Introduction
Astaxanthin (ASX) is a naturally occurring
red-colored oxygenated carotenoid that is present in microalgae,
salmon, trout and shrimp (1).
Owing to its structure, which consists of a long backbone and
hydroxyl and keto moieties at each polar end, ASX has especially
powerful antioxidant capacity compared with other carotenoids
(2). ASX has no adverse effects
(3), and there is evidence of a
reduction in biomarkers of oxidative stress and inflammation with
ASX administration (4).
ASX has been shown to inhibit cancer cell
proliferation by reducing or increasing oxidative stress (5–10).
It has been reported that ASX (100–300 µM) decreases NF-κB and
Wnt/β-catenin levels, thereby inhibiting hepatic cancer cell
proliferation (5). ASX (50 and
100 µM) has been shown to inhibit the proliferation of Kato-III and
SNU cells by suppressing cell cycle progression (6). With respect to antioxidant activity
in anticancer mechanisms, ASX (50–150 µM) is known to induce
apoptosis by increasing the expression levels of apoptotic Bax and
caspase-3, but decreasing anti-apoptotic Bcl-2 levels in colorectal
cancer LS-180 cells (7). Kochi
et al (8) showed that ASX
treatment inhibited azoxymethane-induced neoplastic lesions in the
colonic mucosa of mice. ASX administration reduced serum levels of
hydroperoxides, an oxidative stress marker, but increased the
colonic mucosal levels of antioxidant enzymes (superoxide
dismutase, catalase and glutathione peroxidase). These studies
suggest that ASX reduces oxidative stress-mediated cancer cell
growth.
By contrast, recent studies have reported the
pro-oxidant effects of ASX on cancer cells. In one study, ASX (10,
25, 50 and 100 µg•ml−1) induced cell death in a dose-
and time-dependent manner (24, 48 and 72 h) in human non-small cell
lung cancer A549 cells (9). ASX
(50 µg•ml−1) increased reactive oxygen species (ROS)
levels (195% of untreated cells) after 4 h of culture in A549 cells
(9). In breast cancer MCF-7
cells, ASX (20, 30, 40 and 50 µM) decreased cell viability in a
dose- and time-dependent manner (24, 28 and 72 h culture). Cells
cultured for 48 h with ASX (20 µM) increased ROS levels to 153% of
that of the untreated cells (10). Therefore, ASX may exhibit its
anticancer effect through the production of ROS and ROS-mediated
death signaling pathways.
Necroptosis is programmed necrosis, which is
regulated by necroptotic regulators, such as receptor-interacting
protein kinase 1 (RIP1), RIP3 and mixed lineage kinase domain-like
protein (MLKL) (11,12). Upon stimulation, including the
activation of Toll-like receptors (13), T cell receptors (14) and the TNF receptor superfamily
(15), the serine/threonine
kinase activity of RIP1 and RIP3 increases, and consecutive
phosphorylation of RIP1 and RIP3 forms a RIP1-RIP3 heterodimeric
scaffold complex. However, necrosis represents a form of
non-programmed cell death. Therefore, necrosis is not regulated by
necroptotic regulators, such as RIP1, RIP3 and MLKL (16).
After formation of the RIP1-RIP3 heterodimeric
scaffold complex, free RIP3 is recruited and auto-phosphorylated.
This allows the recruitment of MLKL to form the necrosome (17,18). Phosphorylated MLKL translocates to
the plasma membrane and oligomerizes into complexes, leading to
plasma membrane rupture, thereby acting as an executioner of
necroptosis (13). Dysregulation
of necroptotic proteins results in cancer development (19). RIP3 is lower in breast and
colorectal cancer cells than in healthy cells, and low RIP3
expression levels are associated with poor survival in patients
with cancer (12,20). MLKL levels are lower in gastric
cancer tissues than in normal tissues (21).
ROS activate RIP1 autophosphorylation and contribute
to RIP3 recruitment into the necrosome (22,23). NADPH oxidase produces large
amounts of ROS upon exposure to various stimuli (24). Thus, NADPH oxidase activation may
mediate necroptosis by activating RIP1-RIP3-MLKL signaling in
cancer cells.
The present study aimed to determine whether ASX
induces necroptosis by activating necroptotic proteins (RIP1, RIP3
and MLKL) and cell death [by determining lactate dehydrogenase
(LDH) release, propidium iodide (PI)-positive cells and cell
viability] in gastric cancer AGS cells. In addition, to investigate
whether ASX-induced necroptosis is induced by NADPH oxidase
activation and increased ROS levels, the cells were treated with
the NADPH oxidase inhibitor ML171 or antioxidant N-acetylcysteine
(NAC) prior to ASX treatment. Moreover, to assess whether ASX
affects the viability of normal gastric epithelial cells, normal
RGM-1 cells were treated with ASX, and cell viability and NADPH
oxidase activity were measured.
Materials and methods
Materials
ASX (cat. no. SML0982), necrotatin-1 (Nec-1; the
stable variant; cat. no. 5042970001), z-VAD-fmk (cat. no. 627610),
ML171 (cat. no. 492002) and N-acetylcysteine (NAC; cat. no. A7250)
were purchased from Sigma-Aldrich (Merck KGaA). Dichlorofluorescein
diacetate (DCF-DA; cat. no. D399) was purchased from Molecular
Probes (Thermo Fisher Scientific, Inc.). RPMI-1640 medium (cat. no.
31800022) was purchased from Gibco (Thermo Fisher Scientific,
Inc.). MuLV reverse transcriptase (cat. no. M1705) was obtained
from Promega Corporation. The protease inhibitor complex
(cOmplete™; cat. no. 11697498001) was obtained from Roche
Diagnostics GmbH. Antibodies against caspase-9 (cat. no. sc-81663),
Bcl-2 (cat. no. sc-492), Bax (cat. no. sc-526) and actin (cat. no.
sc-47778) were obtained from Santa Cruz Biotechnology, Inc.
Antibodies against phosphorylated (p)-RIP1 (cat. no. 65746) and
p-MLKL (cat. no. 91689) were obtained from Cell Signaling
Technology, Inc. The antibody against RIP1 (cat. no. 610458) was
obtained from BD Pharmingen (BD Biosciences). Antibodies against
RIP3 (cat. no. ab56164), p-RIP3 (cat. no. ab209384) and MLKL (cat.
no. ab183770) were obtained from Abcam. All other reagents were
obtained from Sigma-Aldrich (Merck KGaA). ASX, NEC-1, z-VAD-fmk and
ML171 were dissolved in dimethyl sulfoxide (DMSO). Cells incubated
with DMSO alone (<0.3%) served as controls (vehicle alone).
Cell line and culture conditions
The human gastric cancer cell line AGS (ATCC
CRL-1739; gastric adenocarcinoma) was purchased from the American
Type Culture Collection and cultured in RPMI-1640 medium with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 2
mM glutamine and antibiotics (100 U•ml−1 penicillin and
100 µg•ml−1 streptomycin). The cells were cultured at
37°C in a humidified atmosphere with 95% air and 5% CO2.
The normal rat gastric epithelial cell line, RGM-1,
was obtained from the RIKEN BioResource Center. RGM-1 cells were
grown in a 1:1 mixture of Dulbecco's modified Eagles medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc.) and Ham's F-12 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 20%
newborn calf serum (Gibco; Thermo Fisher Scientific, Inc.), 100
U•ml−1 penicillin and 100 µg•ml−1
streptomycin. The cells were cultured at 37°C in a humidified
atmosphere with 95% air and 5% CO2.
Experimental protocol
For all treatments, the cells were cultured at 37°C.
For the time-course experiment to determine ROS levels and
necroptotic markers, the cells were treated with 20 µM ASX and
cultured for 0.5, 1 and 2 h (for ROS levels) or 2, 4 and 6 h (for
the mRNA and protein expression levels of RIP1, RIP3, MLKL, and
p-RIP1, p-RIP3 and p-MLKL levels).
For the concentration-course experiment to assess
ROS levels and cell viability, the cells were treated with ASX (5,
10 or 20 µM) for 2 h (for ROS determination) and 24 h (for analysis
of cell viability).
For the assessment of ROS levels, NADPH oxidase
activity, cell viability, LDH release and Hoechst 33342/propidium
oxide (PI) double staining, the cells were pretreated with ML171 (2
µM), NAC (1 mM), Nec-1 (25 µM) or z-VAD-fmk (10 µM) for 1 h and
treated with 20 µM ASX for 2 h (to measure levels of ROS and NADPH
oxidase activity) or 24 h (for cell viability, LDH release and
Hoechst 33342/PI double staining).
To assess the role of RIP1 in ASX-induced cell
death, cells were transfected with RIP1 small interfering (si)RNA
or negative control (NC) siRNA and treated with ASX (20 µM) for 24
h, and the number of viable cells was counted.
