne 4.5.0
software; Bio-Rad Laboratories Inc.). Expression was normalized to
β-actin.
Statistical analysis
Data are presented as the mean ± SD. SPSS 17.0
software (SPSS, Inc.) was used to perform statistical analysis.
Differences between groups were determined using Student's t-test
or one-way analysis of variance with a Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-217 is upregulated in
atherosclerosis
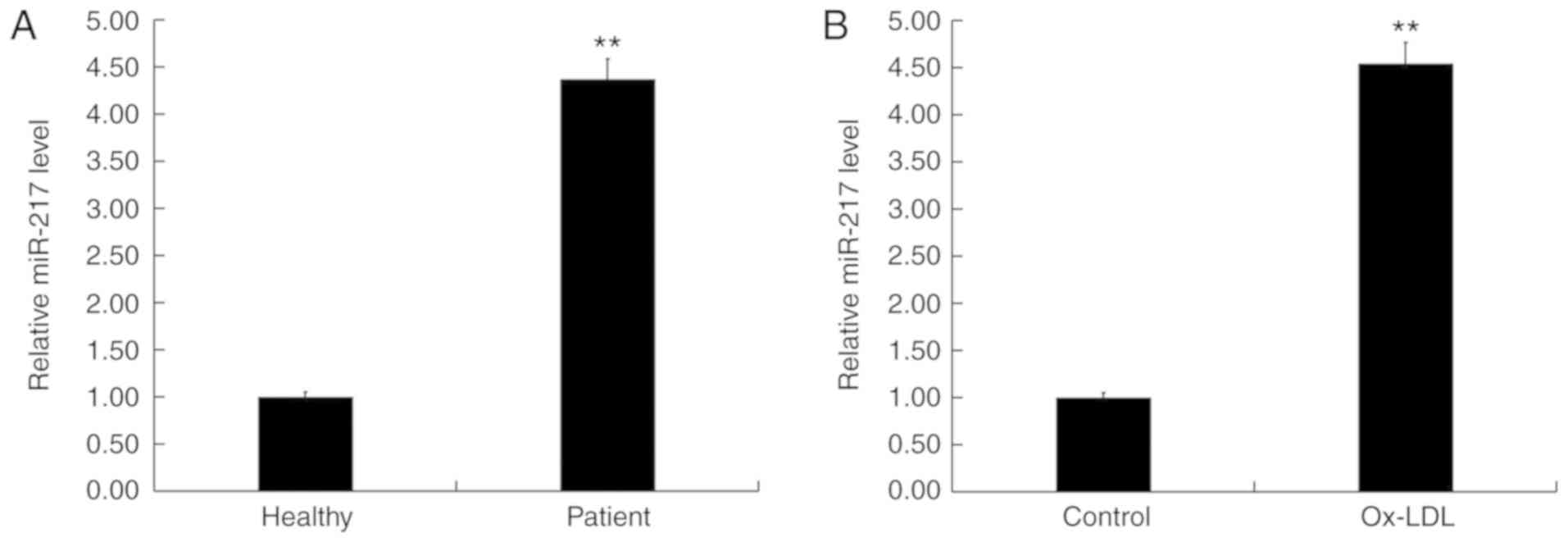
Expression levels of miR-217 were determined in the
blood of 60 patients with atherosclerosis and 60 healthy volunteers
using RT-qPCR. The results indicated that, compared with healthy
volunteers, the level of miR-217 in the blood of patients with
atherosclerosis was significantly increased (Fig. 1A). In addition, the level of
miR-217 was significantly increased in ox-LDL-treated THP-1
macrophages (atherosclerosis model) compared with untreated THP-1
macrophages (Fig. 1B). These data
suggested that miR-217 may be upregulated in atherosclerosis.
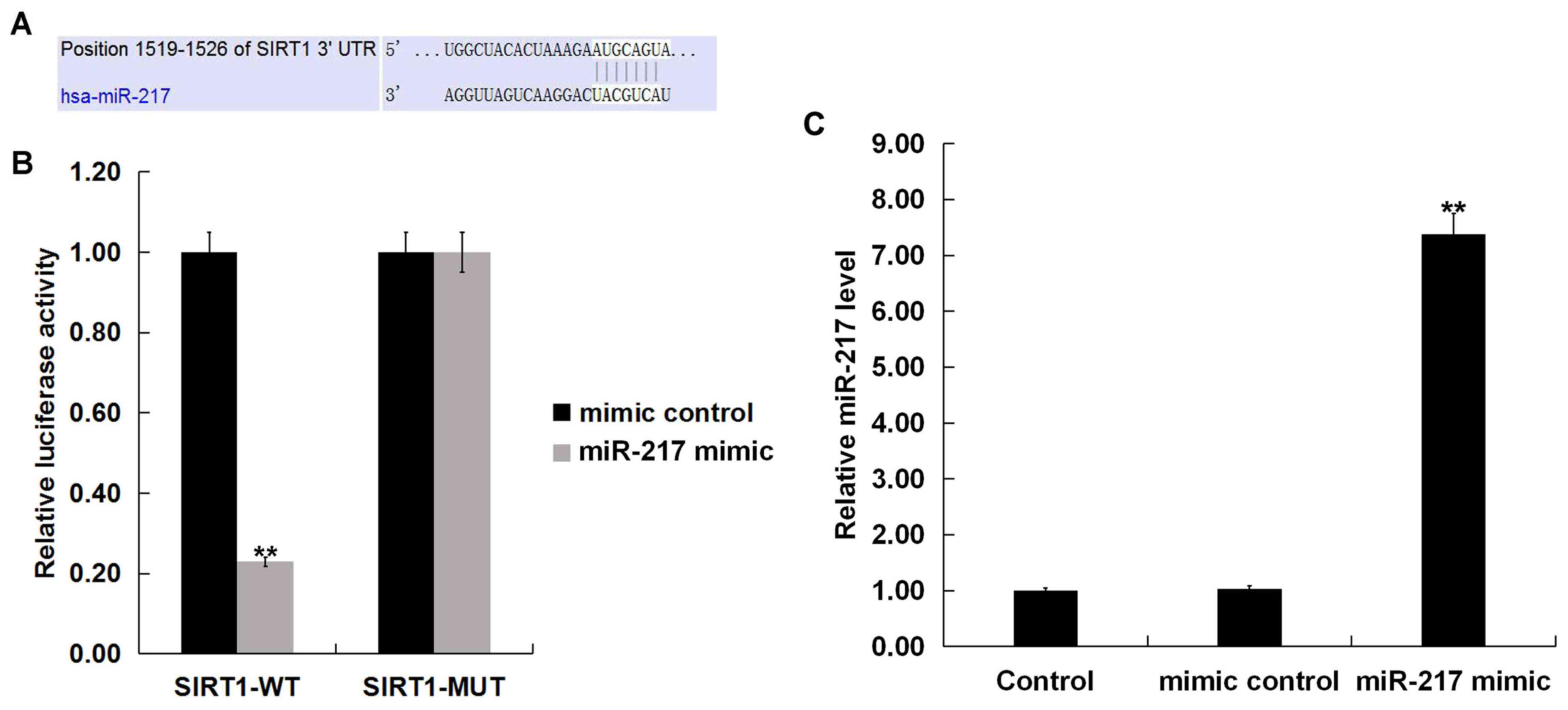
SIRT1 is a target of miR-217
TargetScan identified SIRT1 as a potential miR-217
target (Fig. 2A). Results from the
dual-luciferase reporter assay demonstrated that luciferase
