Introduction
Estrogens are hormones involved in a number of
physiological functions both pre- and postnatally, including brain
development, growth, reproduction and metabolism. The primary
members of the estrogen family include estrone (E1), estriol and
17β-estradiol (17β-E2), which are primarily produced through the
aromatization of testosterone in the granulosa cells in the ovaries
and peripheral tissues such as the placenta, adipose tissue,
osteoblasts, brain and smooth muscle cells (1,2).
Steroidogenesis begins in the ovarian theca cells where cholesterol
is converted to androgens, and is completed by the ovarian
granulosa cells through the conversion of these androgens to
estrogens. Estrogen synthesis begins with the conversion of
cholesterol into pregnenolone by the P450 side chain cleavage
enzyme. Pregnenolone is then converted into progesterone by
3-β-hydroxysteroid dehydrogenase (3β-HSD), or into
17-hydroxypregnenolone by 17-α-hydroxylase/17,20 lyase (CYP17).
Progesterone is then converted into 17-hydroxyprogesterone by the
CYP17 enzyme, whereas 17-hydroxypregnenolone is converted into
17-hydroxyprogesterone by 3β-HSD. Next, CYP17 converts
17-hydroxypregnenolone and 17-hydroxyprogesterone into
dehydroepiandrosterone (DHEA) and androstenedione, respectively
(1,2). Next, different 17β-HSD enzymes
catalyze the synthesis of androstenediol from DHEA and testosterone
from androstenedione. Finally, in the granulosa cells of the
ovaries, the enzyme aromatase converts androstenedione and
testosterone into E1 and E2, respectively (1,2).
Another enzyme, 5α-reductase, converts testosterone into the potent
androgen 5α-dihydrotestosterone. In men, estrogens are primarily
produced in the peripheral tissues and, to a lesser extent in the
testes by the aromatization of testosterone (1,2). The
primary biologically active estrogen is 17β-E2, which demonstrates
a high affinity for estrogen nuclear receptors and regulates vital
physiological processes both pre- and postnatally. Estrone is a
less active estrogen given its low affinity to estrogen receptors
(ERs) and predominates in peri- and post-menopausal women. Estriol
is primarily secreted by the placenta during pregnancy and has a
weak affinity to ERs (1).
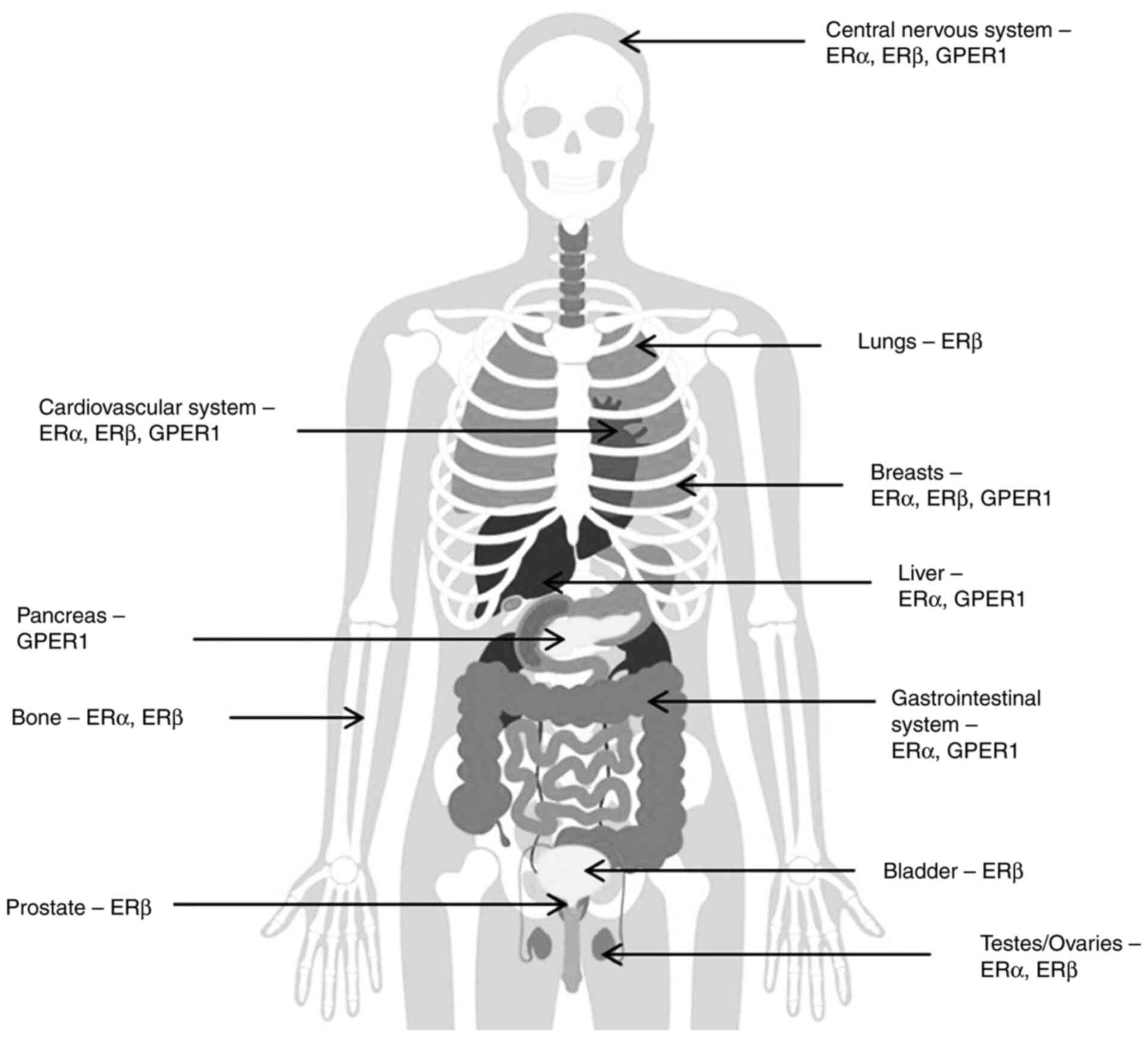
ERα, ERβ and G-protein coupled ER1 (GPER1) are the
primary ERs and are expressed in numerous target tissues. ERα is
primarily present in breast tissues, bones, the uterus, thecal
ovarian cells, testes, epididymis, the prostate and the liver. ERβ
is expressed in the bladder, granulosa ovarian cells, prostate
epithelium, the colon and immune system cells. In adipose tissue
and to a greater extent, in the brain and cardiovascular tissues,
both ERα and ERβ are expressed (3,4)
(Fig. 1). Expression of GPER1 has
been detected in multiple tissues, including in the endometrium,
breast, ovaries, brain, adrenal glands, kidneys, vasculature and
heart endothelium (5). More recent
studies on mice and in vitro have reported the homeostatic
role of GPER1 in physiological actions such as energy homeostasis,
cardiovascular function, bone and cartilage development, immune
system response and neurodevelopment and neurotransmission
(6). Any defects in ER signaling
lead to a number of diseases, including those affecting growth and
puberty, fertility and reproduction abnormalities, cancer,
metabolic diseases, osteoporosis and neurodegeneration (4).
Estrogens and ERs exert their effects via genomic
and non-genomic processes. Estrogens bind to ERs to cause ER
dimerization and translocation to the nucleus, where they function
with specific auxiliary elements known as estrogen response
elements (EREs) located in the promoter regions of target genes.