To determine the effect of ASX on caspase-9
activation, the levels of Bax and Bcl-2, and Annexin V-FITC/PI
double staining, the cells were treated with 20 µM ASX for 24 h.
Western blot analysis was used to determine the levels of
procaspase-9 and cleaved caspase-9. Fluorescence images of AGS
cells were obtained by Annexin V-FITC/PI double staining.
To determine whether ASX induced cell death and
activated NADPH oxidase in normal cells, normal rat gastric
epithelial cells (RGM-1) were treated with 20 µM ASX for 2 h (to
measure NADPH oxidase activity) or different concentrations (5, 10
or 20 µM) of ASX for 24 h (for cell viability).
Determination of intracellular ROS
levels
2′-7′-Dichlorofluorescin diacetate (DCFH-DA) was
used to measure intracellular levels of ROS. This method is used to
detect hydrogen peroxide and hydroxyl radicals (25). The cells were seeded
(6×104 cells per well) in 6-well plates and incubated
with 10 µM DCF-DA for 30 min. The cells were then washed and
scraped into 1 ml PBS. DCF was measured (excitation/emission at
495/535 nm) using a VICTOR5 multilabel counter (PerkinElmer, Inc.).
Intracellular ROS levels were expressed in terms of relative
increases.
Cell viability measurement
The cells were plated in a 24-well plate
(1×104 cells per well) and cultured for 24 h with or
without treatment. Viable cell numbers were determined by direct
counting using a hemocytometer based on a trypan blue exclusion
test (0.2% trypan blue; Sigma-Aldrich; Merck KGaA). The mean number
of viable cells that were not treated with ASX (untreated) was set
to 100%.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was isolated using the TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA was
converted to cDNA by reverse transcription using MuLV reverse
transcriptase. The reaction conditions were as follows: 23°C for 10
min, 37°C for 60 min and 95°C for 5 min. cDNA was used for qPCR.
qPCR was conducted in triplicate using SYBR-Green Real-time PCR
Master Mix (Toyobo Life Science). RIP1 primers (forward,
5′-GGCATTGAAGAAAAATTTAGGC-3′ and reverse,
5′-TCACAACTGCATTTTCGTTTG-3′) were used to generate a 109-bp PCR
product, RIP3 primers (forward, 5′-GACTCCCGGCTTAGAAGGACT-3′ and
reverse, 5′-CTGCTCTTGAGCTGAGACAGG-3′) were used to generate a
180-bp PCR product and MLKL primers (forward,
5′-AGAGCTCCAGTGGCCATAAA-3′ and reverse, 5′-TACGCAGGATGTTGGGAGAT-3′)
were used to generate a 124-bp PCR product. For β-actin, the
forward primer was 5′-ACCAACTGGGACGACATGGAG-3′ and the reverse
primer was 5′-GTGAGGATCTTCATGAGGTAGTC-3′, yielding a 349-bp PCR
product. cDNA was amplified by 45 cycles of denaturation at 95°C
for 30 sec, annealing at 55°C for 30 sec and extension at 72°C for
30 sec. During the first cycle, the 95°C step was extended to 3
min. Amplification specificity was validated by melting curve
analysis generated at the end of each PCR reaction. All genes
presented a single peak in the melting curve, indicating the
absence of primer-dimer formation during the reaction and
specificity of the amplification. Relative changes in gene
expression between untreated cells and cells treated with ASX were
determined using the 2−ΔΔCq method (26). Levels of the target transcript
were normalized to β-actin endogenous control and were constantly
expressed in the group.
Preparation of cell extracts
Preparation of whole-cell extracts, membrane
fractions and cytosolic fractions was performed as previously
described (27). Briefly, the
cells were harvested by treatment with trypsin/EDTA, washed and
centrifuged at 1,000 × g at 21–23°C for 5 min. The cell pellets
were resuspended in lysis buffer containing 150 mM NaCl, 1% NP-40,
0.5% sodium deoxycholate, 0.1% SDS, 25 mM Tris (pH 7.4) and
protease inhibitor complex. The resulting mixture was centrifuged
at 10,000 × g at 21–23°C for 15 min. The supernatants were
collected and used as whole-cell extracts. To prepare the cytosolic
and membrane fractions, the supernatant was separated by
centrifugation at 100,000 × g at 21–23°C for 1 h. The membrane
fraction was obtained by resuspending the pellet in lysis buffer
containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM EDTA and 10%
glycerol. The supernatant was used as the cytosolic fraction. The
protein concentration was determined using the Bradford assay.
Western blot analysis
Whole cell extracts were isolated from cells by
using lysis buffer containing 150 mM NaCl, 1% NP-40, 0.5% sodium
deoxycholate, 0.1% SDS, 25 mM Tris (pH 7.4) and protease inhibitor
complex (Complete; Roche Diagnostics GmbH). The protein
concentration was determined using the Bradford assay. Whole cell
extracts were loaded onto 8–10% SDS-PAGE gels (40–60 µg protein per
lane) and separated by electrophoresis under reducing conditions.
Proteins were transferred onto nitrocellulose membranes via
electroblotting. The membranes were blocked using 3% non-fat dry
milk in Tris-buffered saline and 0.2% Tween-20 (TBST) for 1 h at
room temperature. The membrane was incubated with antibodies
against caspase-9 (1:1,000), actin (1:4,000), RIP1 (1:1,000),
p-RIP1 (1:1,000), RIP3 (1:1,500), p-RIP3 (1:1,000), MLKL (1:1,500)
and p-MLKL (1:1,000) in TBST solution containing 3% dry milk
overnight at 4°C. After washing with TBST, the membrane was
incubated with horseradish peroxidase-conjugated anti-mouse
(1:3,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) or
anti-rabbit (1:4,000; cat. no. sc-2005; Santa Cruz Biotechnology,
Inc.) secondary antibodies in TBST solution containing 3% dry milk
and anti-rabbit) for 2 h at room temperature. The proteins were
visualized by exposure to X-ray film by using an enhanced
chemiluminescence (ECL) detection system (cat. no. sc-2048; Santa
Cruz Biotechnology, Inc.). Actin was used as the loading control.
ImageJ software version 1.52a (National Institutes of Health) was
used for densitometry analysis of western blots.
Measurement of NADPH oxidase
activity
A luciferase assay was used to measure NADPH oxidase
activity in the membrane and cytosolic fractions (28). The assay was performed in 50 mM
Tris-MES buffer (pH 7.0) containing 2 mM KCN, 10 µM lucigenin and
100 µM NADPH. The reaction was initiated by the addition of 10 µg
membrane-extract protein. Photon emission was measured using a
microplate reader (Molecular Devices, LLC). For negative control
experiments, cytosolic fraction protein was used instead of the
membrane-fraction protein.
PI and Hoechst 33342 double
staining
PI is permeable to necrotic cells and is visible as
red fluorescence in nuclear DNA (29). Hoechst staining is a
cell-permeable nuclear counterstain that emits blue fluorescence
when combined with double-stranded DNA (29). Hoechst dyes are very sensitive to
DNA conformation and chromatin status in cells and are, therefore,
used to detect nuclear damage, such as distinguishing condensed
pycnotic nuclei in apoptotic cells. PI and Hoechst 33342 double
staining was used to determine necrosis or apoptosis.
The cells were seeded (6.0×104 cells per
well in a 6-well plate) and cultured overnight. PI and Hoechst
33342 were prepared in PBS to serve as the staining solutions (0.5
mg•ml−1). The cells were treated with 20 µl staining
solution in the dark and then incubated for 10 min at 37°C. The
cells were removed from the medium and fixed for 10 min with 2 ml
cold methanol at 20–22°C. After the removal of methanol, the cells
were washed with PBS (1 ml) for 5 min, examined under a laser
scanning confocal microscope (Zeiss LSM 880; Carl Zeiss AG), and
photographed. The optical filters for excitation were 490–630 nm
for PI and 350–460 nm for Hoechst. The ratio of red and blue
fluorescence density was quantified by ZEN 2.3 (blue edition)
software (Carl Zeiss Microscopy GmbH) and expressed as the
percentage of PI-positive cells.
Annexin V/PI double staining
Annexin V and PI double staining is used to
discriminate between apoptosis and necrosis; PI-positive staining
reflects necrosis and Annexin V-positive/PI-negative staining
reflects apoptosis (30). The
cells were plated (6.0×104 cells per well) in a 6-well culture
plate and then cultured overnight. The cells were then treated with
20 µM ASX for 24 h. The treated cells were then washed with
ice-cold PBS and incubated with Annexin V-FITC and PI (FITC Annexin
V Apoptosis Detection Kit; BD Pharmingen; BD Biosciences) in the
dark according to the manufacturer's recommendations. The cells
were fixed in 2% paraformaldehyde for 10 min at 20–22°C, washed
three times with 1 ml PBS for 5 min, and then examined under a
laser scanning confocal microscope (Zeiss LSM 880; Carl Zeiss AG)
and photographed.