activity was significantly reduced in THP-1 macrophages
co-transfected with SIRT1-WT reporter plasmids and miR-217 mimic
compared with SIRT1-WT and mimic control; no differences were
observed between co-transfections with SIRT1-MUT reporter plasmids
and miR-217 mimic or mimic control (Fig. 2B). miR-217 significantly enhanced
the level of miR-217 in THP-1 macrophages (Fig. 2C). These data indicated that
miR-217 may directly target SIRT1.
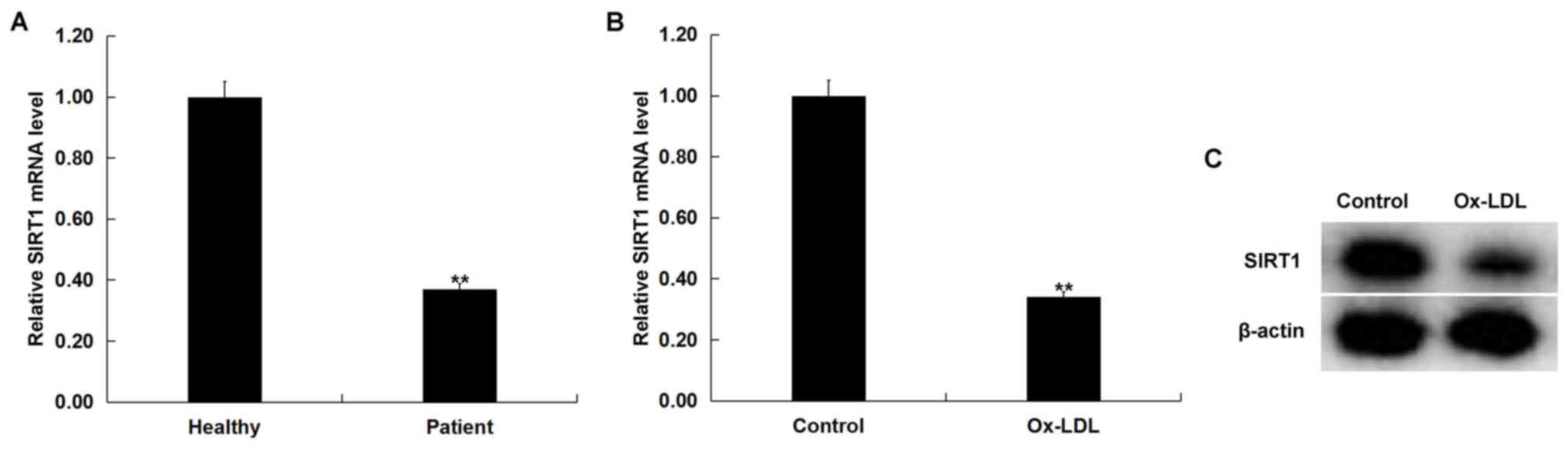
SIRT1 is downregulated in
atherosclerosis
SIRT1 mRNA expression levels were examined in the
blood of 60 patients with atherosclerosis and 60 healthy volunteers
using RT-qPCR. Compared with the healthy volunteers, SIRT1 mRNA
expression level in the blood of patients with atherosclerosis was
significantly decreased compared with healthy volunteers (Fig. 3A). In addition, ox-LDL treatment
significantly decreased SIRT1 mRNA level in THP-1 macrophages
compared with untreated cells (Fig.
3B). ox-LDL treatment markedly decreased SIRT1 protein
expression in THP-1 macrophages compared with the untreated cells
(Fig. 3C). These data suggested
that SIRT1 may be downregulated in atherosclerosis.
