Introduction
Chronic obstructive pulmonary disease (COPD) is a
chronic disease characterized by persistent airflow restriction and
is associated with an abnormal chronic inflammatory response of
airways and lung tissues to harmful gases or particles (1,2).
Typical clinical manifestations of COPD are chronic cough, sputum,
shortness of breath or dyspnea, and wheezing and chest tightness;
however, some COPD patients lack clinical manifestations of chronic
cough, sputum, or other clinical symptoms before airflow
restriction (3,4). Notably, COPD is difficult to
precisely diagnose, and it is estimated that only 60–85% of COPD
patients are diagnosed in the early to intermediate stages of
disease progression (5).
Currently, COPD severity is evaluated primarily according to
clinical symptoms, pulmonary function and complications, especially
for pulmonary function, it is typically measured by forced
expiratory volume in 1 s/forced vital capacity (FEV1/FVC) after
inhalation of a bronchodilator (6,7).
Since pulmonary function decreases with age regardless of COPD,
using pulmonary function tests to diagnose COPD may cause
underdiagnosis in young patients and overdiagnosis in the elderly
(8). Thus, timely and accurate
COPD is very important for its treatment, and can prevent further
destruction of lung tissue.
At present, samples used for COPD diagnosis are
derived from lung tissue, sputum, exhaled gas from patients, and
bronchial biopsies. Acquiring lung tissue causes trauma and other
samples have poor reproducibility and lack standardization
(9,10). In recent years, microRNAs (miRNAs)
have been revealed to be involved in regulating many physiological
and pathological processes including cell differentiation,
apoptosis, cell proliferation, and angiogenesis, among others
(11,12). Shen et al (13) revealed that smoking induced the
downregulation of miR-149-3p, increasing the inflammatory response
in COPD patients through the TLR-4/NF-κB signaling pathway. In
addition, Liu et al (14)
reported that miR-23a was associated with the development of COPD,
and identified miR-23a as a potential biomarker to discriminate
between frequent and non-frequent exacerbators. miRNA-101-3p.1, a
member of the miRNA-101 family, had been identified to interact
with COPD-related genes involved in the mechanisms of COPD
including imbalance between anti-proteolytic and proteolytic
activity, inflammatory response, apoptosis, and oxidative stress
(15). Hassan et al
(16) revealed that chronic
cigarette smoke exposure could induce miR-101 upregulation in the
lung of mice and human bronchial epithelial cells, which may
suppress CFTR which is involved in the pathogenesis of
COPD/emphysema. Notably, miRNA-101-3p.1 was also revealed to be
relevant as a non-invasive diagnostic tool to identify acute
cellular rejection in heart transplant patients. Thus, it was
speculated that miRNA-101-3p.1 may be an ideal diagnostic marker of
COPD and may play an important role in the development of COPD.
In the present study, the level, profile, and
diagnostic accuracy of miRNA-101-3p.1 were investigated in
peripheral blood mononuclear cells (PBMCs) from patients with
stable COPD (SCOPD) and acute exacerbation of COPD (AECOPD).
Furthermore, the molecular mechanism by which miRNA-101-3p.1
regulates COPD progression was elucidated. This research provides
valuable information regarding the accurate diagnosis of COPD.
Patients and methods
Patients and clinical specimens
All study protocols were approved by The Ethics and
Scientific Committees of Zhejiang University. Before study
participation, written informed consent was provided by each
participant. From October 2015 to September 2017, 58 patients with
SCOPD and 46 patients with AECOPD were enrolled in the present
study. In addition, 50 age- and sex-matched healthy subjects with
normal pulmonary function were also enrolled in this study. The
clinical data for all study participants are described in Table I. Based on the Global Initiative
for Chronic Obstructive Lung Disease (GOLD) guideline, the
criterion for COPD was defined as FEV1/FVC<0.7. COPD severity
was classified as follows: GOLD I was defined as FEV1 for predicted
values (FEV1%pre)≥80%; GOLD II was defined as 50%≤FEV1%pre<80%;
GOLD III was defined as 30%≤FEV1%pre<50%; GOLD IV was defined as
FEV1%pre,<30%. Stable COPD (SCOPD) was identified as a COPD
patient that had not undergone acute exacerbation during the last 3
months. Acute exacerbation COPD (AECOPD) patients had a FEV1%pre
20.41±5.24. AECOPD is defined as an acute worsening of respiratory
symptoms such as dyspnea, cough, or sputum purulence severe enough
to warrant hospital admission. Frequent exacerbator was identified
as AECOPD patients with 2.48±0.86 episodes of acute exacerbations
during the preceding 1 year. Exclusion criteria included the
existence of other chronic lung diseases, nervous system diseases,
tumors, diabetes, unstable cardiovascular diseases and liver and
kidney diseases. The COPD assessment test (CAT) consists of 8 items
with scores ranging from 0 to 5 (0=no impairment). The total scores
ranging from 0 to 40 are calculated by adding the score from each
item, higher scores indicating a poorer control of COPD or a more
severe health status impairment.
 | Table I.Clinical characteristics of HS, SCOPD
patients and AECOPD patients. |
Table I.
Clinical characteristics of HS, SCOPD
patients and AECOPD patients.
| Clinical
variable | HS (n=50) | SCOPD (n=58) | AECOPD (n=46) |
|---|
| Age, years |
63.47±11.31 | 67.53±8.49 | 65.13±9.87 |
| Sex |
|
|
|
| Male
[n, (%)] | 41 (82) | 49 (84.48) | 39 (84.78) |
| Female
[n, (%)] | 9 (18) | 9 (15.52) | 7 (15.22) |
| GOLD grade |
|
|
|
| GOLD
I | NA | 8 | NA |
| GOLD
II | NA | 15 | NA |
| GOLD
III | NA | 21 | 24 |
| GOLD
IV | NA | 12 | 22 |
| BMI
(kg/m2) | 28.64±4.92 | 27.65±4.43 | 27.11±4.82 |
| Duration time
(months) | NA |
8.36±4.11 | 10.15±5.26 |
| Past Smoker [n,
(%)] | 8 (16) | 49
(81.48)a | 42
(91.30)a |
| Leukocyte
(×103 µl) |
6.47±2.83 |
8.03±2.68a |
8.27±3.68a |
| Neutrophils
(×103 µl) |
3.69±2.55 |
5.12±2.04a |
5.49±2.81a |
| Lymphocyte
(×103 µl) |
2.07±1.14 |
2.11±1.03 |
1.94±1.17 |
| Fibrinogen
(g/l) |
346.17±153.28 |
365.81±115.93 |
488.36±193.25a,b |
| FEV1 %
predicted | 93.11±8.45 |
54.19±14.67a |
20.41±5.24a,b |
| FVC/FEV1 (%) | 82.33±5.36 |
60.88±11.10a |
27.92±9.33a,b |
| β-receptor blocker
[n, (%)] | NA | 55 (94.83) | 45 (97.83) |
| Steroid drugs [n,
(%)] | NA | 34 (58.62) | 37
(80.43)b |
Blood samples and PBMC isolation
Venous blood samples of participants were collected
in BD CPT™ tubes after 12 h of fasting. The CPT™ tubes (BD
Biosciences,) were used to separate PBMCs and plasma from
granulocytes and erythrocytes following centrifugation.
Subsequently, blood samples were inverted ten times following blood
collection and centrifuged at 1,500 × g for 20 min. Then, the PBMC
layer was gently suspended in the plasma and transferred to conical
tubes and washed with PBS by centrifugation (300 × g; 10 min).
Monocyte purity was >97% as assessed by flow cytometry. PBMCs
were cultured in RPMI-1640 medium with 10% fetal bovine serum (both
from Thermo Fisher Scientific, Inc.) in an incubator at 37°C with
5% CO2.