Transfection of RIP1 siRNA
siGenome SMART pool siRNA for human RIP1
(5′-CCACUAGUCUGACGGAUAA-3, 5-UGAAUGACGUCAACGCAAA-3′,
5′-GCACAAAUACGAACUUCAA-3′ and 5′-GAUGAAAUCCAGUGACUUC-3′) and the NC
siRNA (siGenome None-Targeting siRNA Pool #1;
5′-UAGCGACUAAACACAUCAA, 5′-UAAGGCUAUGAAGAGAUAC-3′,
5′-AUGUAUUGGCCUGUAUUAG-3′ and 5′-AUGAACGUGAAUUGCUCAA-3′) were
obtained from GE Healthcare Dharmacon, Inc. Cells were transfected
with 50 nmol RIP1 or NC siRNAs by using
1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), following the
manufacturer's instructions (Invitrogen; Thermo Fisher Scientific,
Inc.), and then cultured for 24 h. Then, 24 h after transfection of
RIP1 or NC siRNAs, the cells were treated with 20 µM ASX.
Measurement of LDH release
LDH release represents the permeabilization of the
plasma membrane, a necrosis marker (31). LDH release was measured using an
LDH assay kit (cat. no. ab102526; Abcam). Cell lysates were
prepared using lysis buffer containing 0.1 M Tris (pH 7.4) and 10%
Triton X-100. Cell lysates were centrifuged at 10,000 × g for 15
min at 4°C. LDH activity in the culture medium and the cells was
measured, as previously described (32). LDH release was quantified as a
percentage of the total LDH content (LDH in the supernatant + LDH
inside the cells).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. All experiments were repeated three times. Number of
each group was ten (n=10). The statistical differences among groups
were evaluated by one-way analysis of variance (ANOVA) followed by
Tukey's test. Statistical analysis was performed using SPSS 22.0
software (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
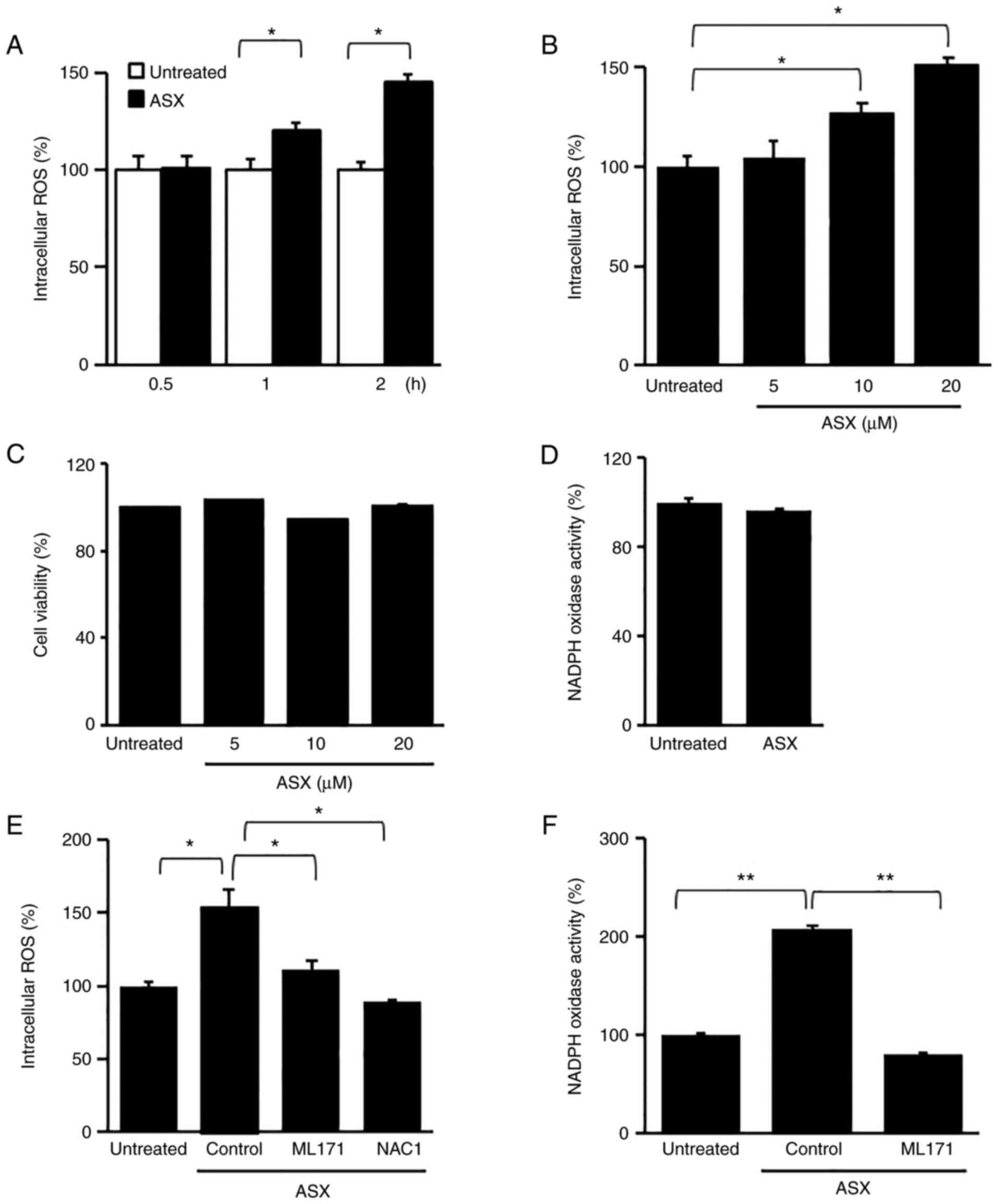
ASX increases ROS levels and decreases
cell viability in AGS cells
To assess the appropriate culture time and
concentration of ASX for ROS production in AGS cells, ROS levels
were determined using different culture times and various
concentrations of ASX. The cells were treated with ASX (20 µM) and
cultured for 2 h. As shown in Fig.
1A, 20 µM ASX increased ROS levels in a time-dependent manner.
Furthermore, ROS levels were highest at 2 h of culture. When the
cells were treated with various concentrations (5, 10 and 20 µM) of
ASX and cultured for 2 h, the highest level of ROS in AGS cells was
obtained with 20 µM ASX (Fig.
1B). Thus, for further studies on ROS levels, cells were
treated with 20 µM ASX for 2 h.
To determine whether ASX induces cell death and
activates NADPH oxidase in normal cells, normal rat gastric
epithelial RGM-1 cells were treated with 20 µM ASX for 2 h (for
determination of NADPH oxidase activity) or different
concentrations (5, 10 or 20 µM) of ASX for 24 h (for cell
viability). ASX had no effect on cell viability or NADPH oxidase in
RGM-1 cells (Fig. 1C and D). The
results showed that ASX did not induce death of normal gastric
epithelial cells.
ASX increases NADPH oxidase activity,
which leads to cell death in AGS cells
To determine whether ASX-induced ROS production was
linked to NADPH oxidase activation in AGS cells, the cells were
pretreated with ML171, a specific inhibitor of NADPH oxidase 1, or
an antioxidant, NAC, for 1 h, and then treated with ASX (20 µM) for
2 h. The ASX-induced increase in ROS levels was decreased by
treatment with ML171 and NAC (Fig.
1E). As shown in Fig. 1F, ASX
increased NADPH oxidase activity, which was reduced by ML171 in AGS
cells. These results indicated that ASX induced NADPH oxidase
activation, leading to increased ROS levels in AGS cells.