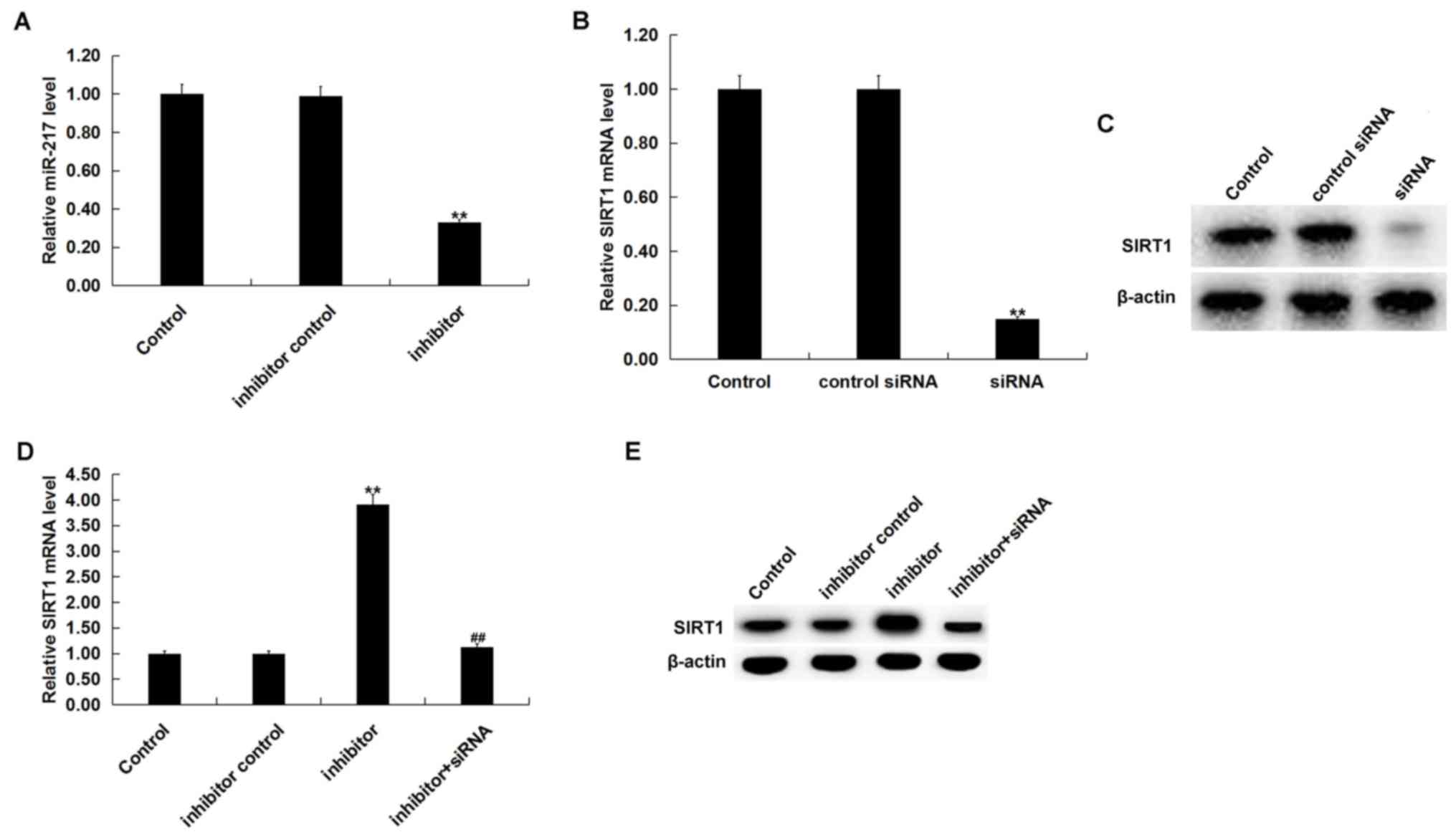
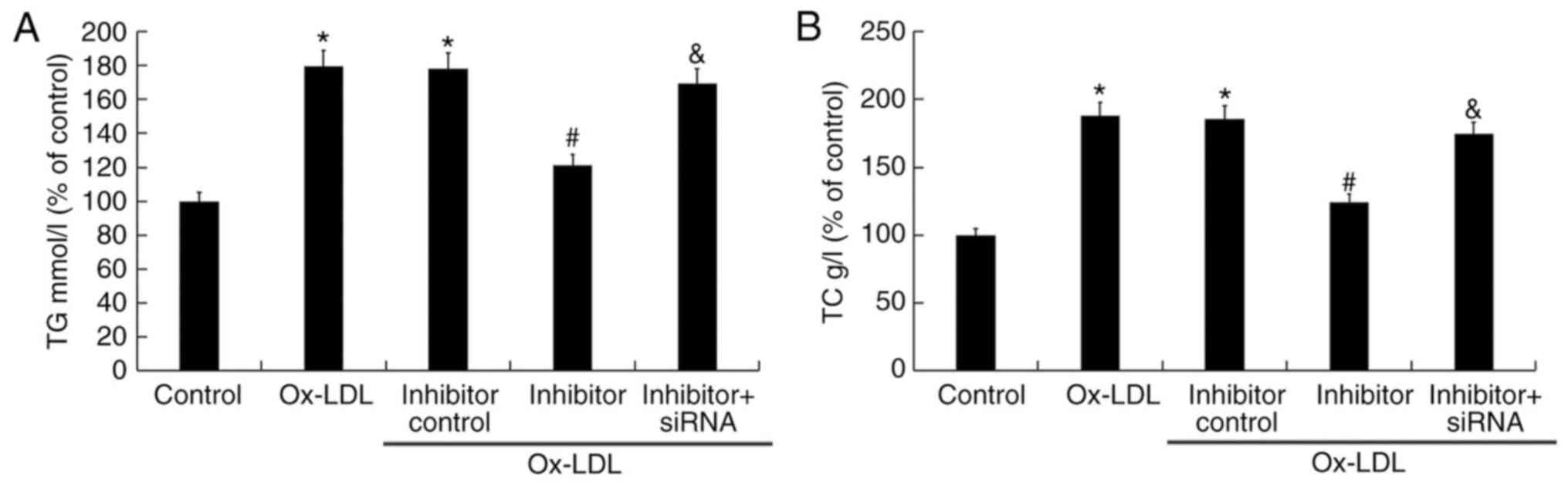
miR-217 downregulation reduces TG and
TC levels triggered by ox-LDL
To determine the role of miR-217 in atherosclerosis,
miR-217 inhibitor, inhibitor control, control-siRNA, SIRT1-siRNA or
miR-217 inhibitor + SIRT1-siRNA was transfected into THP-1
macrophages for 24 h prior to treatment with 25 µg/ml ox-LDL for 24
h. Transfection efficiency was determined by RT-qPCR and/or western
blot analysis. miR-217 inhibitor significantly downregulated the
miR-217 level in THP-1 macrophages compared with the untreated
cells (Fig. 4A), and SIRT1-siRNA
significantly decreased the SIRT1 mRNA level in THP-1 macrophages
compared with untreated cells (Fig.