RNA isolation, reverse transcription,
and reverse transcription- quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted for reverse transcription
using the PAXgene Blood miRNA Kit (Qiagen, Inc.) following the
manufacturer's instructions. Following RNA transcription into cDNA,
it was amplified with specific sense and antisense primers using
the SYBR Premix Ex Taq II kit (Takara Biotechnology Co., Ltd.) for
mRNA and the miRNA PrimeScript RT Enzyme Mix kit (Takara
Biotechnology Co., Ltd.) for miRNA. U6 was used as a miRNA internal
control; GAPDH was used as a gene internal control. The gene
expression level was compared to internal control and calculated
using the equation: Fold expression level=2−ΔΔCq
(17). Independent experiments
were performed in triplicate, as were PCR reactions for each gene.
The primers were as follows: miRNA-101-3p.1,
5′-CTTCAGTTATCACAGTACTGTA-3′; and U6, 5′-AACGCTTCACGAATTTGCGT-3′.
The primers for pVHL were as follows: Forward primer,
5′-ACATCGTCAGGTCGCTCTAC-3′ and reverse primer,
5′-ATCTCCCATCCGTTGATGTG-3′. The primers for UBE2D1 were as follows:
Forward primer, 5′-TAGCGCATATCAAGGTGGAGT-3′ and reverse primer,
5′-TGGTGACCATTGTGACCTCAG-3′. The primers for GAPDH were as follows:
Forward primer, 5′-ACAGTCAGCCGCATCTTCTT-3′ and reverse primer,
5′-GACAAGCTTCCCGTTCTCAG-3′.
Transient transfection with siRNAs,
miRNA mimics or miRNA inhibitor
siRNA, miRNA mimics and a miRNA inhibitor were
designed and synthesized by Sangon Biotech Co., Ltd. PBMCs were
plated onto a 6-well plate at 30–50% confluence. After 24 h, siRNA,
miRNA mimics or miRNA inhibitor was transfected into cells via
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) based on the
manufacturer's protocol. PBMCs were collected after 48 h for
further experiments. The sequences for the miRNA mimics were:
5′-UACAGUACUGUGAUAACUGAAG-3′ (sense) and
5′-UUCUGUCAUGACACUAUUGACU-3′ (antisense). The sequences of miRNA
inhibitor were: 5′-CUUCAGUUAUCACAGUACUGUA-3′. The sequences of the
negative controls (NC) were 5′-GUGGAUAUUGUUGACAUCA-3′ (sense) and
5′-dTdTAAGAGGCUUGCACAGUGCA-3′ (antisense). The sequences of pVHL
siRNA was 5′-CCAAUGGAUUCAUGGAGUA-3′ (sense) and
5′-CCACCCAAAUGUGCAGAAA-3′ (antisense). The sequences of ubiquitin
conjugating enzyme E2 D1 (UBE2D1) siRNA were
5′-UCUAGCGUCCACAGUGGUTT-3′ (sense) and 5′-GCGACAUCUAUGACUCAUTC-3′
(antisense). The NC-siRNA sequences were
5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-AATTCTCCGAACGTGTCACGT-3′
(antisense).
Stable expression of HIF-1α
Lentiviral vectors overexpressing HIF-1α (Ov-HIF-1α)
and siRNA lentiviral vectors inhibiting HIF-1α expression
(Si-HIF-1α) were purchased from Cyagen Biosciences. The lentiviral
vector expressing scrambled RNA acted as a control (NC). PBMCs were
infected with lentiviral vector. Subsequently, flow cytometry
sorted fluorescence-activated PBMCs to select polyclonal cells with
green fluorescent protein signals. RNA levels from these cell
clones was quantified using qRT-PCR. The sequences of siRNA were:
5′-UCAAGUUGCUGGUCAUCAGdTdT-3′ (sense) and
5′-CUGAUGACCAGGAACUUGAdTdT-5′ (antisense). Primers sequences of
HIF-1α were 5′-TCATCCAAGAAGCCCTAACGTG-3′(sense);
5′-TTTCGCTTTCTCTGAGCATTCTG-3′ (antisense).
Western blot analysis
PBMCs were lysed using RIPA lysate buffer (Beyotime,
Institute of Biotechnology) followed by quantification of total
protein concentrations using Pierce BCA Protein Assay Kit (Thermo
Fisher Scientific, Inc.). Total protein (50 µg) was separated using
SDS-PAGE gel preparation kit (Beijing Solarbio Science &
Technology Co., Ltd.). After transfer to PVDF membranes (EMD
Millipore), 5% skim milk was used for blocking. Subsequently, the
cells were incubated overnight at 4°C with a primary antibody and
then a secondary antibody was added for 2 h. Signals were detected
using the ChemiDoc Touch Imaging System (Bio-Rad Laboratories,
Inc.). ImageJ 1.8.0 software (National Institutes of Health) was
used to analyze the protein expression level. The primary
antibodies and secondary antibody were purchased from Cayman
Chemical Company, HuaBio and CST.
3′-UTR luciferase reporter assays
miRNA-101-3p.1 target genes, UBE2D1 and pVHL, were
searched using the bioinformatics tool, TargetScanHuman (http://http://www.targetscan.org). UBE2D1 3′-UTR
fragment (2,020 bp) or pVHL 3′-UTR fragment (3,722 bp) was
amplified by PCR and cloned into psiCHECK-2 vectors (WT). A
GeneTailor Site-Directed Mutagenesis System (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for site-directed mutagenesis of
miRNA-101-3p.1 (MUT). MT or WT vector and control vector psiCHECK-2
vector were cotransfected into PBMCs with miRNA-101-3p.1 mimics in
48-well plates. Forty-eight hours after transfection, PBMCs were
harvested for luciferase assay using Dual-Luciferase Reporter Assay
System (Promega Corporation).
Cell proliferation
Cell Counting Kit-8 (CCK-8; MedChemExpress) was used
to analyze cell proliferation. PBMCs (1,000–1,500/well) were seeded
in 96-well plates and further incubated for 48 h. Next, cell
viability was assessed by adding 100 µl medium containing 10 µl
WST-8. The absorbance at a wavelength of 450 nm was detected using
a microplate reader (Tecan Group, Ltd.).
Statistical analysis
All assays were independently performed in
triplicate. All data are expressed as the mean ± standard
deviation. Statistical Product and Service Solutions (SPSS) 20.0
software (IBM Corp.) was performed for all statistical analyses.
The Chi-square test was used to analyze the count data. The t-test
was used for comparisons of data meeting normal distribution
between two groups, and those did not conform to normal
distribution were compared via non-parametric test. One-way
analysis of variance (ANOVA) followed by Tukey's test was applied
to compare multiple groups. Receiver operating characteristic (ROC)
curves were used to analyze the predictive value and optimal
cut-off value of miRNA-101-3p.1. Two-sided P-values <0.05 were
considered to indicate a statistically significant difference.
Results
Clinical characteristics of
participants
The demographic and clinical characteristics of
participants including 58 patients with SCOPD, 46 patients with
AECOPD and 50 healthy subjects are presented in Table I. The statistical analyses revealed
that there were no significant differences in the distribution of
clinical variables including sex, age, and BMI among the three
groups and routine blood tests (P>0.05) except for fibrinogen
between the SCOPD group and AECOPD group. The fibrinogen levels in
the AECOPD group were significantly higher than that in the SCOPD
group and HS group (P<0.01), but there were no significant
differences between the SCOPD group and HS group due to fewer
participants. Notably, there were more males than females in the
three groups. Among the three groups, there were significant
differences in the smoking status. Specifically, the number of past
smokers in the SCOPD group and AECOPD group was significantly
higher than the healthy subjects (P<0.01). Notably, FEV1%
predicted and FVC/FEV1 (%) were significantly decreased in the
SCOPD group and AECOPD group relative to healthy subjects
(P<0.01).