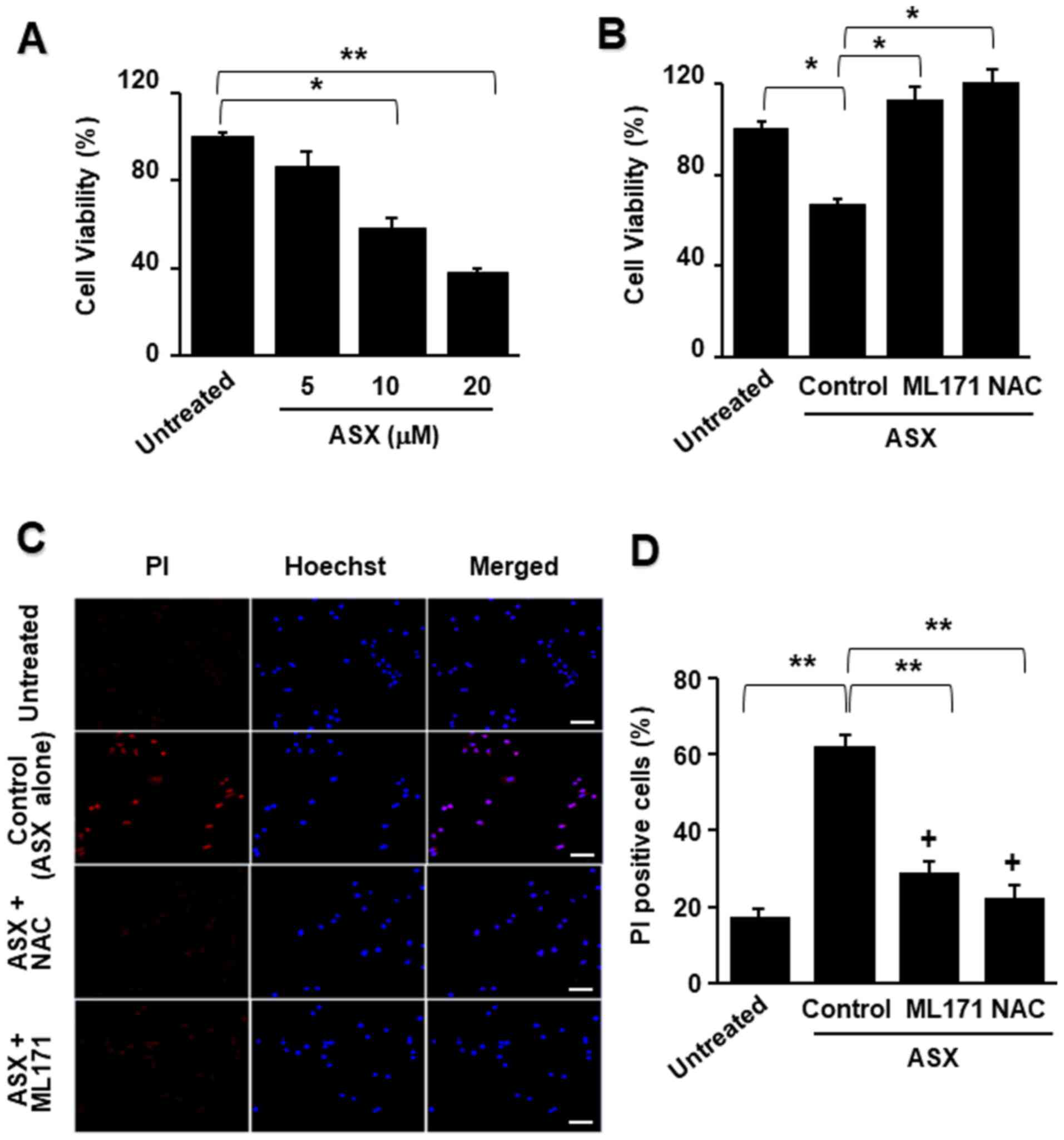
To examine whether ASX induces cell death, cell
viability was assessed after 24 h of culture with various
concentrations of ASX. ASX reduced cell viability in a
dose-dependent manner (Fig.
2A).
Further, to determine whether NADPH oxidase and ROS
induced ASX-associated cell death, the cells were pretreated with
ML171 or NAC for 1 h followed by treatment with ASX for 24 h. As
presented in Fig. 2B, both ML171
and NAC inhibited the ASX-induced decrease in cell viability.
To determine whether ML171 or NAC inhibited
ASX-induced necrosis in AGS cells, PI and Hoechst 33342 double
staining was performed (Fig. 2C).
The density ratio of the red and blue fluorescence was quantified
and expressed as PI-positive cells (%) (Fig. 2D). The number of necrotic AGS
cells increased after ASX treatment, as indicated by the increase
in the ratio of PI-positive cells. Both ML171 and NAC reduced the
number of PI-positive cells, indicating that NAC and ML171 blocked
ASX-induced necrotic cell death. These results suggested that ASX
induced necrosis, which may be mediated by NADPH oxidase and
increased ROS levels.
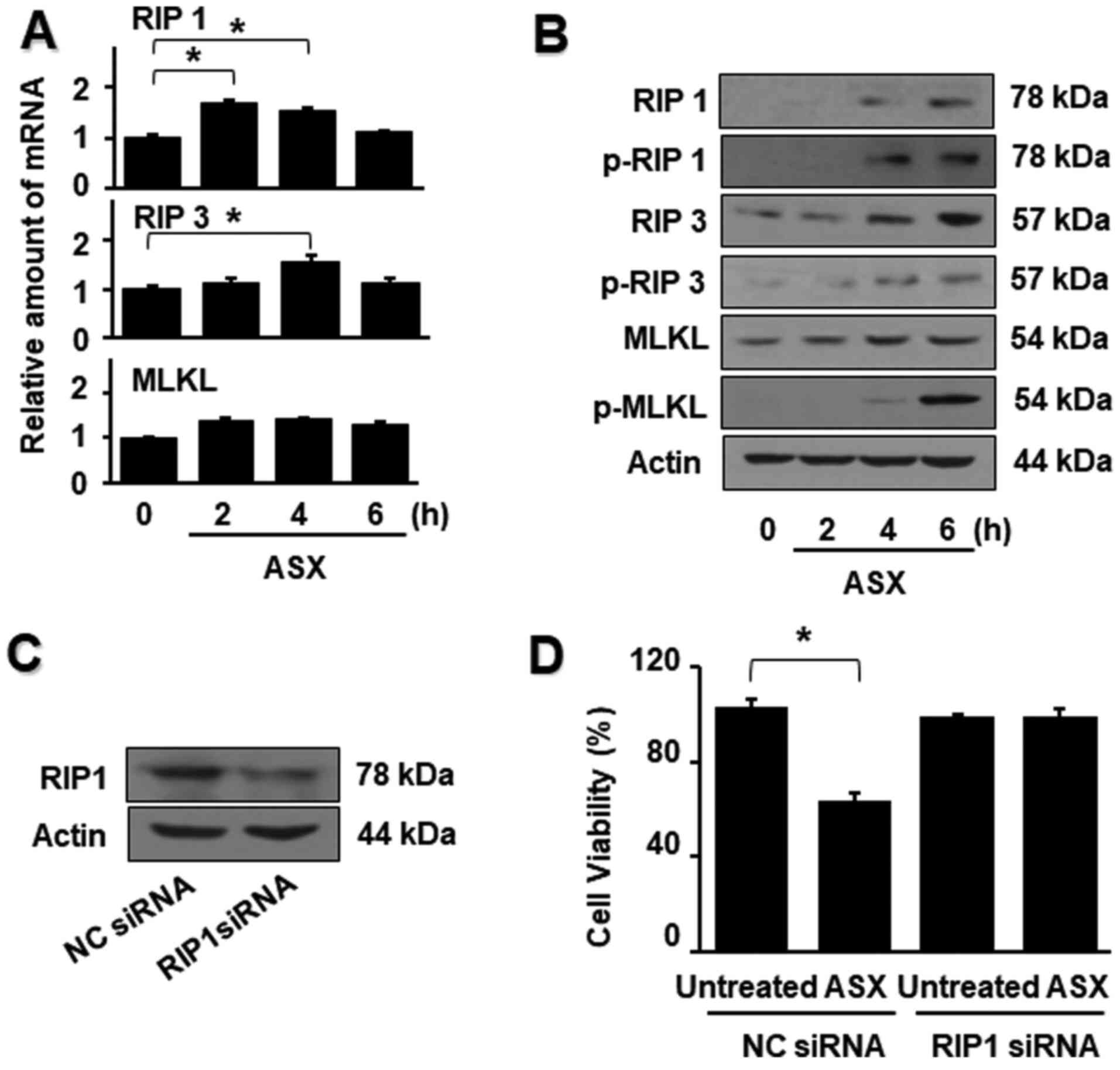
ASX activates necroptotic proteins in
AGS cells
During necroptosis, the mRNA and protein levels of
necroptotic proteins, including RIP1, RIP3 and MLKL, are
upregulated (33,34). In the present study, ASX
significantly increased the mRNA expression of RIP1 and RIP3 at 4 h
compared with the untreated group. However, there were no
significant changes in mRNA expression of MLKL by ASX treatment
(Fig. 3A).
Total and phospho-specific forms of RIP1, RIP3 and
MLKL were confirmed via western blotting to determine whether the
effect of ASX on cell death involves necroptosis (Fig. 3B). It was found that ASX increased
the protein expression of RIP1, RIP3 and MLKL. Moreover, ASX
augmented the activation of RIP1, RIP3 and MLKL, as indicated by
the increased levels of the phosphorylated forms of RIP1 and RIP3.