4B). The protein level of SIRT1 was also reduced in THP-1
macrophages by SIRT1-siRNA treatment (Fig. 4C). Additionally, miR-217 inhibitor
significantly increased the mRNA levels of SIRT1, and the
enhancement was reversed by SIRT1-siRNA (Fig. 4D). The miR-217 inhibitor markedly
enhanced the expression of SIRT1, and this was reversed by
SIRT1-siRNA (Fig. 4E).
The effect of miR-217 on TG and TC levels in the
atherosclerosis cell model was also determined. Compared with the
untreated control group, 25 µg/ml ox-LDL treatment significantly
enhanced the levels of TG and TC, whereas miR-217 inhibitor
significantly reduced TG and TC levels induced by ox-LDL (Fig. 5). In addition, co-treatment with
SIRT1-siRNA reversed the effects of miR-217 inhibitor on the levels
of TG and TC in THP-1 macrophages (Fig. 5).
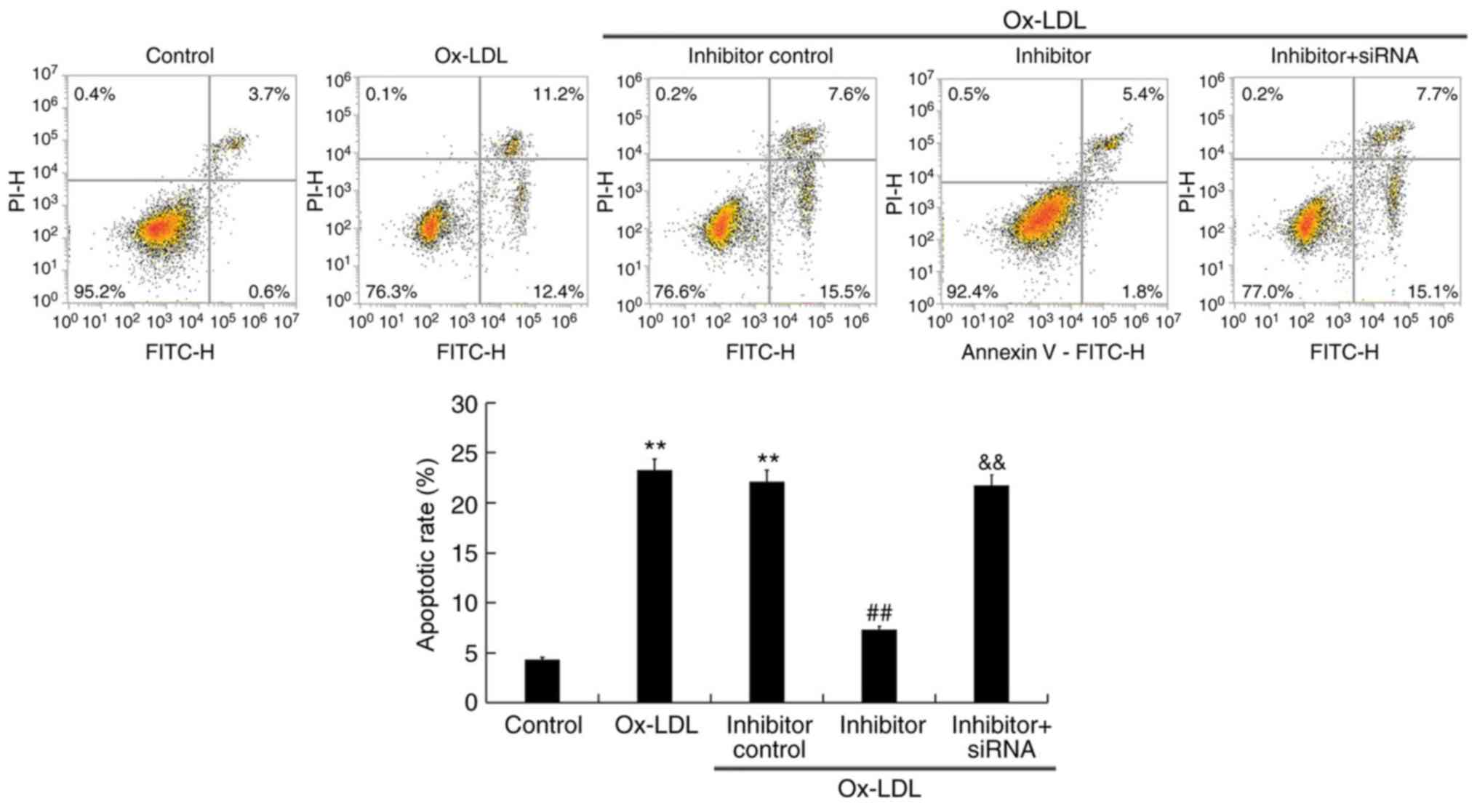
miR-217 downregulation inhibits
ox-LDL-induced apoptosis
The effect of miR-217 on THP-1 cell apoptosis was
analyzed. Ox-LDL treatment significantly induced THP-1 macrophage
apoptosis (Fig. 6), which was
notably inhibited by miR-217 inhibitor transfection, and this
inhibition was reversed by co-transfection with SIRT1-siRNA.