Alternatively, ERs also act via kinases and other molecular
interactions leading to gene expression and functions through a
non-genomic mechanism (7–10). The actions of ERα and ERβ
antagonize each other in specific tissues such as the bones,
breasts and brain, and in prostate cancer cells (8–12).
ERβ has isoforms that can act separately and by different actions
on specific tissues (11). For
instance, the ERβ isoforms ERβ2 and ERβ5 inhibit ERα in breast
cancer cells, exerting a protective role (8,10,12).
Likewise, in breast cancer T47D cells, ERβ expression is
upregulated compared with ERα (10). In addition, in the bone, ERα is
primarily expressed in the cortical bone, whereas ERβ is expressed
mostly in the trabecular bone (11).
ERs: Location, structure and function
Location and structure
Estrogen nuclear receptors belong to the nuclear
receptor superfamily and thus have similar structural properties to
thyroid and steroid hormone receptors. ERα consists of 595 amino
acids and is encoded by the ESR1 gene located on chromosome
6 (6q25.1) (12). ERβ is 530 amino
acids long and is encoded by the ESR2 gene on chromosome 14
(14q23.2) (13). ER, similar to
all the other members of the nuclear receptor family of proteins is
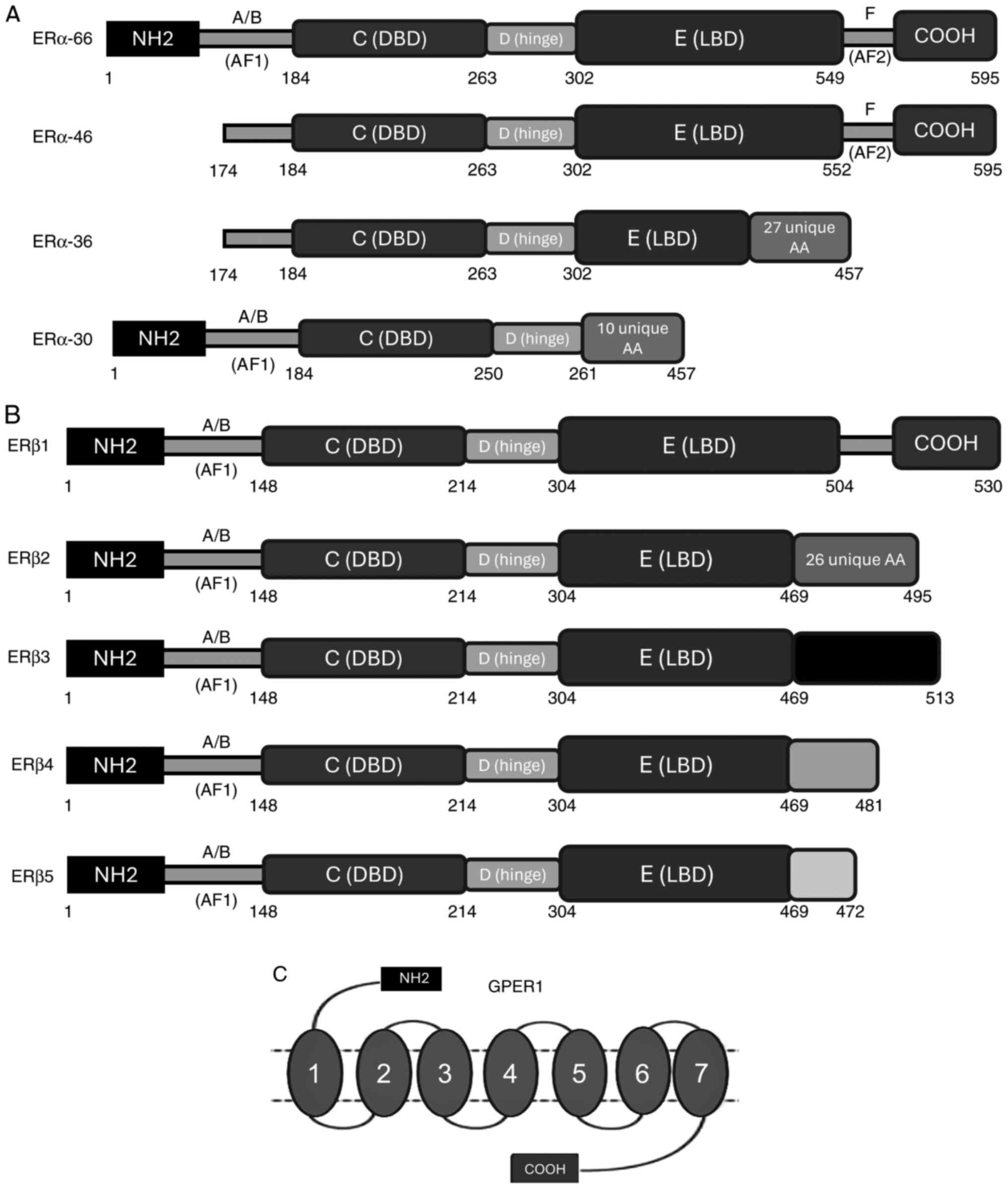
domain-structured. Specifically, ER consists of six domains: A, B,
C, D, E and F (Fig. 2). The A and
B domains, also referred to as the N-terminal domain, connects and
activates the DNA transactivation function domain [activation
function 1 (AF-1)]. The A and B domains are associated with
receptor specificity and contribute to the transcriptional
activation of target genes; they are also required for
ligand-independent transcriptional regulation (12–15).
Domain C, also known as the DNA-binding domain, is a highly
preserved domain that enhances the DNA-binding ability of ER
(12–15). Two zinc fingers are localized on
the C domain, which interact with specific hormonal response
elements, EREs, leading to receptor dimerization. Domain D is the
hinge region between domains C and E, which binds to heat shock
proteins. Domain D also acts to stabilize the DNA-binding function
of the C domain and provides the receptor with flexibility in
binding to EREs (14,15). Finally, the E and F domains are the
ligand-binding domains (LBD), which are located at the C-terminus.
The E and F domains exhibit a complex monitoring ligand-dependent
action through the transcriptional activation function domain
(AF-2) and coactivators in Helix 12. Helix 12 serves a key role in
the LBD, modifying its structure when bound to a ligand; this
results in the active form when ER is bound to an agonist, or the
inactive form when bound to an antagonist, and this structural
change modulates the transcriptional regulation (14,15).
Finally, GPER1, previously known as GPR30, consists of 375 amino
acids and is located on chromosome 7 (7p22.3). GPER1 consists of
seven transmembrane α-helices, which form four extracellular
segments and four cytosolic segments. The binding affinity of GPER1
to estrogen is weaker than that of the nuclear ERs. GPER1 was
discovered 20 years ago and the details of its exact interactions
with estrogens and other steroid hormones remain to be determined
(16).
Different isoforms of ERα arise due to gene splicing
(Fig. 2). ERα isoforms include
ERα-46 and ERα-36, which are the primary reactive estrogen isoforms
(17) ERα-36 is formed due to the
presence of an alternative transcription site at the first intron
and lacks AF1 and AF2 domains. ERα-36 contains exons 2–6 of the
wild-type EΡα and exon 9 (27 amino acids at the C-terminal)
(18,19). ERα-46 is another ERα variant
lacking exon 1; it contains all exons of the wild-type receptor
(18). Numerous variants of ERβ
have also been identified, including ERβ2, ERβ4 and ERβ5, which
lack a different C-terminal sequence in order to bind to different
EREs (20,21).