These results suggested the involvement of RIP1/RIP3/MLKL in
ASX-induced necroptosis in AGS cells.
To further investigate the role of RIP1 in
ASX-induced cell death, a gene silencing approach was used to
specifically knock down RIP1. To confirm the inhibitory effect of
RIP1 siRNA, the protein expression of RIP1 in AGS cells was
measured after transfection with RIP1 or NC siRNA. The protein
levels of RIP1 were notably suppressed in cells transfected with
RIP1 siRNA compared with those transfected with the NC siRNA
(Fig. 3C). ASX did not induce
cell death in cells transfected with RIP1 siRNA, whereas ASX
reduced the viability of cells transfected with the NC siRNA
compared with the untreated group (Fig. 3D). These results indicated that
ASX-induced cell death may occur through RIP1-mediated
necroptosis.
ASX-induced cell death is suppressed
by Nec-1, but not by z-VAD, in AGS cells
To determine whether ASX-induced cell death was
necroptotic, the cells were treated with Nec-1 or z-VAD for 1 h
prior to treatment with ASX for 24 h. Nec-1 is known to be a
selective inhibitor of RIP1 (35). As shown in Fig. 4A and B, ASX-induced cell death and
LDH release were inhibited by the necroptosis inhibitor Nec-1. A
pan-caspase inhibitor, z-VAD, as an inhibitor of apoptosis, did not
affect the ASX-induced increase in LDH release and cell death.
These results suggested that ASX may induce necroptosis, but not
apoptosis.
 | Figure 4.Cell viability, LDH release,
PI/Hoechst 33342 double staining, cleavage of caspase-9, ratio of
Bax/Bcl-2 and Annexin V-FITC/PI double staining of AGS cells
treated with ASX with or without Nec-1 or z-VAD. (A-D) Cells were
pretreated with 25 µM Nec-1 or 10 µM z-VAD for 1 h and then treated
with 20 µM ASX for 24 h. (A) The number of viable cells was
determined using the trypan blue exclusion test. (B) LDH activity
was measured in the culture medium and cells. The LDH release was
quantified as a percentage compared to the total LDH content (LDH
in the supernatant+ LDH inside the cells). The value (cell
viability or LDH release) of the cells that were not treated with
ASX (untreated) was set at 100%. (C) Nuclei were stained with
Hoechst 33342 (blue). Dead cells resulting from necrosis were
displayed in red, due to PI staining. Double staining of cells with
Hoechst 33342 and PI enabled us to distinguish between the typical
features of necrosis. Scale bar, 20 µm. (D) The ratio of red and
blue fluorescence density was quantified and expressed as
PI-positive cells (%). (E-G) AGS cells were treated with 20 µM ASX
for 24 h. (E) The expression levels of procaspase-9 and cleaved
caspase-9 were assessed via western blotting. Actin served as a
loading control. (F) Upper panel, levels of Bax, Bcl-2 and actin
were assessed via western blotting. Lower panel, ratio of Bax/Bcl-2
was determined based on the protein band densities of Bax and
Bcl-2. The ratio of Bax/Bcl-2 activity of cells that were not
treated with ASX (untreated) was considered to be 1. (G)
Fluorescence images of AGS cells were captured following Annexin
V-FITC/PI double staining. Scale bar, 20 µm. Data are presented as
the mean ± standard error of the mean. *P<0.05 and **P<0.01
as determined by one-way ANOVA followed by Tukey's test. LDH,
lactate dehydrogenase; PI, propidium iodide; ASX, astaxanthin;
Nec-1, Necrostatin-1. |
To determine whether ASX triggered necrotic cell
death in AGS cells, PI and Hoechst 33342 double staining was
performed (Fig. 4C). Hoechst
33342 is a bis-benzamide derivative that binds to AT-rich sequences
in the minor groove of double-stranded DNA. Thus, this fluorescent
stain is commonly used to visualize nuclei in microscopic studies
(27). The plasma
membrane-permeable dye PI can be detected in the necrotic cells. In
the present study, Hoechst 33342 stained the nuclei of all cells,
including healthy cells, producing blue fluorescence. However, live
or apoptotic cells are impermeable to PI, which intensely stains
necrotic cells red. As presented in Fig. 4C, AGS cells that underwent ASX
treatment showed more necrotic cells, as determined by the
increased ratio of PI-positive cells (Fig. 4D). Nec-1, a necroptosis inhibitor,
decreased the number of PI-positive cells. However, z-VAD did not
affect the ASX-induced increase in PI-positive cells, as determined
by the ratio of red to blue fluorescence. These results showed that
Nec-1 blocked ASX-induced necrotic cell death in AGS cells,
suggesting that ASX may induce necroptosis in AGS cells.
ASX does not induce apoptosis in AGS
cells
Caspase-9 activation amplifies the apoptosis cascade
by activation of caspase-3 and other apoptosis mediators (36). Thus, the effect of ASX on
caspase-9 activation in cells was determined. ASX did not increase
the levels of active caspase-9 (Fig.
4E). As an index of apoptosis, the ratio of Bax/Bcl-2 was
determined in cells treated with or without ASX (Fig. 4F). The Bax/Bcl-2 ratio was not
affected by ASX. To determine whether ASX triggered apoptotic or
necrotic cell death, double staining with Annexin V-FITC and PI was
performed (Fig. 4G). Annexin V is
a sensitive marker for detecting early apoptosis(30). Apoptotic
cells can be distinguished from necrotic cells by double staining
with PI. Annexin V-FITC and PI double staining easily distinguished
apoptotic cells (shown as green fluorescence) from necrotic cells
(shown as red fluorescence). ASX did not cause increased green
fluorescence, but resulted in increased red fluorescence,
indicating that ASX induced necrosis, but not apoptosis. Taken
together, these results indicated that ASX-mediated cell death was
related to necrosis/necroptosis.
Discussion
ASX shows anticancer effects by increasing or
decreasing ROS levels, depending on the cell type, ASX
concentration or redox state of the cancer cells. A number of
studies have demonstrated that ASX serves as an antioxidant because
it scavenges singlet oxygen and free radicals (37–39). However, a recent study showed that
high-dose administration (5, 15 and 30 mg•kg−1 body
weight) of ASX suppresses the activities of antioxidant enzymes
(catalase and glutathione peroxidase) in the muscle tissues of mice
(40).
As relatively few studies have determined the
pro-oxidative activity of ASX, the mechanisms underlying it are not
well defined. Previous research has shown that NADPH oxidase is a
major source of ROS (41,42). The present study found that ASX
increased NADPH oxidase, indicating that the source of ROS in
ASX-treated cells is NADPH oxidase.
High levels of ROS mediate necroptotic cell death by
increasing the phosphorylation of RIP1 and RIP3, which promotes
necrosome formation (43–45). Regarding ROS and activation of
RIP1/RIP3, RIP1 targets NADPH oxidase and increases ROS production
in the plasma membrane in TNF-α-treated mouse fibroblasts (46). ROS increase the expression of RIP1
and RIP3 and their reciprocal binding (44,47) and stabilize this interaction in
glioma cells stressed via photodynamic therapy (48). Therefore, ROS may regulate
necroptosis by increasing RIP1 and RIP3 protein levels and their
interactions. The current study showed that ASX-induced necroptosis
was mediated via NADPH oxidase and ROS, as well as the activation
of RIP1-RIP3, as Nec-1 (a specific inhibitor of RIP1), NAC (an
antioxidant) and ML171 (an NADPH oxidase inhibitor) significantly
suppressed the number of PI-positive AGS cells.
RIP3 binds to the plasma membrane and induces the
phosphorylation of MLKL, which induces the oligomerization of
p-MLKL to induce plasma membrane rupture and necroptosis (49). In the present study, MLKL was
activated during ASX-induced necroptosis in AGS cells. The dead
cells resulting from necrosis appeared red due to the PI staining.
Hoechst 33342 and PI double staining allowed us to distinguish
between the typical features of necrosis. Based on the PI and
Hoechst 33342 staining results, ASX increased the number of
PI-positive cells, which were inhibited by treatment with NAC,
ML171 and Nec-1. However, z-VAD did not affect the ASX-induced
increase in PI-positive cells. These results demonstrated that
ASX-induced cell death occurred via necroptosis. Moreover, ASX did
not induce caspase-9 activation. Moreover, in Annexin V/PI double
staining, ASX did not increase the number of Annexin-positive
cells, but increased the number of PI-positive cells at 24 h. These
findings showed that ASX did not induce apoptosis, but did induce
necrotic cell death.