The level of miRNA-101-3p.1 in
patients with SCOPD or AECOPD
To explore the profiles of miRNA-101-3p.1 in PBMCs,
serum samples were collected from healthy subjects, SCOPD patients
and AECOPD patients, and total RNA extraction and qRT-PCR were
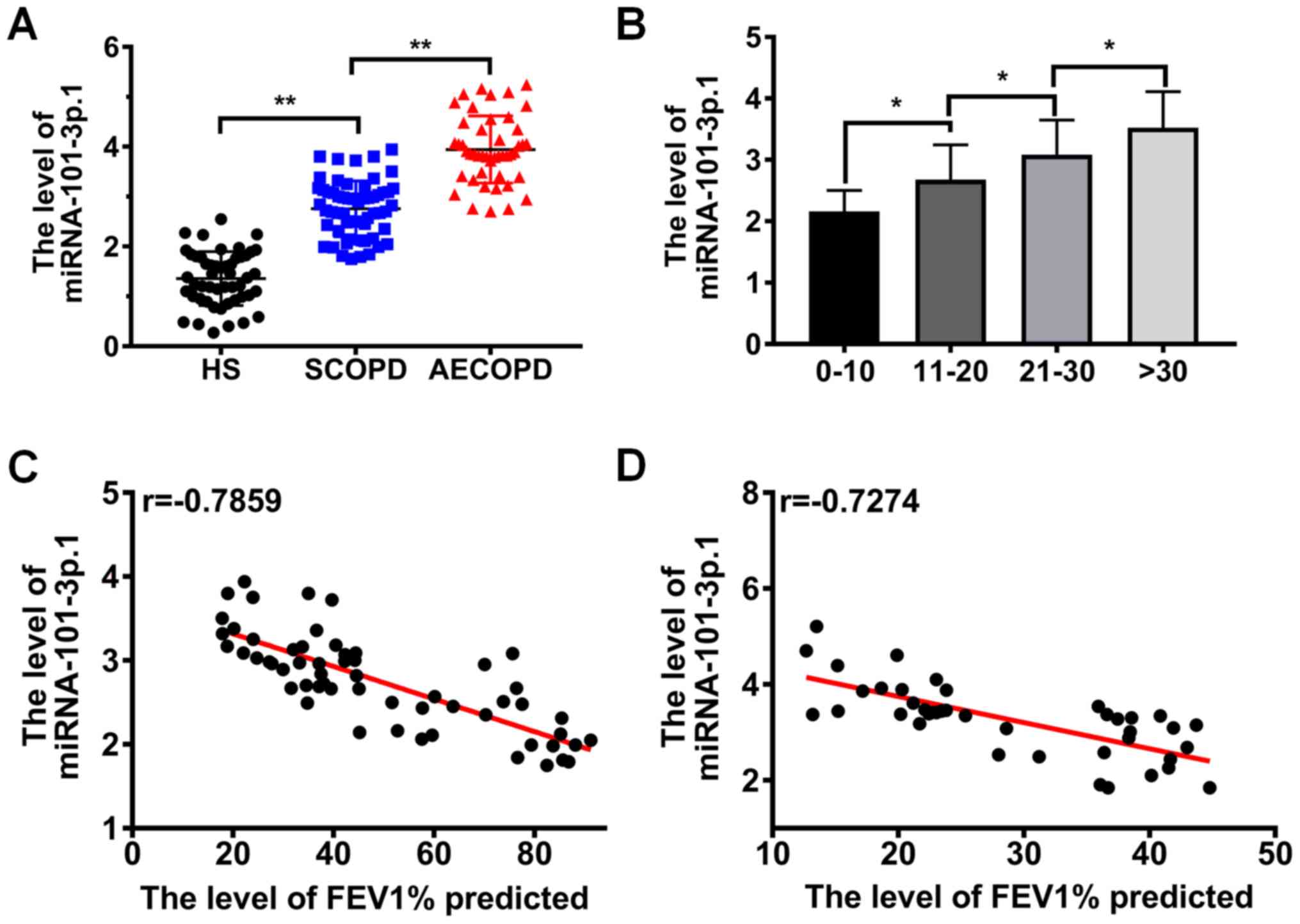
performed. The results presented in Fig. 1A demonstrated that miRNA-101-3p.1
levels in PBMCs were significantly increased in the COPD patients
including SCOPD patients and AECOPD patients compared with healthy
subjects, and were also significantly higher in AECOPD patients
than in SCOPD patients (P<0.05). To further explore the
difference in the level of miRNA-101-3p.1 between the SCOPD
patients and AECOPD patients, the relationship between CAT score
and the level of miRNA-101-3p.1 was analyzed. The results in
Fig. 1B revealed that the
miRNA-101-3p.1 levels were significantly increased with the
increase of CAT score (P<0.05). To explore the correlation
between miRNA-101-3p.1 levels and COPD progression, a correlation
analysis between FEV1% predicted and miRNA-101-3p.1 level was
performed. Pearson correlation analysis, revealed a strong inverse
correlation between FEV1% predicted and the level of miRNA-101-3p.1
in COPD patients (r=−0.7859, P<0.05; Fig. 1C). In addition, a similar result
was observed in AECOPD patients, as revealed in Fig. 1D (r=−0.7274, P<0.05). The
aforementioned data collectively indicated that miRNA-101-3p.1 may
be used as a diagnostic marker in COPD and may be involved in COPD
progression.
Diagnostic accuracy of miRNA-101-3p.1
in SCOPD and AECOPD
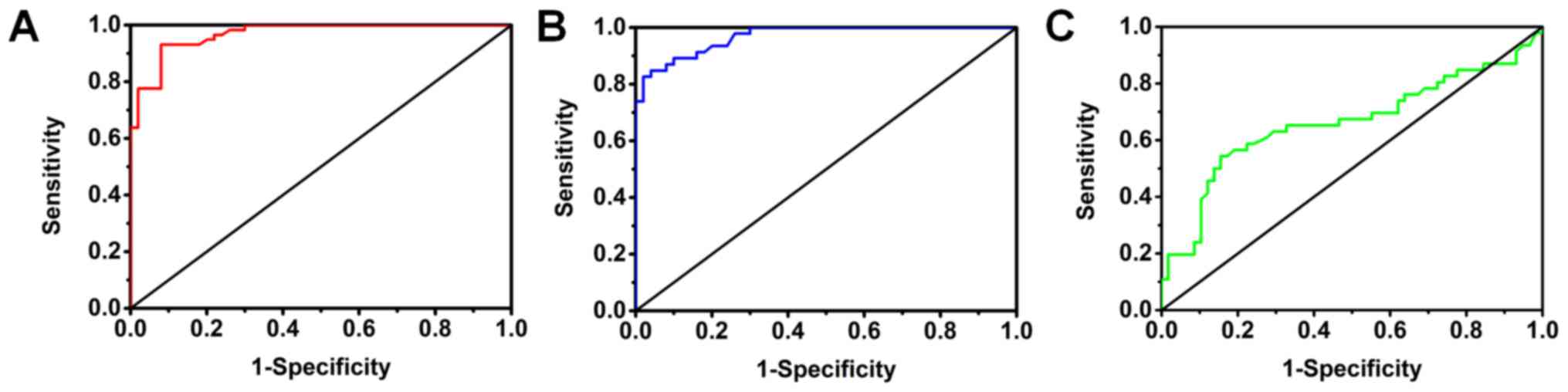
The diagnostic accuracy of miRNA-101-3p.1 as an
independent biomarker to discriminate SCOPD and AECOPD was
evaluated by plotting ROC curves. As is evident in Fig. 2A, miRNA-101-3p.1 could discriminate
between SCOPD patients and healthy subjects with AUC values of
0.968 (95% CI: 0.942–0.995; P<0.05). At the cut-off value of
1.975 for miRNA-101-3p.1, the optimal sensitivity and specificity
of miRNA-101-3p.1 were 93.1 and 92.0%, respectively, while Youden's
index was 0.851. Notably, the results presented in Fig. 2B indicated that miRNA-101-3p.1
could also discriminate between AECOPD patients and healthy
subjects with AUC values of 0.971 (95% CI: 0.945–0.997; P<0.05).