ERβ1 consists of 530 amino acids and is the
full-length version of wild-type ERβ, whereas ERβ2, 3, 4 and 5 have
a single LBD sequence. These differences result in a shorter LBD
and a lack of AF2 domain function. ERβ1 is the only isoform that
can bind to an estrogen-ligand, the remaining truncated ERβ
isoforms have no estrogen-binding ability (20).
Function
Estrogens exert their effects upon binding to their
receptors via complex genomic and non-genomic mechanisms (Fig. 3) (22). The mechanisms of ER expression and
action are complex. Both ERα and ERβ are activated via AF-1 and
AF-2 domains, which are located in the N-terminal domain and LBD,
respectively, and mediate synergistic transcriptional regulation
(14,23). The genomic action of ERs regulates
the transcription of numerous target genes involved in the growth
and differentiation of the cell. When estrogen hormones bind to
ERα, the receptor is activated, dimerizes and translocates to the
nucleus where it interacts with transcriptional coactivators. The
activated form of ERα targets the promoters of the target genes
binding to EREs. Moreover, ERα is able to bind to serum-responsive
elements forming a complex, which then interacts with transcription
factors, including activator protein 1 and specific protein 1, to
modulate the expression of genes lacking EREs in their promoter
regions (9,10,15,24).
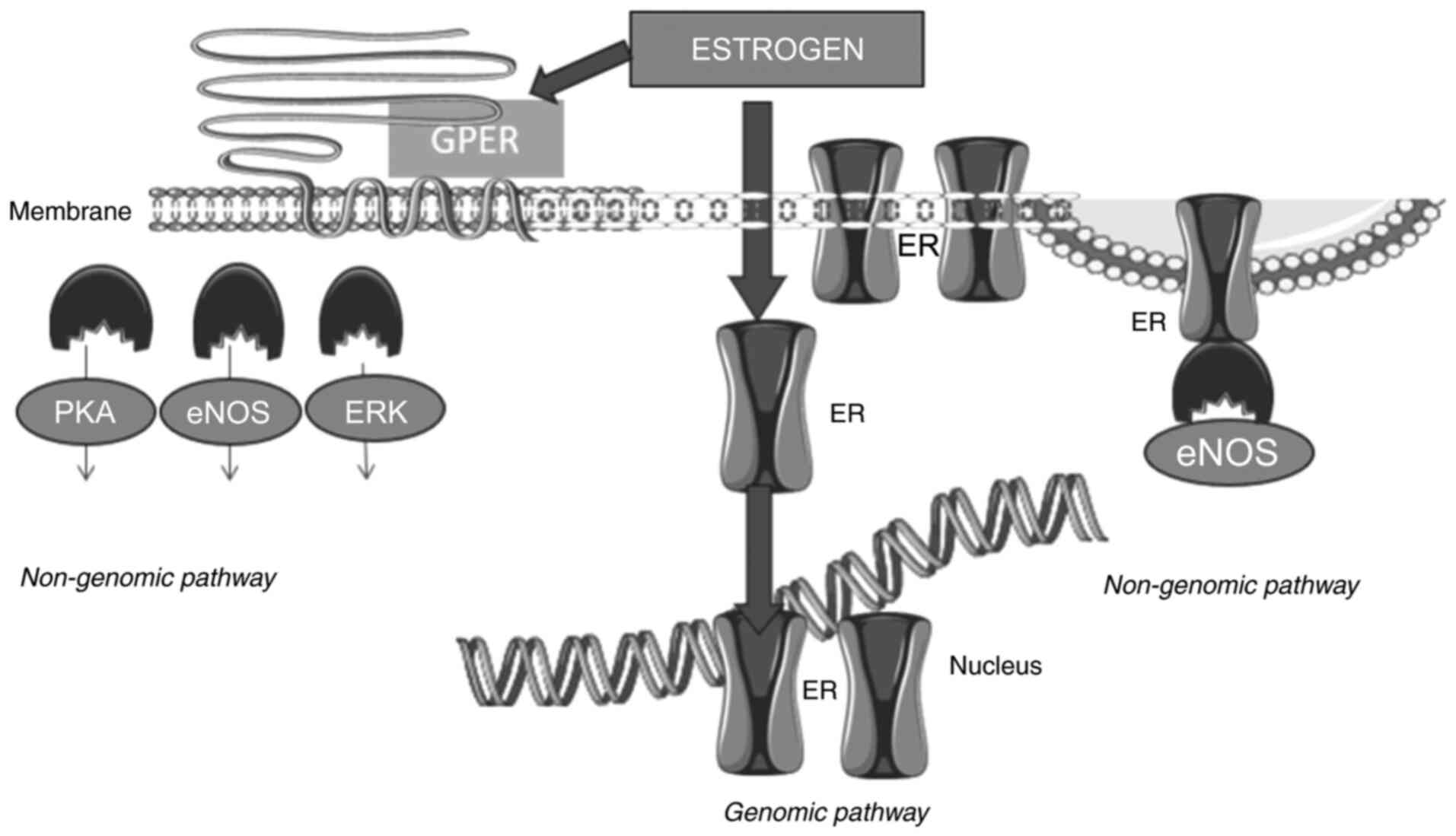 | Figure 3.Estrogen receptor signaling pathway.
By the genomic mechanism, ER after ligand binding and dimerization,
translocates to the nucleus, where it directly binds to the
responsive elements of target genes involved in cell growth,
inflammation, proliferation, survival and protein synthesis. By the
non-genomic mechanism, estrogens bind to ERs modulating eNOS
activity. Estrogens also bind to the GPER located on the membrane
to regulate signaling through eNOS, PKA and extracellular
signal-regulated kinases ERK or MAPK. ER, estrogen receptor; eNOS,
endothelial nitric oxide synthase; GPER, G-protein coupled ER; PKA,
protein kinase A. |
ERα also exerts non-genomic activity (25,26).
Estrogen binds to membrane ERs and regulates ion channel opening,
or activates related enzymes and Ca2+ mobilization that
triggers the activation of nuclear transcription factors. This
involves the fast activation of intracellular signaling pathways
including the cyclic adenosine monophosphate and the growth factor
receptor PI3K/Akt or Ras/MAPK pathways. The ERα-E2 complex binds
with a number of different proteins leading to the generation of a
complex molecule within the cytoplasm. Such proteins include the
Src protein kinase and the p85 subunit of PI3K. Such complexes can
rapidly trigger the activation of the MAPK and Akt pathways
(27). Furthermore, ERs can also
act via a ligand-independent mechanism through interaction with
growth factors such as insulin-like growth factor (IGF) and
epidermal growth factor. These growth factors trigger the
phosphorylation of the ERs via the growth factor membrane receptor
to regulate intracellular signaling pathways. Through this
mechanism, ERs can regulate gene expression without the need for
ligand binding (22,23).
The non-genomic mechanisms of ERα involving the
plasma membrane are based on post-translational alterations. The
two mechanisms, genomic and non-genomic, are not completely
independent. In fact, there is an interchange between the two
mechanisms of action, particularly via the phosphorylation of ERα
or its cofactors. An example of the overlap between the two types
of mechanisms of ER signaling is the estrogenic activation of
cyclin D1 via the AP-1 binding site, which requires MAPK activity
and formation of an ER/Src/PI3K complex (7,28).