In the present study, it was found that ASX induced
an increase in NADPH oxidase activity, ROS production and
phosphorylation of RIP1/RIP3/MLKL, leading to cell death in AGS
cells. Treatment with the NADPH oxidase 1 inhibitor ML171
suppressed ASX-induced ROS production. High levels of ROS induce
mitochondrial dysfunction and increase mitochondrial ROS levels
(50,51). Moreover, treatment with ML171,
antioxidant NAC and Nec-1, a specific inhibitor of RIP1, and
transfection with RIP1 siRNA suppressed ASX-induced necroptosis.
Therefore, it was concluded that NADPH oxidase is important in the
production of ROS and, consequently, in ROS-mediated activation of
RIP1-RIP3-MLK and necroptosis in gastric cancer AGS cells (Fig. 5).
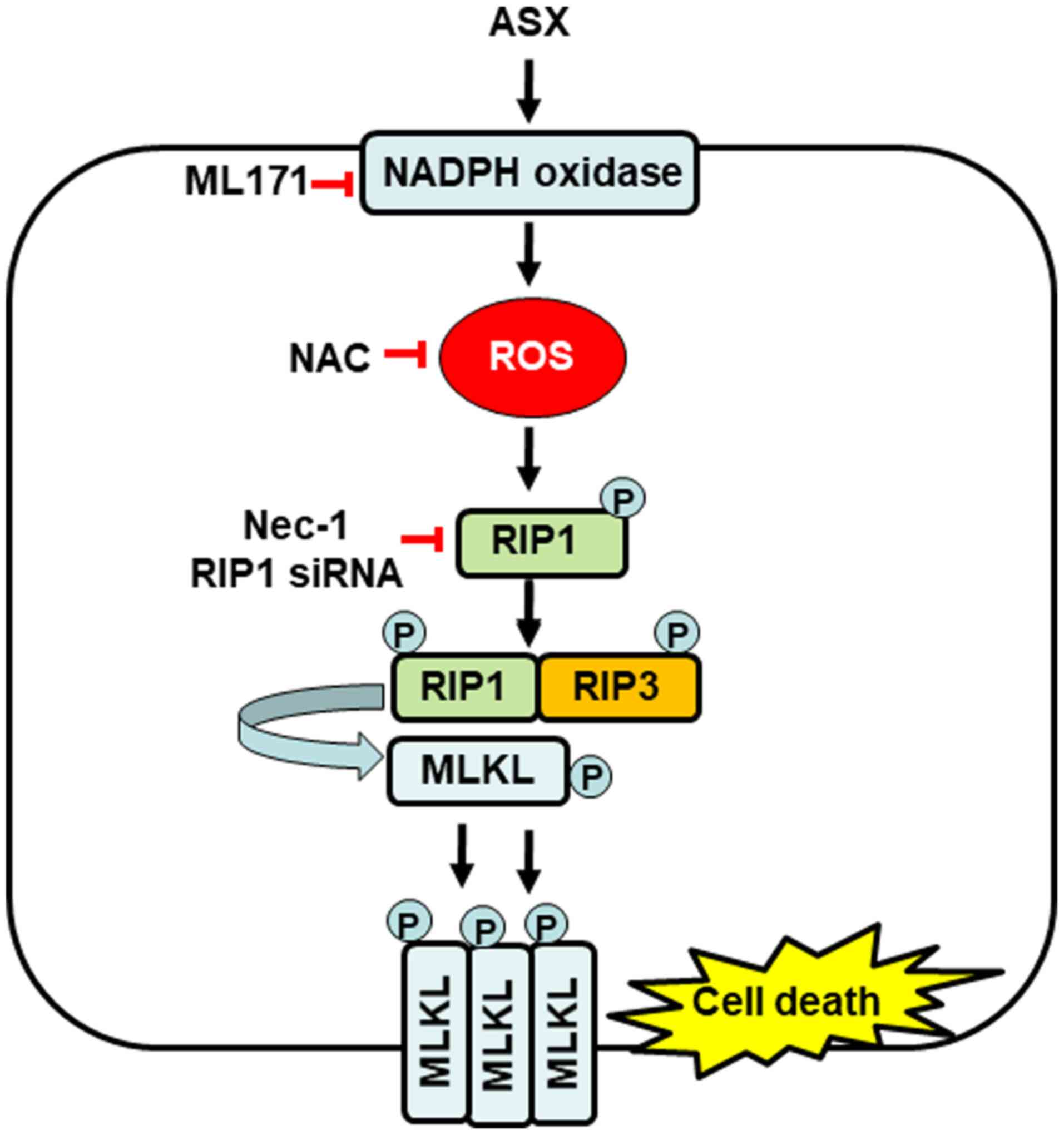 | Figure 5.Proposed mechanism by which ASX
induces necroptosis in gastric cancer AGS cells. High levels of ASX
(20 µM) may activate NADPH oxidase and increase the production of
ROS, which induce the phosphorylation of RIP1, thus, activating
RIP3 and MLKL. Activated MLKL is translocated to the plasma
membrane, where it possibly ruptures the cell membrane and causes
necroptosis. Treatment with NADPH oxidase inhibitor ML171,
antioxidant NAC and Nec-1, a specific inhibitor of RIP1, suppresses
ASX-induced necroptosis in gastric cancer AGS cells. ASX,
astaxanthin; ROS, reactive oxygen species; RIP,
receptor-interacting protein kinase; MLKL, mixed lineage kinase
domain-like protein; p-, phosphorylated; NAC, N-acetyl cysteine;
Nec-1, Necrostatin-1; siRNA, small interfering RNA. |
Regarding the effect of ASX on normal cell
viability, the current study did not find any effect on cell
viability and NADPH oxidase activity in normal RGM-1 cells.
Depending on their concentration and oxygen tension, carotenoids
have been reported to act as antioxidants or pro-oxidants. The
pro-oxidant activity of carotenoids is shown at increased
concentrations under high oxygen tension (52,53). Therefore, ASX-induced activation
of NADPH oxidase and cell death may be affected by high
concentrations of ASX and cellular environmental events in AGS
cells.
For bioavailability of ASX in humans, 6
day-consumption of ASX (daily consumption of 250 g salmon; 5 µg ASX
per gram of salmon flesh) results in plasma ASX concentrations of
39–52 nM (54). Furthermore,
12-week supplementation of 1 or 3 mg ASX (capsules containing
ASX-rich oil) results in plasma concentrations of 18.9 and 62.4 nM,
respectively, in Japanese middle-aged and senior subjects (55). Therefore, the concentration of ASX
(20 µM) used in the present study is much higher than the
concentration that results from dietary intake. These results
suggested that a high concentration of ASX should be used to induce
gastric cell necroptosis. Further studies should be performed to
determine whether high concentrations of ASX induces necroptosis in
various cancer cell lines in vitro and in vivo.
In the present study, AGS cells were used to
determine the anticancer effects of ASX. The gastric adenocarcinoma
AGS cell line is derived from untreated human adenocarcinoma of the
stomach. The AGS cell line is a moderately differentiated human
gastric adenocarcinoma hyperdiploid cell line. The AGS cell line
has been shown to grow in athymic mice (56) and retain the same histochemical
and cytological characteristics as the original malignant cells
obtained from the patient (57).
It is important to characterize human tumor cells in vitro
in a detailed manner, as they serve as useful model systems for
studies involving heterogeneous responses to anticancer drugs
(57). Recently, this cell line
has been widely used as a model system for evaluating cancer cell
apoptosis (58–60).
AGS cells have more genetic changes and higher
growth rates than normal cells (60,61). One example is tripartite motif
containing 25 (TRIM25), a member of TRIM proteins that has been
implicated in carcinogenesis. AGS cells show high expression of
TRIM25, which contributes to higher growth rates compared with
normal gastric epithelial cells (61). AGS cells exhibit abnormal
expression of microRNA-372 (miR-372) (62). miR-372 maintains oncogene
characteristics by targeting tumor necrosis factor-α-induced
protein 1-regulated AGS cell growth (63). In a study by Guan et al
(64) expression of the oncogene
HER-2 in AGS cells was found to be twice that in normal gastric
epithelial cells.
In addition, it is known that ROS are produced by
several nutrients, such as vitamin C, zeaxanthin and β-carotene,
which induce cell death in AGS cells (65–67). Therefore, in the present study,
AGS cells were used to determine the anticancer mechanism of
ASX.
In a study by Dong et al (68), Nec-1 and NADPH oxidase inhibitor
diphenyleneiodonium prevent tumor necrosis factor-α,
benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone and antimycin A
(collectively termed TZA)-induced necroptosis by inhibiting the
phosphorylation of RIP3 and MLKL in HK-2 cells. Recent studies have
demonstrated that NAC suppresses necroptosis induced by high
concentrations of iron or glucose through the inhibition of RIP1
phosphorylation (69,70). These studies suggest that NADPH
oxidase-mediated ROS production may induce necroptosis through
phosphorylation of RIP1, RIP3 and MLKL (69,70). Further studies are needed to
determine whether treatment with ML171, NAC or Nec-1 suppresses
ASX-induced expression and activation of RIP1, RIP3 and MLKL in AGS
cells.