At the cut-off value of 2.25 for miRNA-101-3p.1, the optimal
sensitivity and specificity were 84.8 and 96.0%, respectively,
while Youden's index was 0.808. To further evaluate the diagnostic
accuracy of miRNA-101-3p.1 in discriminating between AECOPD and
SCOPD, a ROC curve indicated that the AUC value of miRNA-101-3p.1
was 0.661 (95% CI: 0.549–0.773; P<0.05). At the cut-off value of
3.265, the optimal sensitivity and specificity were 54.3 and 84.5%,
respectively, while Youden's index was 0.388 (Fig. 2C). Collectively, these data
indicated that the determination in the level of miRNA-101-3p.1 was
effective in detecting SCOPD or AECOPD.
Increasing miRNA-101-3p.1 is
responsible for COPD development
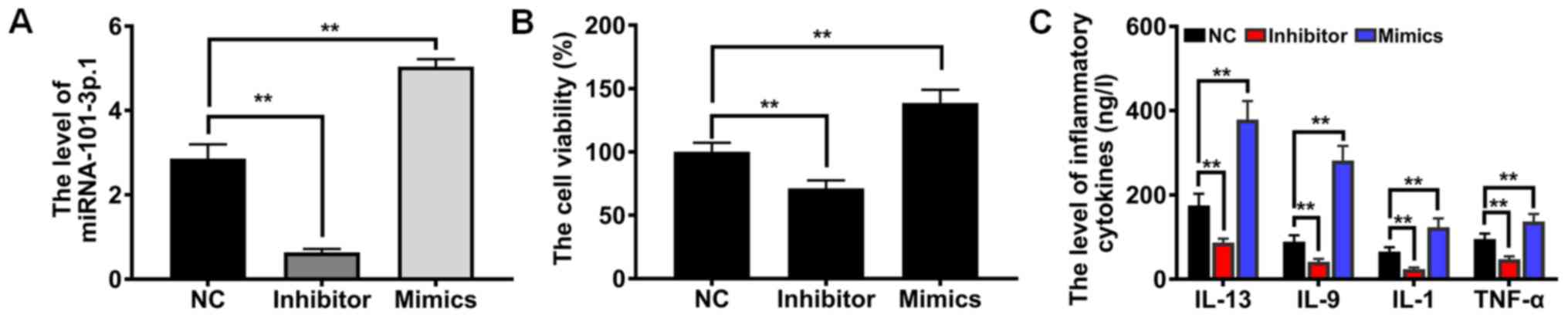
To offer sufficient evidence of the role of
miRNA-101-3p.1 in COPD diagnosis and progression, miRNA-101-3p.1
mimics, miRNA-101-3p.1 inhibitor and miRNA-101-3p.1 negative
control were established (Fig.
3A). Subsequently, the influence on cell proliferation was
detected by CCK-8 assay. As revealed in Fig. 3B, compared with NC group, ectopic
expression of miRNA-101-3p.1 caused a significant enhancement of
proliferation (P<0.01). However, downregulation of
miRNA-101-3p.1 expression resulted in the opposite effect on cell
proliferation. Accumulating evidence indicates that inflammatory
cytokines play crucial roles in COPD pathogenesis. To evaluate the
effects of miRNA-101-3p.1 on pulmonary inflammation responses,
inflammatory cytokines including IL-13, IL-9, IL-1 and TNF-α were
assessed. These results, presented in Fig. 3C, demonstrated that ectopic
expression of miRNA-101-3p.1 significantly enhanced the expression
of inflammatory cytokines, including IL-13, IL-9, IL-1 and TNF-α,
relative to the NC group (P<0.01). Conversely, downregulating
miRNA-101-3p.1 caused marginal expression of IL-13, IL-9, IL-1 and
TNF-α. Collectively, these results provide clear evidence that
increasing miRNA-101-3p.1 promotes cell proliferation and
inflammatory responses.
Increasing miRNA-101-3p.1 causes
downregulation of UBE2D1 and pVHL in PBMCs
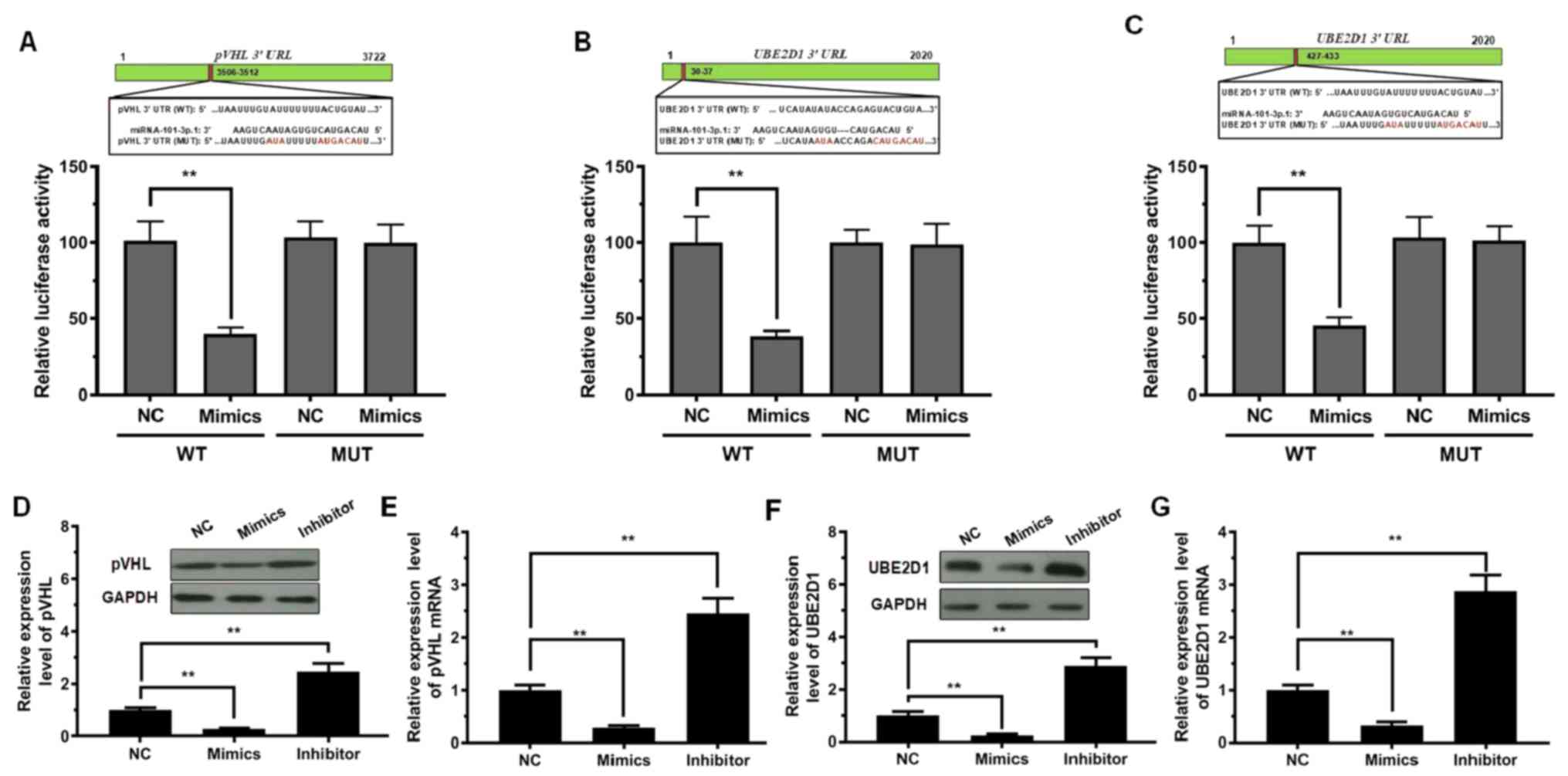
To delineate the molecular mechanism by which
miRNA-101-3p.1 promotes the expression of inflammatory cytokines,
influencing COPD progression, miRNA-101-3p.1 target genes were
searched using the bioinformatics tool, TargetScanHuman (http://http://www.targetscan.org). This tool predicted
1,100 conserved sites. Among these candidates, pVHL and UBE2D1 are
two essential factors involved in cell proliferation, inflammatory
reaction, autophagy, and related processes. Thus, a dual-luciferase
reporter system was used to verify whether miRNA-101-3p.1 mediated
pVHL and UBE2D1 expression. The 3′-UTR regions of pVHL and UBE2D1
mRNA, including the WT site or MUT site (Fig. 4A-C), were co-transfected with
miRNA-101-3p.1 mimics. The results in Fig. 4A indicated that the plasmid with WT
pVHL exhibited a significant decrease in luciferase activity after
transfection with miRNA-101-3p.1 mimics; however, luciferase
activity was not altered after co-transfection with the MUT pVHL
and miRNA-101-3p.1 mimics. Similar results were observed with
UBE2D1 (Fig. 4B and C).