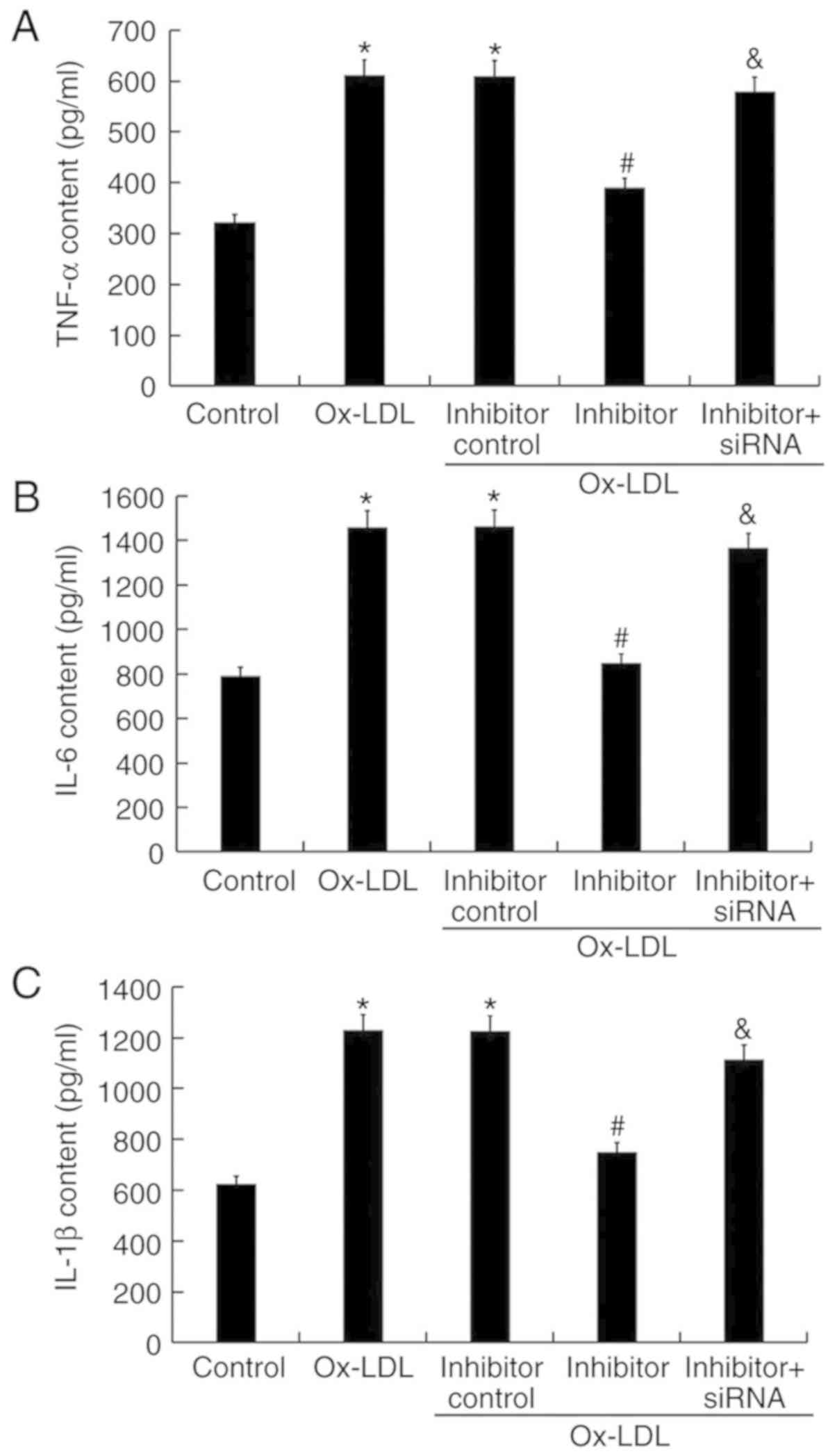
miR-217 downregulation inhibits
ox-LDL-induced inflammatory response
The levels of inflammatory factors were detected by
ELISA. The results demonstrated that treatment with ox-LDL
increased TNF-α, IL-6, and IL-1β content in THP-1 macrophage
culture, which was significantly decreased by miR-217 inhibitor
transfection (Fig. 7). The effects
of miR-217 inhibitor were reversed by SIRT1 silencing (Fig. 7).
Effects miR-217 downregulation on
SIRT1/AMPK-α/NF-κB pathway in ox-LDL-treated THP-1 macrophages
The SIRT1/AMPK-α/NF-κB pathway in ox-LDL-treated
THP-1 macrophages was analyzed. Ox-LDL treatment markedly decreased
SIRT1 protein level, reduced p-AMPK-α protein level, and increased
the phosphorylation level of NF-κB p65 protein in THP-1 cells
(Fig. 8A). Ox-LDL treatment
significantly decreased the level of SIRT1 mRNA (Fig. 8B), reduced the p-AMPK-α/AMPK-α
ratio (Fig. 8C), and increased the
ratio of p-p65/p65 (Fig. 8D) in
THP-1 cells. Compared with the ox-LDL-only treatment group,
co-treatment with the miR-217 inhibitor markedly enhanced SIRT1 and
p-AMPK-α expression, and reduced p-p65 protein level. SIRT1
silencing significantly reversed the effects of miR-217 inhibitor
on the expression of SIRT1, p-AMPK-α and p-p65 in THP-1
macrophages.