In the present review, ERs' effects in three target
tissues as paradigms of the multiple facets of the ER, including
bone and growth disorders, cancer, gender dysphoria and ageing, are
discussed. The antagonistic action of ERs in the aforementioned
tissues complicates the understanding of their mechanisms of action
(4). The increasing prevalence of
breast cancer, particularly hormone receptor-positive breast
cancer, remains a challenge for the development of novel
anti-hormonal therapies other than tamoxifen and aromatase
inhibitors, to minimize the toxicity of long treatment regimens in
patients with breast cancer. A complete understanding of the
mechanisms of action of ERs in the bones may result in the
identification of novel pathways for the treatment of osteoporosis.
Aging of the brain and related diseases such as dementia and
depression are associated with the lack of estrogens, particularly
in women after menopause. ERs serve a key role in aging. Estrogens
are known to exert a neuroprotective role on the brain via ERs.
Decreases of estrogens with age is followed by brain cell
degeneration and decreased of memory and cognition. Gender
dysphoria, a marked incongruence between experienced gender and
biological sex, is commonly hypothesized to arise from discrepant
cerebral and genital sexual differentiation. The exact role of ERs
in gender dysphoria is a challenge that requires further
research.
In the present review, a comprehensive literature
search was performed using PubMed and the search terms ‘ER’,
‘structure’, ‘function’, ‘bones’, ‘cancer’, ‘breast cancer’,
‘brain’ and ‘gender dysphoria’ along with the appropriate Boolean
modifiers.
ERs in the bones
Estrogens are vital for skeletal growth and for the
maintenance of bone mass in both males and females. Estrogen action
is mediated via ERα and ERβ in both the growth plate and
mineralized bone from early in human development to adulthood
(29). During puberty, estrogens
begin exhibiting their roles in the pubertal growth spurt by
binding to ERs on osteoblasts and chondrocytes. Estrogens promote
osteoblastic activity and inhibit osteoclastic activity via
advancing of the growth plate, bone proliferation and epiphyseal
fusion (29,30). The fact that estrogen deficiency
leads to osteopenia and presumably osteoporosis in postmenopausal
women is well established (29).
Both estrogens and androgens also act directly and/or via the
ERα gene in osteoblasts by altering the physiological
balance of the osteoprotegerin/receptor activator of NF-κB ligand
system (30). Another mechanism
involved in the osteoprotective actions of estrogens is achieved
via the upregulation of the Fas ligand (FasL) gene.
FasL action is mediated by ERα, resulting in increased
apoptosis in differentiated osteoclasts (31). Moreover, androgens also contribute
to skeletal growth and bone mineral density either via a direct
action on the growth plate or indirectly through their
aromatization to estrogens (30).
Estrogen deficiency leads to an increase in
osteoblast and osteocyte apoptosis in both humans and rodents.
There are different patterns of expression of ERs on specific bone
cells, including osteoblasts and osteoclasts, and on cartilage
cells known as chondrocytes, and these patterns of expression are
influenced by age and sex. ERα chondrocyte knock-out mice
have been reported to exhibit increased longitudinal bone growth
and wider growth plates (32).
However, ERα deletion from the osteoclast lineage does not
affect the cortical bone mass (33). ERα knock-out mice
demonstrated trabecular bone loss and high bone turnover, which may
be related to increased osteoclast counts in females compared with
males. However, deletion of the ERα gene in osteoblast
progenitors in female mice demonstrated no effect on trabecular
bone mass (11,34,35).
Furthermore, ERβ is also highly expressed in osteoclasts (23). Studies in humans reported similar
findings (11,34,35).
Expression of ERα and ERβ also differs in bone compartments in
humans, where ERβ is expressed in higher levels on the trabecular
bone whereas ERα is primarily expressed in the cortical bone
(11,34,35).
The clinical implications of ER function were first
described approximately four decades ago. The first case of
estrogen resistance in a male patient of tall stature, with
markedly delayed skeletal maturation and osteoporosis, was
described in 1986. This patient was reported to possess a
disruptive mutation in the ERα gene (36) and the administration of estrogen
did not improve the condition of the patient (36). A similar clinical presentation was
observed in male patients with an aromatase deficiency. The
aromatase gene CYP19, is located on chromosome 15q21.1 and
is expressed in a number of tissues, including the placenta,
ovaries, testes, adipose tissues and the brain. Specific promoters
in each tissue type lead to different splice variants and different
expression profiles of the CYP19 gene in the respective
tissues (37). The aromatase
enzyme serves an essential role in sex steroid physiology by
converting C19 androgenic steroids (androstenedione and
testosterone) to C18 estrogens (E2 and E1), which promote growth
acceleration, skeletal maturation and epiphyseal fusion. Aromatase
gene inactivating mutations in males cause delayed bone maturation
and decreased bone mineralization. In contrast with estrogen
resistance, administration of estrogen in patients with aromatase
deficiency, promotes the closure of growth plates (38). Conversely, females with an
aromatase deficiency demonstrate different degrees of virilization,
from an infant with ambiguous genitalia to a woman with polycystic
ovarian syndrome (39).
ERs and cancer
Estrogen nuclear receptors are involved in tumor
development and progression in numerous types of cancer (10). Gynecological, endocrine gland,
gastrointestinal and lung cancer may exhibit either up- or
downregulation of ERs. Both ERα and ERβ are expressed in numerous
types of tumors but usually in different cancer types (40). ERα expression appears to
predominate in breast, ovarian, endometrial and endocrine gland
cancer, whereas ERβ is upregulated in gastrointestinal and lung
cancer (10). Conversely, ERβ
inhibits the proliferation of colon and lung cancer, whereas ERα
exerts an onco-protective role in breast cancer, with decreased ERα
levels associated with a poorer prognosis (40). The polymorphic expression of ERs in
different types of cancer supports the notion that estrogenic
function is tissue-dependent. However, the exact mechanisms of
action in the different types of tissues remain incompletely
understood. The present review focuses on breast cancer, with a
summary of ER expression in other types of cancer provided in
Table I (41–57).
 | Table I.ERα, ERβ and GPER actions in cancer
tissues. |
Table I.