Subsequently, the expression levels of pVHL mRNA and
protein were detected during ectopic expression and silencing of
miRNA-101-3p.1. As reported in Fig. 4D
and E, in contrast to the NC group, pVHL and UBE2D1 levels were
significantly reduced with ectopic expression of miRNA-101-3p.1.
Conversely, silencing of miRNA-101-3p.1 resulted in a significant
increase in pVHL and UBE2D1 expression. Significantly, the
downregulation of pVHL and UBE2D1 was observed with ectopic
expression of miRNA-101-3p.1, while upregulation of pVHL and UBE2D1
was observed when downregulating miRNA-101-3p.1 (Fig. 4F and G).
miRNA-101-3p.1 activates the
EGFR/PI3K/AKT signaling pathway
To further elucidate the miRNA-101-3p.1-regulated
mechanism accounting for the expression of inflammatory cytokines,
HIF-1α expression was investigated, since it is involved in chronic
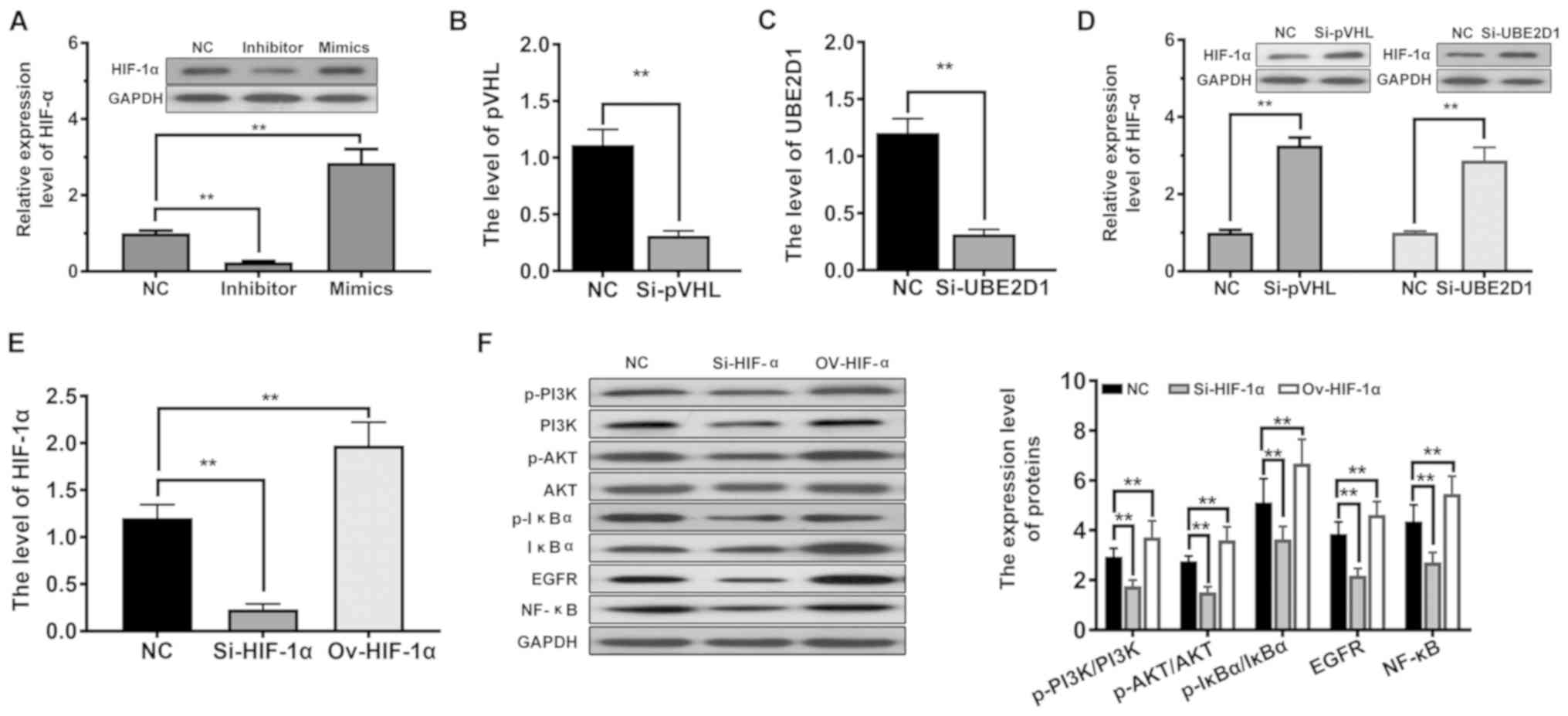
hypoxia. As revealed in Fig. 5A,
ectopic expression of miRNA-101-3p.1 significantly increased the
level of HIF-1α (P<0.01), whereas inhibition of miRNA-101-3p.1
expression had the opposite effect. Next, the decreased expression
of pVHL and UBE2D1 was established (Fig. 5B and C). Following the decreased
expression of pVHL, the HIF-1α level was significantly enhanced in
PBMCs (P<0.01, Fig. 5D).
Similar results were obtained for UBE2D1, as presented in Fig. 5D. These data indicated that
miRNA-101-3p.1 may participate in the regulation of HIF-1α in
inflammatory cytokines. Subsequently, the expression of HIF-1α was
altered by siRNA or overexpression (Fig. 5E). Then the expression of p-PI3K,
p-AKT, p-IκBα, EGFR and NF-κB was assessed by western blotting. As
revealed in Fig. 5F, increased
HIF-1α levels were correlated with significantly increased levels
of p-PI3K/PI3K, p-AKT/AKT, p-IκBα/IκBα, EGFR and NF-κB (P<0.01).
The opposite effects were observed on the activation of the
EGFR/PI3K/AKT signaling pathway in the absence of HIF-1α. It is
concluded from all of these results that increasing miRNA-101-3p.1
aggravates COPD through HIF-1α-dependent activation of the
EGFR/PI3K/AKT signaling pathway.