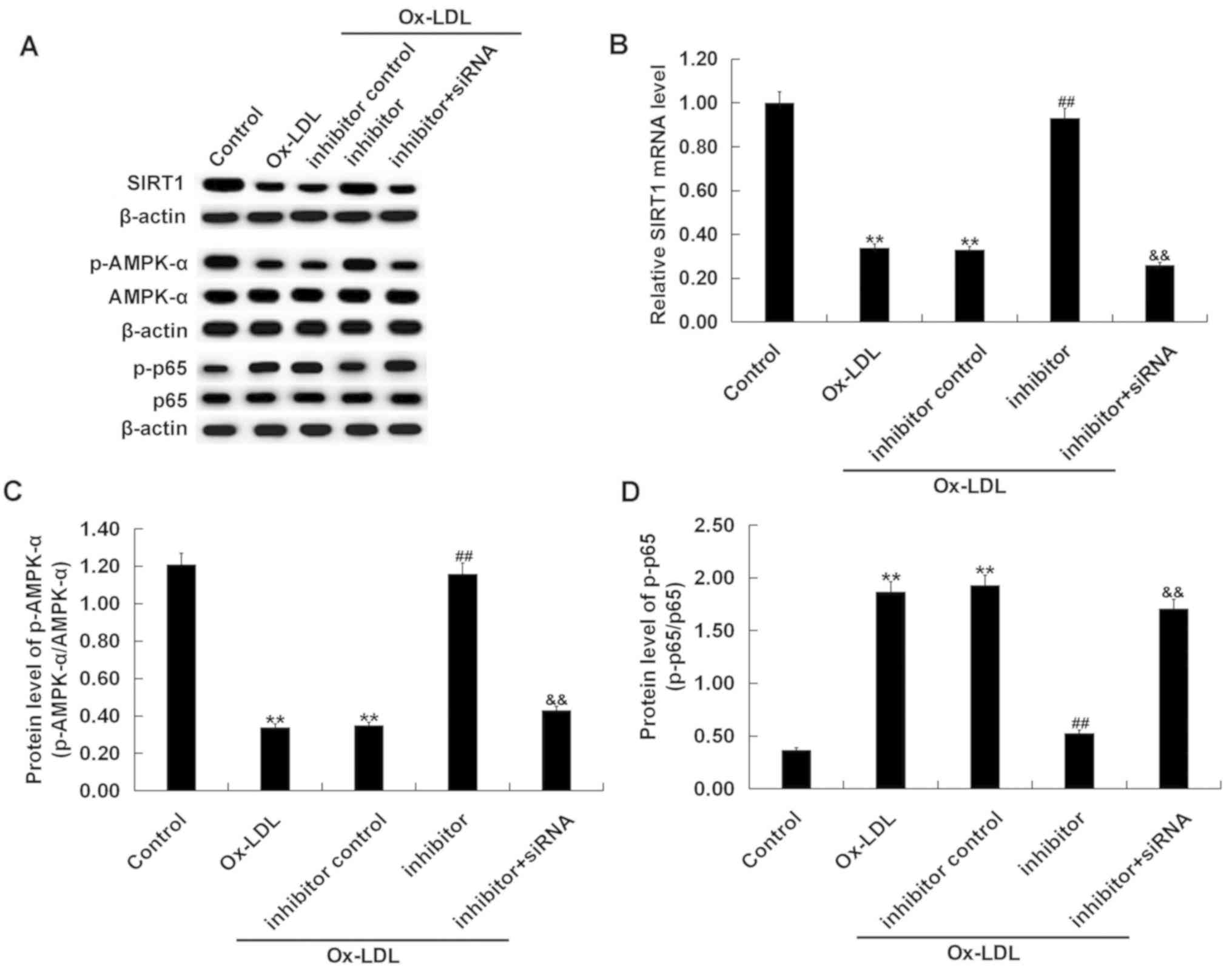 | Figure 8.Effects of miR-217 inhibitor on
SIRT1/AMPK-α/NF-κB pathway in THP-1 macrophages. THP-1 macrophages
were pre-transfected with miR-217 inhibitor, inhibitor control, or
miR-217 inhibitor + SIRT1-siRNA and treated with ox-LDL. (A) The
protein levels of SIRT1, p-AMPK-α, AMPK-α, p-p65, and p65 in THP-1
macrophages was determined using western blotting. (B) the mRNA
level of SIRT1 in THP-1 macrophages was determined using reverse
transcription-quantitative PCR. The ratio of (C) p-AMPK-α/AMPK-α
and (D) p-p65/p65 was calculated. **P<0.01 vs. Control;
##P<0.01 vs. ox-LDL; &&P<0.01
vs. Inhibitor. AMPK-α, 5′-AMP-activated kinase α; miR-217,
microRNA-217; ox-LDL, oxidized low density lipoprotein; p,
phosphorylated; SIRT1, sirtuin 1. |
Discussion
The results of the present study demonstrated that
miR-217 was significantly upregulated in the blood of patients with
atherosclerosis and ox-LDL-treated THP-1 macrophages. SIRT1 was
demonstrated to be a direct target of miR-217, and it was
downregulated in the blood of patients with atherosclerosis and
ox-LDL-treated THP-1 macrophages. Further analysis indicated that
ox-LDL-induced apoptosis and TNF-α, IL-1β and IL-6 expression
levels in THP-1 macrophages were repressed by miR-217 inhibitor
transfection. The results of this study suggested that the effects
of miR-217 inhibitor on ox-LDL-treated THP-1 macrophages were
eliminated by SIRT1 silencing with siRNA. The data obtained in the
current study supported the hypothesis that miR-217/SIRT1 axis may
be a novel therapeutic target for treating atherosclerosis.
Atherosclerosis is one of the leading causes of
morbidity and mortality in the world, and it is a major threat
worldwide (33). Therefore,
finding new targets for the treatment of atherosclerosis is of
great importance. The key cell events of atherosclerosis include
hyperlipidemia, monocyte recruitment, differentiation into
macrophages, foam cell formation and induced inflammation (34). A previous study suggested that
inflammation drives the formation, development and rupture of
atherosclerotic plaques (35).
Therefore, the study of potential targets for atherosclerotic
inflammatory processes may provide new therapeutic strategies for
atherosclerosis (36). Previous
studies have also demonstrated the roles of miRNAs in the
pathogenesis and development of atherosclerosis. Li et al
reported that miR-30c-5p inhibits endothelial cell pyroptosis by
inhibiting forkhead box O3 in atherosclerosis (37). Wei et al reported that
miR-342-5p promotes inflammatory macrophage activation during
atherosclerosis (38).
Additionally, miR-126, miR-150, miR-155 and miR-142-3p have been
demonstrated to serve critical roles in the development of
atherosclerosis (39–42). miR-217, which has been widely
studied in cancer (15–18), has been revealed to be upregulated
in atherosclerosis (19,20). However, the role and molecular
mechanism of miR-217 in atherosclerosis remain largely unknown. The
present study aimed to determine whether miR-217 is involved in the
development of atherosclerosis through the regulation of
macrophages.