ERα, ERβ and GPER actions in cancer
tissues.
|
| Role of
receptors |
|---|
|
|
|
|---|
| Cancer type | ERα | ERβ | GPER |
|---|
| Ovarian | Tumor promoter
(41) | Tumor suppressor
(41); | GPER1 tumor
suppressor (43); |
|
|
| ERβ and ERβ2
suppress ERα (42); | GPER30 tumor
promoter (44) |
|
|
| ERβ5 tumor promoter
(42) |
|
| Prostatic | Tumor promoter
(42) | Tumor suppressor
ERβ5 (45); | Tumor suppressor
(46) |
|
|
| Tumor promoter
(45) |
|
|
Gastric/intestinal | Tumor suppressor
(47); | ERβ5 tumor promoter
(49) | Tumor promoter
(50); |
|
| ERα36 tumor
promoter (48) |
| Tumor suppressor
(50) |
| Liver | Tumor suppressor
(51); | Tumor promoter
(51) | Tumor suppressor
(52) |
|
| Tumor promoter
(51) |
|
|
| Pancreatic | Tumor promoter
(53) | Tumor promoter
(53) | Tumor suppressor
(53) |
| Melanoma | Tumor promoter
(54) | Tumor suppressor
(54) | Tumor suppressor
(54) |
| Lung | Tumor promoter
(55) | Tumor promoter
(56) | Tumor suppressor
(57) |
Breast cancer is the most common cancer among women,
accounting for 30% of all new female cancer cases, with a rising
global incidence in both pre- and postmenopausal women (58). Breast tumors are categorized based
on histopathological analysis as either lobular or ductal, in
situ or invasive carcinoma (59). According to the molecular profile,
breast cancer can be classified as positive or negative for ERα,
progesterone receptor (PR) and human epidermal growth factor
receptor 2 (HER2). The latter classification of breast cancers
relies on three primary subtypes as follows: Luminal, which is
ER+PR+HER2−, HER2-positive, which
is ER−PR−HER2+, or
triple-negative, which is
ER−PR−HER2−. The majority of
breast cancer cases (~80%) are ERα-positive. The luminal type is
separated into two subclasses, A and B. Both subclasses express ERα
but vary in terms of aggressiveness. Luminal A cases are typically
low-grade, whereas luminal B can be of higher grade and with worse
prognosis. Luminal B cases demonstrate upregulated expression of
HER2, reduced ERα expression and increased cancer cell
proliferation (60). Knowing the
molecular profile of breast cancer allows for the selection of the
most appropriate treatment, management and follow-up for
patients.
Physiologically, ERα is expressed in the ducts and
lobules of the breast gland, while ERβ is expressed in the luminal,
myoepithelial and stromal cells. ERα serves a major role in breast
development, while ERβ depletion has no effect on mammary gland
growth and development (60). The
etiology of breast cancer is multifactorial. Mutations in tumor
suppressor genes such as BRCA1, BRCA2, CHEK2, TP53 or
PTEN, or gain of function mutations in oncogenes such as
EGFR, IGF1R and HER2 only account for a small
percentage of breast cancer cases (58). Given that ERα is found only in 10%
of breast tissue cells, mutations in the ERα gene are not
the primary cause of breast cancer formation. However, ERα
dysregulation is involved in breast cancer development and
progression. ERα is found in ~80% of breast cancer cells (61), which shows the importance of the
role of estrogens in hormone-dependent breast cancer. Numerous
studies have linked the occurrence of breast cancer with exposure
to estrogen replacement therapy in menopausal women, and the
involvement of ERs has been hypothesized (62,63).
The pathophysiological mechanisms of the
carcinogenic effects of estrogen in the breast are still under
evaluation. Estrogens, through ER-dependent and independent
mechanisms, can promote the upregulation or downregulation of
numerous molecules involved in cell proliferation, differentiation
and apoptosis (40). ERα functions
as a transcription factor for several tumor-associated genes,
including IGF1 receptor (IGF1R), cyclin D1, the anti-apoptotic
protein BCL-2 and vascular endothelial growth factor (62–64).
Moreover, estrogens, primarily 17β-E2, can stimulate ERα-mediated
genes such as the LRP16 gene, which interferes with and
downregulates ERα-mediated transcription of E-cadherin (64). The reduction of E-cadherin enhances
the invasiveness of breast cancer (64). Furthermore, estrogens may
upregulate the expression of Wnt11 via an ER-dependent
mechanism in breast cancer (65).
Likewise, studies have reported that phosphorylation of ERα at
serine residues 118 and 167 in breast cancer cells induces
expression of genes that impact tumor progression and treatment
responsiveness (65–67). Conversely, estrogens can promote
cell proliferation via non-ER related cell membrane phosphorylation
of target molecules such as Akt (67). Such knowledge may allow the
development of innovative cancer treatments based on non-ER-related
mechanisms of estrogen action.
ERα is expressed in ~80% of breast cancer cells,
meaning that these breast cancer cases respond to endocrine
therapy. Conversely, patients with ESRα mutant-positive
metastases demonstrate resistance to hormonal therapy. Missense
mutations in ESRα have been identified in >51 residues
most of which are within the ER-LBD. Such mutations are either
hormone-dependent, hormone-independent or neutral. Y537S and D538G
are the most common mutations and are characterized as
hormone-independent mutations (68). Other mutations such as K303R and
E380Q result in estrogen hypersensitivity and are characterized as
hormone-dependent. Finally, neutral mutations such as S432L and
V534E have also been reported (69). ERα splice variants are also
involved in breast cancer. ERα-46 is expressed in ~70% of breast
cancer tissues and inhibits cell proliferation and ERα-66-regulated
target gene transcription. ERα-46 appears to enhance endocrine
responses by inhibiting selected ERa-66 endocrine responses. Via
this mechanism, ERα-46 appears to increase the response to
endocrine therapy (70). ERα-36
regulates non-genomic signaling. It has been reported that patients
with breast cancer with tumors expressing high levels of ERα-36
benefit less from tamoxifen therapy compared with those with low
levels of ERα-36 expression. Thus, ERα-36 is suggested to act as an
antagonist of tamoxifen contributing to hormone therapy resistance
and cancer metastases (71).
Genomic studies of breast cancer have reported the
role of epigenetics in ERα dysregulation. The loss of ERα
expression can be the result of hypermethylation of CpG islands
within the ERα promoter region. Deacetylation and histone
methylation of the ER promoter are also epigenetic events involved
in ERα deregulation. Demethylating agents and methylation
inhibitors may thus serve as potential treatments for ERα-negative
or endocrine therapy-resistant tumors (72).
ERβ is expressed in breast cancer cells, but to a
lesser degree than ERα. There are conflicting studies regarding the
role of ERβ in breast cancer. Some studies have identified a
protective effect of ERβ expression in breast cancer cells by
antagonizing the action of Erα (73). Moreover, the expression of ERβ is
associated with a better prognosis and with an increased response
to endocrine therapy (74). Other
studies have reported opposing results, demonstrating that ERβ
expression is associated with adverse outcomes in breast cancer and
is not predictive of resistance to tamoxifen (75,76).
More recently, studies have focused on the protective effects of
ERβ-5 in patients with breast cancer and the positive association
with longer relapse-free survival times. ERβ-5 has also been
reported to induce the sensitivity of breast cancer cell lines to
chemotherapy-induced apoptosis (77,78).
Finally, GPER1 expression is detected in 60% of
breast cancer cases, primarily in Luminal A cases. GPER1
downregulation in the tumor cells is associated with poorer outcome
(66). Likewise, studies have
reported that expression of GPER1 decreases during tamoxifen
treatment. Thus, breast cancers with low expression of GPER1 show
endocrine treatment resistance (79).
The exact mechanisms of action and antagonism of ERs
in different types of cancer is an ongoing area of research, which
may highlight novel strategies for the development of therapeutics
for the management of specific types of cancer. Research on novel
treatments for combating endocrine-resistant tumors is also
ongoing.