Discussion
COPD a progressive disease characterized by
inflammation and airflow obstruction, is not fully reversible, and
is responsible for an increasing number of deaths (18,19).
Despite the improved understanding of its pathophysiology, causes
of development and management, the molecular mechanisms underlying
COPD development are still not fully understood. Effective
diagnostic methods for COPD are urgently required. In recent years,
dysregulation of miRNAs has been reported to be involved in the
development of various pulmonary diseases, including COPD, lung
cancer, and emphysema (20,21).
Notably, a set of studies reported that miRNAs could affect
different biological processes involved in COPD, such as
inflammation, tissue repair, and the development of airway lesions
(13,22). Investigating miRNAs as contributors
to COPD initiation and pathogenesis has potential for advancements
in COPD diagnosis. In previous studies, miRNA-101-3p.1 was revealed
to be closely related to pulmonary diseases and inflammatory
responses (23,24). The present study, was conducted to
evaluate the diagnostic potential of miRNA-101-3p.1 to identify
SCOPD and AECOPD using PBMCs and to reveal the molecular mechanism
by which miRNA-101-3p.1 facilitates COPD progression.
In the present study, it was determined that the
miRNA-101-3p.1 level in PBMCs of COPD patients was significantly
increased. Especially, the level of miRNA-101-3p.1 in AECOPD
patients was significantly higher than that in COPD patients.
Further studies revealed that the level of miRNA-101-3p.1 was
significantly correlated with the increase of CAT score and the
deterioration of pulmonary function. Diagnostically, ROC curves
revealed that miRNA-101-3p.1 AUC values were sensitive and specific
for discriminating SCOPD and AECOPD. Furthermore, the biological
function of miRNA-101-3p.1 was explored and it was revealed that
miRNA-101-3p.1 could promote cell proliferation and induce the
expression of inflammatory cytokines. Results from target
prediction and validation assays indicated that miRNA-101-3p.1
directly inhibited pVHL and UBE2D1 expression by binding to their
3′UTRs. Further studies revealed that pVHL and UBE2D1
co-upregulated HIF-1α, and then promoted activation of the
EGFR/PI3K/AKT signaling pathway. Collectively, this provides
convincing evidence that miRNA-101-3p.1 could act as a highlight
biomarker for the diagnosis of SCOPD and AECOPD, and that
miRNA-101-3p.1 facilitates COPD progression by activating the
EGFR/PI3K/AKT signaling pathway.
Currently, miRNAs as biomarkers have been widely
used for risk assessment, diagnosis, prognosis, and dynamic
detection of various diseases, including cancer and cardiovascular
disease (25,26). Ramshankar and Krishnamurthy
(27) revealed 7 differentially
expressed miRNAs by screening lung cancer and COPD patients,
demonstrating that miRNAs could be used as biomarkers to
differentiate lung cancer and COPD. In the present study, it was
revealed that the level of miRNA-101-3p.1 in PBMCs of COPD patients
was significantly increased compared with healthy subjects, while
the miRNA-101-3p.1 level of AECOPD patients was higher than in
SCOPD patients. This observation is consistent with the more
serious manifestation of AECOPD. However, Su et al (28) revealed that miRNA-101 was
downregulated in hepatocellular carcinoma. Such a discrepancy may
be caused by the different contexts in these studies, including
tissue types and hepatitis B virus infection. Furthermore, the
present research revealed that the miRNA-101-3p.1 level was
significantly enhanced with the increase of CAT scores and
deterioration of pulmonary function, indicating that the level of
miRNA-101-3p.1 may reflect the COPD severity. COPD development may
be explained by high levels of miRNA-101-3p.1, leading to
progressive pulmonary function damage in COPD patients.
Furthermore, these results also indicated that miRNA-101-3p.1 may
be an ideal biomarker for the differentiation of SCOPD and AECOPD.
Therefore, ROC curve analyses were performed and it was revealed
that miRNA-101-3p.1 was able to discriminate SCOPD and AECOPD from
healthy subjects. Because the level of miRNA-101-3p.1 was
significantly higher in SCOPD patients and AECOPD patients than
that in healthy patients, the ROC curve revealed high AUC values.
Furthermore, similar AUC values in SCOPD patients and AECOPD
patients may be affected by the fewer number of COPD patients
enrolled and differences in severity of COPD. However, the analysis
did not have satisfactory accuracy to discriminate between SCOPD
and AECOPD due to the influence of COPD patients with GOLD III or
GOLD IV. This finding is largely consistent with a previous study
in which biomarkers were unable to discriminate AECOPD from COPD
(29,30). At present, the clinical diagnosis
of SCOPD and AECOPD is still mainly dependent on comprehensive
analysis of clinical symptoms, laboratory assay and imaging
examinations, which is susceptible to the subjective judgment of
doctors and the behavioral performance of patients (31,32).
miRNA-101-3p.1 as an indicator is helpful in judging disease
severity, patient susceptibility, disease status, and disease
progression, all of which are clinically significant for guiding
the rational use of drugs, prognostic evaluation and predicting
treatment response.
Chronic airway inflammation is an important factor
contributing to airway remodeling and progressive airway
obstruction in COPD patients (33). Clinical and pathological studies
indicated that overexpression of inflammatory cytokines can cause
histopathological injury and inhibit the proliferation of airway
cells, causing airway reconstruction and pulmonary function decline
(34,35). The present results demonstrated
that increasing miRNA-101-3p.1 promoted PBMC proliferation, a
finding largely consistent with a study by Kim et al
(36) in which miR-101-3p played
an important role in promoting proliferation and inhibiting
endothelial cell apoptosis. Increased PBMC proliferation promotes
the infiltration of large numbers of mononuclear cells in the lung.
Mononuclear cells then differentiate into macrophages, leading to a
continuous deterioration of inflammatory response. Several recent
studies have revealed that upregulation of IL-13, IL-9, IL-1, and
TNF-α induces inflammatory cell infiltration, including
macrophages, neutrophils and dendritic cells that contribute to the
airway remodeling and destruction of lung tissue in COPD (37–39).
Furthermore, Zhang et al (40) also revealed that HIF-1α
overexpression aggravated COPD pathological changes by upregulating
the level IL-13, IL-9, IL-1, and TNF-α. In the present study, it
was also determined that increasing miRNA-101-3p.1 induced the
expression of inflammatory cytokines, including IL-13, IL-9, IL-1
and TNF-α, indicating that miRNA-101-3p.1 may be involved in
pulmonary inflammation. Systemic inflammation is also considered to
be closely associated with increased mortality of COPD patients.
Therefore, such observations further support the conclusion that
miRNA-101-3p.1 participates in the development of COPD.
The functions of miRNAs are considered to strongly
depend on post-transcriptional regulation of target proteins
(41). In the present study,
bioinformatics analysis and functional verification indicated that
miRNA-101-3p.1 directly inhibited pVHL and UBE2D1 expression by
binding to their 3′UTR. Several recent studies demonstrated that
pVHL and Cul2 can form a complex and cooperate with E2
ubiquitin-binding enzyme to induce the ubiquitination and
degradation of HIF-1α (42,43).