SIRT1, an NAD+-dependent deacetylase, is
involved in apoptosis (21) and
serves important roles in the regulation of inflammatory responses
(22–25). For example, SIRT1 protein levels
are downregulated by IL1β/NFκB signaling in acetaminophen
hepatotoxicity, resulting in inflammation and oxidative stress
(23). The inhibition of SIRT1
leads to oxidative stress and inflammation in patients with
coronary artery disease (24). Wu
et al reported that SIRT1 is involved in the invasion and
metastasis of human esophageal cancer cells by inducing epithelial
mesenchymal transition through the regulation of Snail expression
(43). Yang et al
demonstrated that SIRT1 inhibition promotes atherosclerosis through
impaired autophagy (26). These
results indicated that miR-217 may serve an important role in the
development of atherosclerosis though the regulation of apoptosis
and inflammatory responses in macrophages by regulating the
expression of SIRT1.
To explore the potential function of miR-217 in
atherosclerosis, loss-of-function experiments were performed using
miR-217 inhibitor. miR-217 downregulation significantly inhibited
the ox-LDL-induced increase of TG and TC levels, apoptosis and the
upregulation of pro-inflammatory factors (TNF-α, IL-6, and IL-1β)
in THP-1 macrophages. AMPKα/SIRT1 pathway activation may inhibit
NF-κB-related inflammation (44).
AMPK activation mediates vascular inflammation and leukocyte
adhesiveness (45). Therefore,
AMPK may have potential roles in cardiovascular disease through its
anti-inflammatory effect (46). To
explore the molecular mechanism of the effect of miR-217 inhibitor
on ox-LDL treated THP-1 macrophages, the SIRT1/AMPK-α/NF-κB pathway
was analyzed in the present study. The results indicated that
ox-LDL treatment significantly inhibited AMPKα/SIRT1 pathway
activation, promoting NF-κB pathway activation, which could lead to
an inflammatory response in THP-1 macrophages; the effects of the
miR-217 inhibitor on ox-LDL treated THP-1 macrophages were
eliminated by SIRT1 silencing.
In conclusion, the results of the present study
demonstrated that miR-217 was upregulated in atherosclerosis, and
its inhibition may relieve atherosclerosis through inhibiting
macrophage apoptosis and inflammation response by restoring SIRT1
expression. miR-217 may be a potential therapeutic target for the
treatment of atherosclerosis. However, the present study is only a
preliminary study of the role of miR-217 in atherosclerosis; more
experimental research is needed to confirm the current results. For
instance, the role of SIRT1 alone in atherosclerosis should be
investigated. The association between miR-217/SIRT1 and the
clinicopathological characteristics of patients with
atherosclerosis should be explored. Additionally, miR-217/SIRT1 in
atherosclerosis should be studied in vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ contributed to study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. JC, QH, ZC, XL and MC contributed to data collection,
statistical analysis.
Ethics approval and consent to
participate
The protocols were approved by the Ethics Committee
of Wuhan Central Hospital (Wuhan, China), and informed consent was
obtained from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Libby P and Theroux P: Pathophysiology of
coronary artery disease. Circulation. 111:3481–3488. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Williams KJ and Tabas I: Atherosclerosis
and inflammation. Science. 297:521–522. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tabas I: Macrophage death and defective
inflammation resolution in atherosclerosis. Nat Rev Immunol.
10:36–46. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hansson GK and Libby P: The immune
response in atherosclerosis: A double-edged sword. Nat Rev Immunol.
6:508–519. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hansson GK and Hermansson A: The immune
system in atherosclerosis. Nat Immunol. 12:204–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghildiyal M and Zamore PD: Small silencing
RNAs: An expanding universe. Nat Rev Genet. 10:94–108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soifer HS, Rossi JJ and Saetrom P:
MicroRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2017. View Article : Google Scholar
|
|
11
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Connell RM, Rao DS, Chaudhuri AA and
Baltimore D: Physiological and pathological roles for microRNAs in
the immune system. Nat Rev Immunol. 10:111–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hosin AA, Prasad A, Viiri LE, Davies AH
and Shalhoub J: MicroRNAs in atherosclerosis. J Vasc Res.
51:338–3349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang LP, Wang JP and Wang XP: HOTAIR
contributes to the growth of liver cancer via targeting miR-217.
Oncol Lett. 15:7963–7972. 2018.PubMed/NCBI
|
|
16
|
Safaralizadeh R, Ajami N, Nemati M,
Hosseinpourfeizi M, Azimzadeh Isfanjani A and Moaddab SY:
Dysregulation of miR-216a and miR-217 in gastric cancer and their
clinical significance. J Gastrointest Cancer. 50:78–83. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu AN, Qu HJ, Yu CY and Sun P: Knockdown
of LINC01614 inhibits lung adenocarcinoma cell progression by
upregulating miR-217 and downregulating FOXP1. J Cell Mol Med.
22:4034–4044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan J, Wu G, Chen J, Xiong L, Chen G and
Li P: Downregulated miR-217 expression predicts a poor outcome in
acute myeloid leukemia. Cancer Biomark. 22:73–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu H, Li X, Song Y and Wang Z:
MicroRNA-217 attenuates intima-media complex thickness of ascending
aorta measured by ultrasound bio-microscopy and inhibits
inflammation and lipid metabolism in atherosclerotic models of
ApoE−/− mice. Lipids Health Dis. 17:1702018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu K, Xuekelati S, Zhou K, Yan Z, Yang X,
Inayat A, Wu J and Guo X: Expression profiles of six
atherosclerosis-associated microRNAs that cluster in patients with
hyperhomocysteinemia: A clinical study. DNA Cell Biol. 37:189–198.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nogueiras R, Habegger KM, Chaudhary N,
Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT
and Tschöp MH: Sirtuin 1 and sirtuin 3: Physiological modulators of
metabolism. Physiol Rev. 92:1479–1514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
da Cunha MSB and Arruda SF:
Tucum-do-Cerrado (Bactris setosa Mart.) may promote anti-aging
effect by upregulating SIRT1-Nrf2 pathway and attenuating oxidative
stress and inflammation. Nutrients. 9(pii): E12432017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rada P, Pardo V, Mobasher MA,
García-Martínez I, Ruiz L, González-Rodríguez Á, Sanchez-Ramos C,
Muntané J, Alemany S, James LP, et al: SIRT1 controls acetaminophen
hepatotoxicity by modulating inflammation and oxidative stress.