ERs in gender dysphoria
ERα and ERβ expression are upregulated during the
development of the central nervous system (CNS) (80). ERα is primarily located in the
hypothalamic nuclei, the periaqueductal grey, the parabrachial
nucleus, the medial amygdala, the preoptic area and on the bed
nucleus of the stria terminalis. ERα is also expressed to a lesser
degree in the locus coeruleus. ERβ is also expressed in the
aforementioned areas, but is primarily expressed in the lateral
septum, the basolateral amygdala and the trigeminal nuclei. Lower
levels of ERβ are also observed in the hippocampus, the locus
coeruleus, the cerebral cortex and the cerebellum. The most
recently discovered ER, GPER1, is also expressed in the brain, with
high levels in the olfactory bulbs, the hypothalamus, cortex, the
hippocampus, and the Purkinje and granule cells of the cerebellum
(80). ERs function in the brain
primarily through non-genomic pathways and to a lesser extent via
the genomic pathways, where ERs regulate brain development, sexual
behavior, cognitive ability, temperature homeostasis and appetite
(81–83).
The masculinization of the brain in humans is
primarily mediated by the androgen receptor (AR) and partly by ERs
(81). In humans, the expression
of aromatase is highest in the thalamus (82). During embryogenesis, fetal
testosterone passes into the brain and is metabolized by the enzyme
aromatase to E2, which exerts its action by binding to ERs, which
masculinizes specific brain regions. It has been previously
reported in mouse knockout studies that ERβ has a defeminization
role in male brains and in male behavior (83). The effects of sex steroids on the
brain during development determine sexual differentiation. A
suggested pathway for brain masculinization is the direct
initiation of gene expression via the activation of AR and ERs.
Gender dysphoria, a marked discordance between experienced gender
and biological sex, is commonly hypothesized to be attributed to
discrepant of typical male or female brain and genital sexual
differentiation (84). The
contribution of ERs to the development of gender incongruence has
been widely studied with contradicting and only partially
convincing results (85,86). Interactions of genes and
polymorphisms may contribute to the polygenic bases of gender
dysphoria. However, genetics is not the only determining factor to
change sexual orientation. The majority of studies that
investigated the implication of the genetic component focused on
polymorphisms related to ERα and ERβ (Table II) (85–92).
 | Table II.Studies on the contribution of ERs in
gender dysphoria. |
Table II.
Studies on the contribution of ERs in
gender dysphoria.
| First author,
year | Gender | ERs | Findings | (Refs.) |
|---|
| Henningsson et
al, 2005 | MTF | ERβ | Significant
association with a dinucleotide CA polymorphism in the ERβ gene.
The higher number of CA repeats implies greater transcription
activation and therefore lower feminization or greater
defeminization | (85) |
| Hare et al,
2009 | MTF | ERβ | Unable to replicate
the significant association between longer CA repeat lengths in the
ERβ gene | (86) |
| Ujike et al,
2009 | MTF and FTM | ERβ and ERα | No difference in
allelic or genotypic distribution of the genes, thus no evidence
that genetic variants of sex hormone-related genes confer
individual susceptibility to transsexualism | (87) |
| Fernández et
al, 2014 | MTF and FTM | ERβ | No difference in
allelic or genotypic distribution of the sex hormone-related genes
between transgender patients and controls | (88) |
| Cortés-Cortés et
al, 2017 | MTF and FTM | ERα | The polymorphism
XbaI-rs9340799 and the haplotypes L-C-G and L-C-A are associated
with FTM in adults | (89) |
| Fernández et
al, 2018 | MTF and FTM | ERα and ERβ | The key receptors
implicated in sexual differentiation of the brain have a specific
allele combination for ERβ and ERα in MTFs. The FTM gender is
associated with specific polymorphisms of ERβ and ERα. Thus, ERα
and ERβ serve a key role in the sexual differentiation of the
brain | (90) |
| Foreman et
al, 2019 | MTF | ERα and ERβ | Overrepresented
alleles and genotypes are proposed to under-masculinize/feminize on
the basis of their reported effects in other disease contexts | (91) |
| Ramírez et
al, 2021 | MTF and FTM | ER
coactivators | The coactivators
SRC-1 and SRC-2 could be considered as candidates for increasing
the list of potential genes for gender incongruence. The role of
SRCs in ER-mediated gene expression is known | (92) |
Based on the limited available published data, the
primary receptors involved in sexual differentiation of the brain
have a specific allele combination for ERβ, ERα and AR in trans
females, whose gender incongruence is associated with a specific
genotypic combination of ERs and AR variants. Specific
polymorphisms of ERβ and ERα have also been reported in trans
males. Both ERα and ERβ serve a key role in the typical sexual
differentiation of the human brain. Estrogens, primarily E2,
regulate the growth, development and function of the reproductive
system and the CNS. The mechanisms of ERs and their interactions
with estrogens in gender differentiation are not completely
understood. The two receptors ERα and ERβ bind with the E2 ligand,
resulting in the dimerization of the receptor, resulting in the
necessary fundamental changes in the LBD and thus allowing for
coactivators to be engaged. This step is required for the
transcriptional regulation of gender-determination genes dependent
on E2 (93,94). Moreover, the E2-ER complex
interacts with steroid receptor coactivators (SRCs) to activate the
transcription of the target genes. SRCs can also influence the
ability of the transcription factors to activate or inhibit the
expression of numerous genes (95). There are three SRC members: SRC-1,
SRC-2 and SRC-3. It has been suggested that variations at the DNA
level in SRCs can affect the function of the E2-ER complex and
therefore modify the transcription of the genes regulated by E2.
The coactivators SRC-1 and SRC-2 are hypothesized to serve a key
role in the potential expression of the gene for gender
incongruence (92). Overall, it is
hypothesized that gender incongruence has a complex underlying
pathophysiology that involves interactions between sex steroids,
sex steroid receptors, coactivators and genes (92). Furthermore, reduced androgen levels
and androgen signaling may serve a role in the acquisition of a
female gender identity of male-to-female transsexuals, whereas a
higher number of CA repeats in the ERβ gene lead to reduced ERβ
signaling and inhibit the ERβ dimerization on the male brain, which
results in increased transcriptional activation and thus lower
feminization, may explain the acquisition of a male gender identity
in female-to-male transexuals.
ERs and the aging brain
Estrogens have been recognized as potent
neuroprotective agents. Estrogens, via their ERs, mediate gene
transcription. The participation of ERs in regulating
differentiation, proliferation and inflammation of neurons and
synapses is fundamental to general physiology. The levels of sex
hormones decrease with age, although this decrease is more
pronounced during the menopause in women (96). The levels of ERs in the brain
decrease just after birth and their actions in the CNS become
slowly restricted to different regions of the brain. ERα is
expressed in the amygdala and the hypothalamus, whereas ERβ is
distributed in the cerebral cortex in the cerebellum, hippocampus,
periventricular nuclei, bed nucleus of the stria terminalis,
substantia nigra, ventral tegmental area and the dorsal raphe
nucleus (80,97–99).
Brain-derived neurotrophic factor (BDNF), found in the brain,
regulates synaptic genesis and development (60,80).
BDNF protein levels increase following ERβ activation in
postmenopausal mice, which serves a crucial role in promoting the
survival and differentiation of neurons (60). Both ERα and in particular ERβ,
contribute to neuroprotection via the striatum in the basal ganglia
of the brain, which regulates muscle tone and coordinates motor
ability (99). Hippocampal GPER1
also contributes to estrogen-dependent conciliation memory via the
regulation of hippocampal synaptic plasticity and neuroprotection.
BDNF expression induced by selective GPER1 activation via the
E2-PI3K/Akt and E2-MAPK signaling pathways promotes synaptic
plasticity, exerting a neuroprotective effect (99).