In conclusion, ASX increased intracellular ROS
levels by activating NADPH oxidase, thereby, inducing necroptosis
in gastric cancer AGS cells. NADPH oxidase-mediated ROS production
may represent an underlying mechanism of ASX-induced necroptosis in
gastric cancer AGS cells. Consequently, ASX could be used to treat
gastric cancer based on its role in necroptotic signaling.
Acknowledgements
Not applicable.
Funding
This study was financially supported by a grant from
the National Research Foundation of Korea, funded by the Korean
Government (grant no. NRF-2021R1A2B5B02002353).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HK conceived and designed the experiments. JWL
assisted in designing the experiment. SK and HL performed the
experiments. SK and JWL analyzed the data. JWL and HK confirm the
authenticity of all raw data. SK wrote the paper. HK reviewed and
edited the paper. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yamashita E: Astaxanthin as a medical
food. Funct Food Health Dis. 3:254–258. 2013. View Article : Google Scholar
|
|
2
|
Kidd P: Astaxanthin, cell membrane
nutrient with diverse clinical benefits and anti-aging potential.
Altern Med Rev. 16:355–364. 2011.PubMed/NCBI
|
|
3
|
Zhang L and Wang H: Multiple mechanisms of
anti-cancer effects exerted by astaxanthin. Mar Drugs.
13:4310–4330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fassett RG and Coombes JS: Astaxanthin: A
potential therapeutic agent in cardiovascular disease. Mar Drugs.
9:447–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Dai W, Xia Y, Chen K, Li S, Liu T,
Zhang R, Wang J, Lu W, Zhou Y, et al: Astaxanthin inhibits
proliferation and induces apoptosis of human hepatocellular
carcinoma cells via inhibition of NF-κB P65 and Wnt/B-catenin in
vitro. Mar Drugs. 13:6064–6081. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JH, Park JJ, Lee BJ, Joo MK, Chun HJ,
Lee SW and Bak YT: Astaxanthin inhibits proliferation of human
gastric cancer cell lines by interrupting cell cycle progression.
Gut Liver. 10:369–374. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hormozi M, Ghoreishi S and Baharvand P:
Astaxanthin induces apoptosis and increases activity of antioxidant
enzymes in LS-180 cells. Artif Cells Nanomed Biotechnol.
47:891–895. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kochi T, Shimizu M, Sumi T, Kubota M,
Shirakami Y, Tanaka T and Moriwaki H: Inhibitory effects of
astaxanthin on azoxymethane-induced colonic preneoplastic lesions
in C57/BL/KsJ-db/db mice. BMC Gastroenterol. 14:2122014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanasawet S, Sukketsiri W,
Chonpathompikunlert P, Klaypradit W, Sroyraya M and Hutamekalin P:
Apoptotic effect of astaxanthin from white shrimp shells on lung
cancer A549 cells. Trop J Pharm Res. 19:1835–1842. 2020. View Article : Google Scholar
|
|
10
|
Sowmya PR, Arathi BP, Vijay K, Baskaran V
and Lakshminarayana R: Astaxanthin from shrimp efficiently
modulates oxidative stress and allied cell death progression in
MCF-7 cells treated synergistically with β-carotene and lutein from
greens. Food Chem Toxicol. 106:58–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen D, Yu J and Zhang L: Necroptosis: An
alternative cell death program defending against cancer. Biochim
Biophys Acta. 1865:228–236. 2016.PubMed/NCBI
|
|
12
|
Feng X, Song Q, Yu A, Tang H, Peng Z and
Wang X: Receptor-interacting protein kinase 3 is a predictor of
survival and plays a tumor suppressive role in colorectal cancer.
Neoplasma. 62:592–601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He S, Liang Y, Shao F and Wang X:
Toll-like receptors activate programmed necrosis in macrophages
through a receptor-interacting kinase-3-mediated pathway. Proc Natl
Acad Sci USA. 108:20054–20059. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ch'en IL, Tsau JS, Molkentin JD, Komatsu M
and Hedrick SM: Mechanisms of necroptosis in T cells. J Exp Med.
208:633–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu JV, Chen HC and Walsh CM: Necroptotic
signaling in adaptive and innate immunity. Semin Cell Dev Biol.
35:33–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vanden Berghe T, Linkermann A,
Jouan-Lanhouet S, Walczak H and Vandenabeele P: Regulated necrosis:
The expanding network of non-apoptotic cell death pathways. Nat Rev
Mol Cell Biol. 15:135–147. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mifflin L, Ofengeim D and Yuan J:
Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic
target. Nat Rev Drug Discov. 19:553–571. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Liu T, Lei T, Zhang D, Du S, Girani
L, Qi D, Lin C, Tong R and Wang Y: RIP1/RIP3-regulated necroptosis
as a target for multifaceted disease therapy (Review). Int J Mol
Med. 44:771–786. 2019.PubMed/NCBI
|
|
19
|
Lalaoui N and Brumatti G: Relevance of
necroptosis in cancer. Immunol Cell Biol. 95:137–145. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon
JH, Koo JS, Kim SI, Kim SJ, Son MK, Hong SS, et al:
Methylation-dependent loss of RIP3 expression in cancer represses
programmed necrosis in response to chemotherapeutics. Cell Res.
25:707–725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun W, Yu W, Shen L and Huang T: MLKL is a
potential prognostic marker in gastric cancer. Oncol Lett.
18:3830–3836. 2019.PubMed/NCBI
|
|
22
|
Zhang Y, Su SS, Zhao S, Yang Z, Zhong CQ,
Chen X, Cai Q, Yang ZH, Huang D, Wu R, et al: RIP1
autophosphorylation is promoted by mitochondrial ROS and is
essential for RIP3 recruitment into necrosome. Nat Commun.
8:143292017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moriwaki K and Chan FKM: RIP3: A molecular
switch for necrosis and inflammation. Genes Dev. 27:1640–1649.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Skonieczna M, Hejmo T, Poterala-Hejmo A,
Cieslar-Pobuda A and Buldak RJ: NADPH oxidases: Insights into
selected functions and mechanisms of action in cancer and stem
cells. Oxid Med Cell Longev. 2017:94205392017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oparka M, Walczak J, Malinska D, van Oppen
LM, Szczepanowska J, Koopman WJ and Wieckowski MR: Quantifying ROS
levels using CM-H2DCFDA and HyPer. Methods. 109:3–11. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee J, Lim JW and Kim H: Lycopene inhibits
oxidative stress-mdiated inflammatory responses in
ethanol/palmitoleic acid-stimulatd pancreatic acinar AR42J cells.
Int J Mol Sci. 22:21012021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Minkenberg I and Ferber E:
Lucigenin-dependent chemiluminescence as a new assay for
NAD(P)H-oxidase activity in particulate fractions of human
polymorphonuclear leukocytes. J Immunol Methods. 71:61–67. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Crowley LC, Marfell BJ and Waterhouse NJ:
Analyzing cell death by nuclear staining with Hoechst 33342. Cold
Spring Harb Protoc. Sep 1–2016.(Epub ahead of print). doi:
10.1101/pdb.prot087205. View Article : Google Scholar
|
|
30
|
Sawai H and Domae N: Discrimination
between primary necrosis and apoptosis by necrostatin-1 in Annexin
V-positive/propidium iodide-negative cells. Biochem Biophys Res
Commun. 411:569–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan FK-M, Moriwaki K and De Rosa MJ:
Detection of necrosis by release of lactate dehydrogenase activity.
Methods Mol Biol. 979:65–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
López E, Figueroa S, Oset-Gasque MJ and
González MP: Apoptosis and necrosis: Two distinct events induced by
cadmium in cortical neurons in culture. Br J Pharmacol.
138:901–911. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu K, Liang W, Ma Z, Xu D, Cao S, Lu X,
Liu N, Shan B, Qian L and Yuan J: Necroptosis promotes
cell-autonomous activation of proinflammatory cytokine gene
expression. Cell Death Dis. 9:5002018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoon S, Kovalenko A, Bogdanov K and
Wallach D: MLKL, the protein that mediates necroptosis, also
regulates endosomal trafficking and extracellular vesicle
generation. Immunity. 47:51–65.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Christofferson DE, Li Y, Hitomi J, Zhou W,
Upperman C, Zhu H, Gerber SA, Gygi S and Yuan J: A novel role for
RIP1 kinase in mediating TNFα production. Cell Death Dis.