In addition, HIF-1α can activate the transcription of
oxygen-sensitive genes to maintain survival under hypoxic
conditions, and COPD is considered to be a chronic hypoxic lung
disease (44–46). Therefore, the relationship between
miRNA-101-3p.1 and HIF-1α was explored. It was revealed that HIF-1α
expression significantly increased with ectopic expression of
miRNA-101-3p.1 or decreasing expression of pVHL and UBE2D1,
indicating that miRNA-101-3p.1 aggravates COPD in a
HIF-1α-dependent manner. This also explains why miRNA-101-3p.1 has
high resolution for COPD diagnosis. However, the regulatory
mechanism in which UBE2D1 downregulates the HIF-1α level is still
unclear. UBE2D1 may participate in pVHL-mediated HIF-1α degradation
as an E2 ubiquitin-binding enzyme. Subsequently, the effect of
HIF-1α on the EGFR/PI3K/AKT signaling pathway, an
inflammatory-related signaling pathway, was investigated. Notably,
HIF-1α overexpression was propitious to the upregulation of
p-PI3K/PI3K, p-AKT/AKT, p-IκBα/IκBα, EGFR and NF-κB, demonstrating
the activation of the EGFR/PI3K/AKT signaling pathway. In agreement
with previous findings, HIF-1α upregulated the expression of
inflammatory factors by activating the EGFR/PI3K/AKT pathway
(40). Collectively, the present
study revealed that increasing miRNA-101-3p.1 aggravates COPD
through HIF-1α-dependent activation of the EGFR/PI3K/AKT signaling
pathway.
In summary, the value of miRNA-101-3p.1 for
diagnosis of SCOPD and AECOPD was determined, and the molecular
mechanism by which miRNA-101-3p.1 facilitates COPD progression was
explored. The results revealed that the level of miRNA-101-3p.1 in
PBMCs of COPD patients was significantly increased, especially in
AECOPD patients and was significantly correlated with the increase
of CAT score and deterioration of pulmonary function. Furthermore,
by binding to their 3′UTRs, miRNA-101-3p.1 directly inhibited pVHL
and UBE2D1 expression and co-upregulated HIF-1α, which activated
the EGFR/PI3K/AKT signaling pathway. The ability of miRNA-101-3p.1
to differentiate COPD will allow for more accurate diagnosis of
individual patients, complementing standard clinical
techniques.
Acknowledgements
Not applicable.
Funding
The present research was supported by Hospital
Research Fund (B1519) and Clinical Research Fund of Zhejiang
Medical Association (2016ZYC-A20).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SC, ZZ and LC designed the study, and wrote and
revised the manuscript. SC and JZ performed the experiments and
analyzed the data. All authors read and approved the final
manuscript and agree to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of
any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All study protocols were approved by The Ethics and
Scientific Committees of Zhejiang University. Before study
participation, written informed consent was provided by each
participant
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marçôa R, Rodrigues DM, Dias M, Ladeira I,
Vaz AP, Lima R and Guimarães M: Classification of chronic
obstructive pulmonary disease (COPD) according to the new global
initiative for chronic obstructive lung disease (GOLD) 2017:
Comparison with GOLD 2011. COPD. 15:21–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCullagh BN, Comellas AP, Ballas ZK,
Newell JD Jr, Zimmerman MB and Azar AE: Antibody deficiency in
patients with frequent exacerbations of chronic obstructive
pulmonary disease (COPD). PLoS One. 12:e01724372017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Wang C and Yang X: Efficacy of
salmeterol and formoterol combination treatment in mice with
chronic obstructive pulmonary disease. Mol Med Rep. 15:1538–1545.
2018.
|
|
4
|
Mohammed J, Derom E, Van Oosterwijck J, Da
Silva H and Calders P: Evidence for aerobic exercise training on
the autonomic function in patients with chronic obstructive
pulmonary disease (COPD): A systematic review. Physiotherapy.
104:36–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Engel M, Endesfelder D, Schloter-Hai B,
Kublik S, Granitsiotis MS, Boschetto P, Stendardo M, Barta I, Dome
B, Deleuze JF, et al: Influence of lung CT changes in chronic
obstructive pulmonary disease (COPD) on the human lung microbiome.
PLoS One. 12:e01808592017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan DM, Ullah A, Randhawa FA, Iqtadar S,
Butt NF and Waheed K: Role of vitamin D in reducing number of acute
exacerbations in chronic obstructive pulmonary disease (COPD)
patients. Pak J Med Sci. 33:610–4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allers M, Langejuergen J, Gaida A, Holz O,
Schuchardt S, Hohlfeld JM and Zimmermann S: Measurement of exhaled
volatile organic compounds from patients with chronic obstructive
pulmonary disease (COPD) using closed gas loop GC-IMS and
GC-APCI-MS. J Breath Res. 10:0260042016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spero K, Khorfan F and Bayasi G: The over
diagnosis of COPD in hospitalized patients. Chest. 150:921A2016.
View Article : Google Scholar
|
|
9
|
Yang IA, Brown JL, George J, Jenkins S,
McDonald CF, McDonald VM, Phillips K, Smith BJ, Zwar NA and
Dabscheck E: COPD-X Australian and New Zealand guidelines for the
diagnosis and management of chronic obstructive pulmonary disease:
2017 update. Med J Aust. 207:436–442. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peat R, Furlong J, Byrne T, Young R,
Kangombe A, Elkin T, Renwick S, Russell D, Oelbaum S, Burhan H and
Walke PP: P198 Anchoring copd screening to drug services in heroin
and crack smokers to improve diagnosis. Thorax. 71:A192.1–A192.
2016. View Article : Google Scholar
|
|
11
|
Wang Q, Yu H, Yu H, Ma M, Ma YL and Li R:
miR-223-3p/TIAL1 interaction is involved in the mechanisms
associated with the neuroprotective effects of dexmedetomidine on
hippocampal neuronal cells in vitro. Mol Med Rep.
19:805–812. 2019.PubMed/NCBI
|
|
12
|
Jin R, Hu S, Liu X, Guan R, Lu L and Lin
R: Intranasal instillation of miR-410 targeting IL-4/IL-13
attenuates airway inflammation in OVA-induced asthmatic mice. Mol
Med Rep. 19:895–900. 2019.PubMed/NCBI
|
|
13
|
Shen W, Liu J, Zhao G, Fan M, Song G and
Zhang Y, Weng Z and Zhang Y: Repression of Toll-like receptor-4 by
microRNA-149-3p is associated with smoking-related COPD. Int J
Chron Obstruct Pulmon Dis. 12:705–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Qu J, Xue W, He L, Wang J, Xi X,
Liu X, Yin Y and Qu Y: Bioinformatics-based identification of
potential microRNA biomarkers in frequent and non-frequent
exacerbators of COPD. Int J Chron Obstruct Pulmon Dis. 13:1217–28.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheng Y, Li J, Zou C, Wang S, Cao Y, Zhang
J, Huang A and Tang H: Downregulation of miR-101-3p by hepatitis B
virus promotes proliferation and migration of hepatocellular
carcinoma cells by targeting Rab5a. Arch Virol. 159:2397–2410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hassan F, Nuovo GJ, Crawford M, Boyaka PN,
Kirkby S, Nana-Sinkam SP and Cormet-Boyaka E: MiR-101 and miR-144
regulate the expression of the CFTR chloride channel in the lung.
PLoS One. 7:e508372012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arbillaga-Etxarri A, Gimeno-Santos E,
Barberan-Garcia A, Benet M, Borrell E, Dadvand P, Foraster M, Marín
A, Monteagudo M, Rodriguez-Roisin R, et al: Socio-environmental
correlates of physical activity in patients with chronic
obstructive pulmonary disease (COPD). Thorax. 72:796–802. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoogendoorn M, Feenstra TL, Asukai Y,
Briggs AH, Hansen RN, Leidl R, Risebrough N, Samyshkin Y, Wacker M
and Rutten-van Mölken MP: External validation of health economic
decision models for chronic obstructive pulmonary disease (COPD):
Report of the third COPD modeling meeting. Value Health.