Behavioral manifestations such as emotion and
cognition are controlled by sex steroid hormones and have been
studied by functional magnetic resonance imaging (fMRI). During the
follicular and luteal phases in healthy women with a normal
menstrual cycle, fMRI demonstrated differences in several areas of
the brain, such as the amygdala, anterior hippocampus and several
cortical regions (100). The role
of ERs in neurodevelopment is vital in the adult brain, in addition
to their more established roles in sexual behavior and
reproduction.
During aging, several modifications and adjustments
to tissues in the brain occur that result in adverse events. The
levels of both ERα and ERβ, found in synapses of the cornu ammonis
neurons in the hippocampus of female rats, decrease as the animal
ages (101). As the expression of
ERs is associated with neuroprotection, this level of
neuroprotection may decrease as an individual ages, particularly
during pre-menopause and menopause, increasing the risk of adverse
neurological events in women, whereas men, who exhibit a gradual
slow decrease in testosterone levels, may experience longer
neuroprotective effects (102).
Complete loss of estrogen during menopause is associated with
neurological changes, since perimenopausal females have an
increased risk of depression that is reduced when they receive
treatment with estrogens (102,103).
Menopausal women demonstrate a decrease in cognitive
function and in both verbal and working memory. According to the
Women's Health Initiative Memory Study, the incidence of memory and
cognitive impairment in menopausal women was 4.5% (104). Moreover, there is evidence of
changes in the cognitive test performance of women that are
consistently related to the reproductive period and menopausal
transition. Population-based studies reported an increased risk of
dementia by up to 23% in females with late menarche, early
menopause and a short reproductive period (96,105). A recent study investigated the
factors associated with a decrease in cognition and found a
synergistic association with an early age of menopause and vascular
risk impacting the cognitive function in menopausal women for >3
years of follow-up after menopause (106). The same study reported that
hormone treatment with estrogens attenuated this association to
preserve cognition (106).
Conversely, an age-related shift in ERα or ERβ may underlie a
decreased response to E2, suggesting that estrogen treatment may
not always lead to cognitive preservation. The effects of ERα or
ERβ on cognitive function are dose-dependent (107). Furthermore, hormone replacement
will interact with locally produced E2, such that the effective
dose of E2 to optimize hippocampal function will depend on local E2
and the relative expression of functional ERα and Erβ (96).
Neurodegenerative diseases, such as Alzheimer's
disease (AD), are associated with estrogen depletion. A higher
prevalence of AD and a more rapid cognitive decline were reported
in women compared with men (106). Furthermore, a meta-analysis
reported that estrogen replacement treatment could benefit
postmenopausal women with neurodegenerative disease (108). Numerous studies have reported the
neuroprotective function of estrogens, including acting as
antioxidants, enabling DNA repair, inducing growth factor
expression and regulating cerebral blood flow. Parkinson's disease
(PD) affects motor skills, followed by cognitive impairment. Women
with lower estrogen levels are more likely to develop PD compared
with those with higher estrogen levels (60,108). The role of estrogen-based
treatments in this group of women for the prevention of dementia,
loss of motor ability and depression is still under evaluation.
Conclusions and future perspectives
Undoubtedly, an understanding of the role of ERs in
different tissues, the estrogen signaling mechanisms and the
interactions between the three ERs (ERα, Erβ and GPER1) is required
for the development of novel therapeutics. Bridging the gap between
animal and human studies may open future research avenues for the
development of novel treatments for ER-related diseases. Notably,
ER signaling members in cancer may be targets for next-generation
ER-targeted treatments. The rising incidence of breast cancer and
also other types of cancer, related to ER pathology, is a hallmark
for the need for more targeted treatments focused on ERs. Existing
hormonal auxiliary treatments for osteoporosis are promising.
However, the association between hormone replacement therapy and
the development of breast cancer highlights the need for more
targeted treatments focusing on ER signaling pathways. Although
notable advancements have been made regarding sex hormone signaling
in the brain, a deeper understanding of the spatiotemporal
expression and ER signaling in the brain is still required. Further
research on the physiological and pathophysiological aging of the
brain and the differences between sexes observed in these processes
is required. In vivo and integrated in vitro studies
assessing the neuroprotective effects of estrogen highlight ERs as
potential therapeutic targets in neurodegenerative diseases and
aging.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MT wrote, edited and reviewed the article. AK and KP
wrote the structure, location, function of ERs sections of the
article. NS wrote, edited, reviewed and supervised the writing of
the manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Green S, Walter P, Kumar V, Krust A,
Bornert JM, Argos P and Chambon P: Human oestrogen receptor cDNA:
Sequence, expression and homology to v-erb-A. Nature. 320:134–139.
1986. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jameson L, De Groot LJ, de Kretser DM,
Giudice LC, Grossman AB, Melmed S, Potts JT and Weir GC:
Endocrinology: Adult and Pediatric. 7th edition. W.B Saunders;
Philadelphia, PH: pp. 2200–2350. 2016
|
|
3
|
Fuentes N and Silveyra P: Estrogen
receptor signaling mechanisms. Adv Protein Chem Struct Biol.
116:135–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Faltas CL, LeBron KA and Holz MK:
Unconventional estrogen signaling in health and disease.
Endocrinology. 161:bqaa0302020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hazell GG, Yao ST, Roper JA, Prossnitz ER,
O'Carroll AM and Lolait SJ: Localisation of GPR30, a novel G
protein-coupled oestrogen receptor, suggests multiple functions in
rodent brain and peripheral tissues. J Endocrinol. 202:223–236.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arterburn JB and Prossnitz ER: G
Protein-Coupled Estrogen Receptor GPER: Molecular pharmacology and
therapeutic applications. Annu Rev Pharmacol Toxicol. 63:295–320.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Björnström L and Sjöberg M: Mechanisms of
estrogen receptor signaling: Convergence of genomic and nongenomic
actions on target genes. Mol Endocrinol. 19:833–842. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Williams C, Edvardsson K, Lewandowski SA,
Ström A and Gustafsson JA: A genome-wide study of the repressive
effects of estrogen receptor beta on estrogen receptor alpha
signaling in breast cancer cells. Oncogene. 27:1019–1032. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klinge CM, Jernigan SC, Smith SL,
Tyulmenkov VV and Kulakosky PC: Estrogen response element sequence
impacts the conformation and transcriptional activity of estrogen
receptor alpha. Mol Cell Endocrinol. 174:151–166. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clusan L, Ferrière F, Flouriot G and
Pakdel F: A basic review on estrogen receptor signaling pathways in
breast cancer. Int J Mol Sci. 24:68342023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bord S, Horner A, Beavan S and Compston J:
Estrogen receptors alpha and beta are differentially expressed in
developing human bone. J Clin Endocrinol Metab. 86:2309–2314. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gosden JR, Middleton PG and Rout D:
Localization of the human oestrogen receptor gene to chromosome
6q24-q27 by in situ hybridization. Cytogenet Cell Genet.