3:e3202012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McDonnell MA, Wang D, Khan SM, Vander
Heiden MG and Kelekar A: Caspase-9 is activated in a cytochrome
c-independent manner early during TNFalpha-induced apoptosis in
murine cells. Cell Death Differ. 10:1005–1015. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dose J, Matsugo S, Yokokawa H, Koshida Y,
Okazaki S, Seidel U, Eggersdorfer M, Rimbach G and Esatbeyoglu T:
Free radical scavenging and cellular antioxidant properties of
astaxanthin. Int J Mol Sci. 17:1032016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kogure K: Novel Antioxidative activity of
astaxanthin and its synergistic effect with vitamin E. J Nutr Sci
Vitaminol (Tokyo). 65 (Suppl 1):S109–S112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Landon R, Gueguen V, Petite H, Letourneur
D, Pavon-Djavid G and Anagnostou F: Impact of astaxanthin on
diabetes pathogenesis and chronic complications. Mar Drugs.
18:3572020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou Y, Baker JS, Chen X, Wang Y, Chen H,
Davison GW and Yan X: High-dose astaxanthin supplementation
suppresses antioxidant enzyme activity during moderate-intensity
swimming training in mice. Nutrients. 11:12442019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rastogi R, Geng X, Li F and Ding Y: NOX
activation by subunit interaction and underlying mechanisms in
disease. Front Cell Neurosci. 10:3012017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Emanuele S, D'Anneo A, Calvaruso G,
Cernigliaro C, Giuliano M and Lauricella M: The double-edged sword
profile of redox signaling: Oxidative events as molecular switches
in the balance between cell physiology and cancer. Chem Res
Toxicol. 31:201–210. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jia Y, Wang F, Guo Q, Li M, Wang L, Zhang
Z, Jiang S, Jin H, Chen A, Tan S, et al: Curcumol induces
RIPK1/RIPK3 complex-dependent necroptosis via JNK1/2-ROS signaling
in hepatic stellate cells. Redox Biol. 19:375–387. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ,
Lin SC, Dong MQ and Han J: RIP3, an energy metabolism regulator
that switches TNF-induced cell death from apoptosis to necrosis.
Science. 325:332–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Belizário J, Vieira-Cordeiro L and Enns S:
Necroptotic cell death signaling and execution pathway: Lessons
from knockout mice. Mediators Inflamm. 2015:1280762015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim YS, Morgan MJ, Choksi S and Liu ZG:
TNF-induced activation of the Nox1 NADPH oxidase and its role in
the induction of necrotic cell death. Mol Cell. 26:675–687. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cho YS, Challa S, Moquin D, Genga R, Ray
TD, Guildford M and Chan FK: Phosphorylation-driven assembly of the
RIP1-RIP3 complex regulates programmed necrosis and virus-induced
inflammation. Cell. 137:1112–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lu B, Gong X, Wang ZQ, Ding Y, Wang C, Luo
TF, Piao MH, Meng FK, Chi GF, Luo YN, et al: Shikonin induces
glioma cell necroptosis in vitro by ROS overproduction and
promoting RIP1/RIP3 necrosome formation. Acta Pharmacol Sin.
38:1543–1553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Chen X, Gueydan C and Han J:
Plasma membrane changes during programmed cell deaths. Cell Res.
28:9–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Peoples JN, Saraf A, Ghazal N, Pham TT and
Kwong JQ: Mitochondrial dysfunction and oxidative stress in heart
disease. Exp Mol Med. 51:1–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tapiero H, Townsend DM and Tew KD: The
role of carotenoids in the prevention of human pathologies. Biomed
Pharmacother. 58:100–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shin J, Song MH, Oh JW, Keum YS and Saini
RK: Pro-oxidant actions of carotenoids in triggering apoptosis of
cancer cells: A review of emerging evidence. Antioxidants.
9:5322020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rüfer CE, Moeseneder J, Briviba K,
Rechkemmer G and Bub A: Bioavailability of astaxanthin
stereoisomers from wild (Oncorhynchus spp.) and aquacultured
(Salmo salar) salmon in healthy men: A randomised,
double-blind study. Br J Nutr. 99:1048–1054. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Miyazawa T, Nakagawa K, Kimura F, Satoh A
and Miyazawa T: Plasma carotenoid concentrations before and after
supplementation with astaxanthin in middle-aged and senior
subjects. Biosci Biotechnol Biochem. 75:1856–1858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Barati T, Haddadi M, Sadeghi F,
Muhammadnejad S, Muhammadnejad A, Heidarian R, Arjomandnejad M and
Amanpour S: AGS cell line xenograft tumor as a suitable gastric
adenocarcinoma model: Growth kinetic characterization and
immunohistochemistry analysis. Iran J Basic Med Sci. 21:678–681.
2018.PubMed/NCBI
|
|
57
|
Barranco SC, Townsend CM Jr, Casartelli C,
Macik BG, Burger NL, Boerwinkle WR and Gourley WK: Establishment
and characterization of an in vitro model system for human
adenocarcinoma of the stomach. Cancer Res. 43:1703–1709.
1983.PubMed/NCBI
|
|
58
|
Park HS, Hong NR, Ahn TS, Kim H, Jung MH
and Kim BJ: Apoptosis of AGS human gastric adenocarcinoma cells by
methanolic extract of Dictamnus. Pharmacogn Mag. 11 (Suppl
2):S329–S336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tsai CL, Chiu YM, Ho TY, Hsieh CT, Shieh
DC, Lee YJ, Tsay GJ and Wu YY: Gallic Acid induces apoptosis in
human gastric adenocarcinoma cells. Anticancer Res. 38:2057–2067.
2018.PubMed/NCBI
|
|
60
|
Ngabire D, Seong YA, Patil MP,
Niyonizigiye I, Seo YB and Kim GD: Induction of apoptosis and G1
phase cell cycle arrest by Aster incisus in AGS gastric
adenocarcinoma cells. Int J Oncol. 53:2300–2308. 2018.PubMed/NCBI
|
|
61
|
Zhu Z, Wang Y, Zhang C, Yu S, Zhu Q, Hou K
and Yan B: TRIM25 blockade by RNA interference inhibited migration
and invasion of gastric cancer cells through TGF-β signaling. Sci
Rep. 6:190702016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li X, Zhang X, Liu X, Tan Z, Yang C, Ding
X, Hu X, Zhou J, Xiang S, Zhou C, et al: Caudatin induces cell
apoptosis in gastric cancer cells through modulation of
Wnt/β-catenin signaling. Oncol Rep. 30:677–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhou C, Li X, Zhang X, Liu X, Tan Z, Yang
C and Zhang J: microRNA-372 maintains oncogene characteristics by
targeting TNFAIP1 and affects NFκB signaling in human gastric
carcinoma cells. Int J Oncol. 42:635–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Guan SS, Chang J, Cheng CC, Luo TY, Ho AS,
Wang CC, Wu CT and Liu SH: Afatinib and its encapsulated polymeric
micelles inhibits HER2-overexpressed colorectal tumor cell growth
in vitro and in vivo. Oncotarget. 5:4868–4880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lim JY, Kim D, Kim BR, Jun JS, Yeom JS,
Park JS, Seo JH, Park CH, Woo HO, Youn HS, et al: Vitamin C induces
apoptosis in AGS cells via production of ROS of mitochondria. Oncol
Lett. 12:4270–4276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sheng YN, Luo YH, Liu SB, Xu WT, Zhang Y,
Zhang T, Xue H, Zuo WB, Li YN, Wang CY, et al: Zeaxanthin induces
apoptosis via ROS-regulated MAPK and AKT signaling pathway in human
gastric cancer cells. OncoTargets Ther. 13:10995–11006. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Park Y, Choi J, Lim JW and Kim H:
β-Carotene-induced apoptosis is mediated with loss of Ku proteins
in gastric cancer AGS cells. Genes Nutr. 10:4672015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Dong W, Li Z, Chen Y, Zhang L, Ye Z, Liang
H, Li R, Xu L, Zhang B, Liu S, et al: NADPH oxidase inhibitor,
diphenyleneiodonium prevents necroptosis in HK-2 cells. Biomed Rep.
7:226–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tian Q, Qin B, Gu Y, Zhou L, Chen S, Zhang
S, Zhang S, Han Q, Liu Y and Wu X: ROS-mediated necroptosis is
involved in iron overload-induced osteoblastic cell death. Oxid Med
Cell Longev. 2020:12953822020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Deragon MA, McCaig WD, Patel PS, Haluska
RJ, Hodges AL, Sosunov SA, Murphy MP, Ten VS and LaRocca TJ:
Mitochondrial ROS prime the hyperglycemic shift from apoptosis to
necroptosis. Cell Death Discov. 6:1322020. View Article : Google Scholar : PubMed/NCBI
|