Antioxid Redox Signal. 28:1187–1208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan SH, Hung CH, Shih JY, Chu PM, Cheng
YH, Lin HC and Tsai KL: SIRT1 inhibition causes oxidative stress
and inflammation in patients with coronary artery disease. Redox
Biol. 13:301–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng YY, Kao CL, Ma HI, Hung CH, Wang CT,
Liu DH, Chen PY and Tsai KL: SIRT1-related inhibition of
pro-inflammatory responses and oxidative stress are involved in the
mechanism of nonspecific low back pain relief after exercise
through modulation of Toll-like receptor 4. J Biochem. 158:299–308.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang X, Wei J, He Y, Jing T, Li Y, Xiao Y,
Wang B, Wang W, Zhang J and Lin R: SIRT1 inhibition promotes
atherosclerosis through impaired autophagy. Oncotarget.
8:51447–51461. 2017.PubMed/NCBI
|
|
27
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nagy L, Tontonoz P, Alvarez JG, Chen H and
Evans RM: Oxidized LDL regulates macrophage gene expression through
ligand activation of PPARgamma. Cell. 93:229–240. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han Y, Ma J, Wang J and Wang L: Silencing
of H19 inhibits the adipogenesis and inflammation response in
ox-LDL-treatedRaw264.7 cells by up-regulating miR-130b. Mol
Immunol. 93:107–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsui L and Wang IJ: Analysis and
quantification of oxidized low-density lipoprotein-induced lipid
droplets in macrophages through high-content screening: Application
for antiatherogenic drugs discovery. Assay Drug Dev Technol.
17:223–230. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du XJ, Lu JM and Sha Y: MiR-181a inhibits
vascular inflammation induced by ox-LDL via targeting TLR4 in human
macrophages. J Cell Physiol. 233:6996–7003. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Herrington W, Lacey B, Sherliker P,
Armitage J and Lewington S: Epidemiology of atherosclerosis and the
potential to reduce the global burden of atherothrombotic disease.
Circ Res. 118:535–546. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ryo A, Suizu F, Yoshida Y, Perrem K, Liou
YC, Wulf G, Rottapel R, Yamaoka S and Lu KP: Regulation of
NF-kappaB signaling by Pin1-dependent prolyl isomerization and
ubiquitin-mediated proteolysis of p65/RelA. Mol Cell. 12:1413–1426.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Imanishi T and Akasaka T: Novel strategies
to target inflammatory processes in atherosclerosis. Curr Pharm
Design. 19:1616–1625. 2013. View Article : Google Scholar
|
|
37
|
Li P, Zhong X, Li J, Liu H, Ma X, He R and
Zhao Y: MicroRNA-30c-5p inhibits NLRP3 inflammasome-mediated
endothelial cell pyroptosis through FOXO3 down-regulation in
atherosclerosis. Biochem Biophys Res Commun. 503:2833–2844. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wei Y, Nazari-Jahantigh M, Chan L, Zhu M,
Heyll K, Corbalán-Campos J, Hartmann P, Thiemann A, Weber C and
Schober A: The microRNA-342-5p fosters inflammatory macrophage
activation through an Akt1- and microRNA-155-dependent pathway
during atherosclerosis. Circulation. 127:1609–1619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zernecke A, Bidzhekov K, Noels H,
Shagdarsuren E, Gan L, Denecke B, Hristov M, Köppel T, Jahantigh
MN, Lutgens E, et al: Delivery of microRNA-126 by apoptotic bodies
induces CXCL12-dependent vascular protection. Sci Signal.
2:ra812009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Liu D, Chen X, Li J, Li L, Bian
Z, Sun F, Lu J, Yin Y, Cai X, et al: Secreted monocytic miR-150
enhances targeted endothelial cell migration. Mol Cell. 39:133–144.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nazari-Jahantigh M, Wei Y, Noels H, Akhtar
S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, et
al: MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in
macrophages. J Clin Invest. 122:4190–4202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qin B, Shu Y, Long L, Li H, Men X, Feng L,
Yang H and Lu Z: MicroRNA-142-3p induces atherosclerosis-associated
endothelial cell apoptosis by directly targeting rictor. Cell
Physiol Biochem. 47:1589–1603. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu Y, Xin D, Liu C and Wang F: SIRT1
participates in epithelial-mesenchymal transition of EC-9706 and
Eca-109 cells in vitro by regulating Snail expression. Nan Fang Yi
Ke Da Xue Xue Bao. 38:1325–1330. 2018.(In Chinese). PubMed/NCBI
|
|
44
|
Tian Y, Ma J, Wang W, Zhang L, Xu J, Wang
K and Li D: Resveratrol supplement inhibited the NF-κB inflammation
pathway through activating AMPKα-SIRT1 pathway in mice with fatty
liver. Mol Cell Biochem. 422:75–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jung CH, Lee MJ, Kang YM, Lee YL, Seol SM,
Yoon HK, Kang SW, Lee WJ and Park JY: C1q/TNF-related protein-9
inhibits cytokine-induced vascular inflammation and leukocyte
adhesiveness via AMP-activated protein kinase activation in
endothelial cells. Mol Cell Endocrinol. 419:235–243. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zabielska MA, Borkowski T, Slominska EM
and Smolenski RT: Inhibition of AMP deaminase as therapeutic target
in cardiovascular pathology. Pharmacol Rep. 67:682–688. 2015.
View Article : Google Scholar : PubMed/NCBI
|