20:397–403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao E, Maj T, Kryczek I, Wei L, Ke W,
Zhao L, Wei S, Crespo J, Wan S, Vatan L, et al: Cancer mediates
effector T cell dysfunction by targeting microRNAs and EZH2 via
glycolysis restriction. Nat Immunol. 17:95–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pichiorri F, Suh SS, Rocci A, De Luca L,
Taccioli C, Santhanam R, Zhou W, Benson DM Jr, Hofmainster C, Alder
H, et al: Downregulation of p53-inducible microRNAs 192, 194, and
215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma
development. Cancer Cell. 18:367–381. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Leary L, Sevinç K, Papazoglou IM, Tildy
B, Detillieux K, Halayko AJ, Chung KF and Perry MM: Airway smooth
muscle inflammation is regulated by microRNA-145 in COPD. FEBS
Lett. 590:1324–1334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu Y, Yao Y, Jiang X, Zhong X, Wang Z, Li
C, Kang P, Leng K, Ji D, Li Z, et al: SP1-induced upregulation of
lncRNA SPRY4-IT1 exerts oncogenic properties by scaffolding
EZH2/LSD1/DNMT1 and sponging miR-101-3p in cholangiocarcinoma. J
Exp Clin Canc Res. 37:812018. View Article : Google Scholar
|
|
24
|
Liang H, Yu T, Han Y, Jiang H, Wang C, You
T, Zhao X, Shan H, Yang R, Yang L, et al: LncRNA PTAR promotes EMT
and invasion-metastasis in serous ovarian cancer by competitively
binding miR-101-3p to regulate ZEB1 expression. Mol Cancer.
17:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hung CH, Hu TH, Lu SN, Kuo FY, Chen CH,
Wang JH, Huang CM, Lee CM, Lin CY, Yen YH and Chiu YC: Circulating
microRNAs as biomarkers for diagnosis of early hepatocellular
carcinoma associated with hepatitis B virus. Int J Cancer.
138:714–720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karakas M, Schulte C, Appelbaum S, Ojeda
F, Lackner KJ, Münzel T, Schnabel RB, Blankenberg S and Zeller T:
Circulating microRNAs strongly predict cardiovascular death in
patients with coronary artery disease-results from the large
AtheroGene study. Eur Heart J. 38:516–523. 2017.PubMed/NCBI
|
|
27
|
Ramshankar V and Krishnamurthy A: Lung
cancer detection by screening-presenting circulating miRNAs as a
promising next generation biomarker breakthrough. Asian Pac J
Cancer Prev. 14:2167–2172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su H, Yang JR, Xu T, Huang J, Xu L, Yuan Y
and Zhuang SM: MicroRNA-101, down-regulated in hepatocellular
carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer
Res. 69:1135–1142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YR, Chen V, Hollander Z, Leipsic JA,
Hague CJ, Demarco ML, FitzGerald JM, McManus BM, Ng RT and Sin DD:
C-reactive protein and N-terminal prohormone brain natriuretic
peptide as biomarkers in acute exacerbations of COPD leading to
hospitalizations. PLoS One. 12:e01740632017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song Y, Chen R, Zhan Q, Chen S, Luo Z, Ou
J and Wang C: The optimum timing to wean invasive ventilation for
patients with AECOPD or COPD with pulmonary infection. Int J Chron
Obstruct Pulmon Dis. 11:535–542. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mouronte-Roibás C, Leiro-Fernández V,
Ruano-Raviña A, Ramos-Hernández C, Abal-Arca J4, Parente-Lamelas I,
Botana-Rial M, Priegue-Carrera A and Fernández-Villar A: Chronic
obstructive pulmonary disease in lung cancer patients: Prevalence,
underdiagnosis, and clinical characterization. Respiration.
95:414–421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cosio BG, Soriano JB, López-Campos JL,
Calle-Rubio M, Soler-Cataluna JJ, de-Torres JP, Marín JM,
Martínez-Gonzalez C, de Lucas P, Mir I, et al: Defining the
Asthma-COPD overlap syndrome in a COPD cohort. Chest. 149:45–52.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Green CE and Turner AM: The role of the
endothelium in asthma and chronic obstructive pulmonary disease
(COPD). Resp Res. 18:202017. View Article : Google Scholar
|
|
34
|
Leiro-Fernández V, Priegue Carrera A and
Fernández-Villar A: Efficacy of double bronchodilation (LABA+LAMA)
in patients with chronic obstructive pulmonary disease (COPD) and
lung cancer. Arch Bronconeumol. 52:622–623. 2016.(In English,
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kokturk N, Baha A, Oh YM, Young Ju J and
Jones PW: Vitamin D deficiency: What does it mean for chronic
obstructive pulmonary disease (COPD)? a compherensive review for
pulmonologists. Clin Respir J. 12:382–397. 2016. View Article : Google Scholar
|
|
36
|
Kim JH, Lee DK, Kim J, Choi S, Park W, Ha
KS, Kim TH, Choe J, Won MH, Kwon YG and Kim YM: A miRNA-101-3p/Bim
axis as a determinant of serum deprivation-induced endothelial cell
apoptosis. Cell Death Dis. 8:e28082017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zou SC, Pang LL, Mao QS, Wu SY and Xiao
QF: IL-9 exacerbates the development of chronic obstructive
pulmonary disease through oxidative stress. Eur Rev Med Pharmacol
Sci. 22:8877–8884. 2018.PubMed/NCBI
|
|
38
|
Kang MJ, Choi LM, Kim BH, Lee CM, Cho WK,
Choe G, Kim DH, Lee CG and Elias JA: IL-18 induces emphysema and
airway and vascular remodeling via IFN-γ, IL-17A, and IL-13. Am J
Respir Crit Care Med. 11:1205–1217. 2012. View Article : Google Scholar
|
|
39
|
Wang Y, Xu J, Meng Y, Adcock IM and Yao X:
Role of inflammatory cells in airway remodeling in COPD. Int J
Chron Obstruct Pulmon Dis. 13:3341–3348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang HX, Yang JJ, Zhang SA, Zhang SM,
Wang JX, Xu ZY and Lin RY: HIF-1α promotes inflammatory response of
chronic obstructive pulmonary disease by activating EGFR/PI3K/AKT
pathway. Eur Rev Med Pharmacol Sci. 22:6077–6084. 2018.PubMed/NCBI
|
|
41
|
Wang J, Liang H, Ge H, Guo XL, Gu D and
Yuan Y: MicroRNA-363-3p inhibits hepatocarcinogenesis by targeting
HMGA2 and is associated with liver cancer stage. Mol Med Rep.
19:935–942. 2019.PubMed/NCBI
|
|
42
|
Min JH, Yang H, Ivan M, Gertler F, Kaelin
WG Jr and Pavletich NP: Structure of an HIF-1alpha-pVHL complex:
Hydroxyproline recognition in signaling. Science. 296:1886–1889.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miller F, Kentsis A, Osman R and Pan ZQ:
Inactivation of VHL by tumorigenic mutations that disrupt dynamic
coupling of the pVHL.hypoxia-inducible transcription factor-1alpha
complex. J Biol Chem. 280:7985–7996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Semba H, Takeda N, Isagawa T, Sugiura Y,
Honda K, Wake M, Miyazawa H, Yamaguchi Y, Miura M, Jenkins DM, et
al: HIF-1α-PDK1 axis-induced active glycolysis plays an essential
role in macrophage migratory capacity. Nat Commun. 7:116352016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shih JW, Chiang WF, Wu ATH, Wu MH, Wang
LY, Yu YL, Hung YW, Wang WC, Chu CY, Hung CL, et al: Long noncoding
RNA LncHIFCAR/MIR31HG is a HIF-1α co-activator driving oral cancer
progression. Nat Commun. 8:158742017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sundh J and Ekström M: Risk factors for
developing hypoxic respiratory failure in COPD. Int J Chron
Obstruct Pulmon Dis. 12:2095–2100. 2017. View Article : Google Scholar : PubMed/NCBI
|