43:218–220. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Enmark E, Pelto-Huikko M, Grandien K,
Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M and
Gustafsson JA: Human estrogen receptor beta-gene structure,
chromosomal localization and expression pattern. J Clin Endocrinol
Metab. 82:4258–4265. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar R, Zakharov MN, Khan SH, Miki R,
Jang H, Toraldo G, Singh R, Bhasin S and Jasuja R: The dynamic
structure of the estrogen receptor. J Amino Acids. 2011:8125402011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hewitt SC and Korach KS: Estrogen
receptors: Structure, mechanisms and function. Rev Endocr Metab
Disord. 3:193–200. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barton M, Filardo EJ, Lolait SJ, Thomas P,
Maggiolini M and Prossnitz ER: Twenty years of the G
protein-coupled estrogen receptor GPER: Historical and personal
perspectives. J Steroid Biochem Mol Biol. 176:4–15. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saito K and Cui H: Estrogen receptor alpha
splice variants, post-translational modifications and their
physiological functions. Cells. 12:8952023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Flouriot G, Brand H, Denger S, Metivier R,
Kos M, Reid G, Sonntag-Buck V and Gannon F: Identification of a new
isoform of the human estrogen receptor-alpha (hER-alpha) that is
encoded by distinct transcripts and that is able to repress
hER-alpha activation function 1. EMBO J. 19:4688–4700. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: Identification, cloning and expression of human
estrogen receptor-alpha36, a novel variant of human estrogen
receptor-alpha66. Biochem Biophys Res Commun. 336:1023–1027. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Warner M, Fan X, Strom A, Wu W and
Gustafsson JÅ: 25 years of ERβ: A personal journey. J Mol
Endocrinol. 68:R1–R9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monteiro FL, Stepanauskaite L, Archer A
and Williams C: Estrogen receptor beta expression and role in
cancers. J Steroid Biochem Mol Biol. 242:1065262024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia M, Dahlman-Wright K and Gustafsson JÅ:
Estrogen receptor alpha and beta in health and disease. Best Pract
Res Clin Endocrinol Metab. 29:557–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arao Y and Korach KS: The physiological
role of estrogen receptor functional domains. Essays Biochem.
65:867–875. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stender JD, Kim K, Charn TH, Komm B, Chang
KC, Kraus WL, Benner C, Glass CK and Katzenellenbogen BS:
Genome-wide analysis of estrogen receptor alpha DNA binding and
tethering mechanisms identifies Runx1 as a novel tethering factor
in receptor-mediated transcriptional activation. Mol Cell Biol.
30:3943–3955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song RX, Zhang Z and Santen RJ: Estrogen
rapid action via protein complex formation involving ERalpha and
Src. Trends Endocrinol Metab. 16:347–353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kelly MJ and Levin ER: Rapid actions of
plasma membrane estrogen receptors. Trends Endocrinol Metab.
12:152–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Castoria G, Migliaccio A, Bilancio A, Di
Domenico M, de Falco A, Lombardi M, Fiorentino R, Varricchio L,
Barone MV and Auricchio F: PI3-kinase in concert with Src promotes
the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J.
20:6050–6059. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pedram A, Razandi M, Aitkenhead M, Hughes
CC and Levin ER: Integration of the non-genomic and genomic actions
of estrogen. Membrane-initiated signaling by steroid to
transcription and cell biology. J Biol Chem. 277:50768–50775. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Delmas PD: Treatment of postmenopausal
osteoporosis. Lancet. 359:2018–2026. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lindberg MK, Erlandsson M, Alatalo SL,
Windahl S, Andersson G, Halleen JM, Carlsten H, Gustafsson JA and
Ohlsson C: Estrogen receptor alpha, but not estrogen receptor beta,
is involved in the regulation of the OPG/RANKL
(osteoprotegerin/receptor activator of NF-kappa B ligand) ratio and
serum interleukin-6 in male mice. J Endocrinol. 171:425–433. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura T, Imai Y, Matsumoto T, Sato S,
Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, et
al: Estrogen prevents bone loss via estrogen receptor alpha and
induction of Fas ligand in osteoclasts. Cell. 130:811–823. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Börjesson AE, Windahl SH, Karimian E,
Eriksson EE, Lagerquist MK, Engdahl C, Antal MC, Krust A, Chambon
P, Sävendahl L and Ohlsson C: The role of estrogen receptor-α and
its activation function-1 for growth plate closure in female mice.
Am J Physiol Endocrinol Metab. 302:E1381–E1389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martin-Millan M, Almeida M, Ambrogini E,
Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA and Manolagas SC:
The estrogen receptor-alpha in osteoclasts mediates the protective
effects of estrogens on cancellous but not cortical bone. Mol
Endocrinol. 24:323–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manolagas SC, O'Brien CA and Almeida M:
The role of estrogen and androgen receptors in bone health and
disease. Nat Rev Endocrinol. 9:699–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Almeida M, Han L, Martin-Millan M, O'Brien
CA and Manolagas SC: Oxidative stress antagonizes Wnt signaling in
osteoblast precursors by diverting beta-catenin from T cell factor-
to forkhead box O-mediated transcription. J Biol Chem.
282:27298–27305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith EP, Boyd J, Frank GR, Takahashi H,
Cohen RM, Specker B, Williams TC, Lubahn DB and Korach KS: Estrogen
resistance caused by a mutation in the estrogen-receptor gene in a
man. N Engl J Med. 331:1056–1061. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rochira V and Carani C: Aromatase
deficiency in men: A clinical perspective. Nat Rev Endocrinol.
5:559–568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morishima A, Grumbach MM, Simpson ER,
Fisher C and Qin K: Aromatase deficiency in male and female
siblings caused by a novel mutation and the physiological role of
estrogens. J Clin Endocrinol Metab. 80:3689–3698. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Conte FA, Grumbach MM, Ito Y, Fisher CR
and Simpson ER: A syndrome of female pseudohermaphrodism,
hypergonadotropic hypogonadism and multicystic ovaries associated
with missense mutations in the gene encoding aromatase (P450arom).
J Clin Endocrinol Metab. 78:1287–1292. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen GG, Zeng Q and Tse GM: Estrogen and
its receptors in cancer. Med Res Rev. 28:954–974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chan KKL, Siu MKY, Jiang YX, Wang JJ, Wang
Y, Leung THY, Liu SS, Cheung ANY and Ngan HYS: Differential
expression of estrogen receptor subtypes and variants in ovarian
cancer: Effects on cell invasion, proliferation and prognosis. BMC
Cancer. 17:6062017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ciucci A, Zannoni GF, Travaglia D,
Petrillo M, Scambia G and Gallo D: Prognostic significance of the
estrogen receptor beta (ERβ) isoforms ERβ1, ERβ2 and ERβ5 in
advanced serous ovarian cancer. Gynecol Oncol. 132:351–359. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ignatov T, Modl S, Thulig M, Weißenborn C,
Treeck O, Ortmann O, Zenclussen A, Costa SD, Kalinski T and Ignatov
A: GPER-1 acts as a tumor suppressor in ovarian cancer. J Ovarian
Res. 6:512013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Smith HO, Arias-Pulido H, Kuo DY, Howard
T, Qualls CR, Lee SJ, Verschraegen CF, Hathaway HJ, Joste NE and
Prossnitz ER: GPR30 predicts poor survival for ovarian cancer.
Gynecol Oncol. 114:465–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ramírez-de-Arellano A, Pereira-Suárez AL,
Rico-Fuentes C, López-Pulido EI, Villegas-Pineda JC and Sierra-Diaz
E: Distribution and effects of estrogen receptors in prostate
cancer: Associated molecular mechanisms. Front Endocrinol
(Lausanne). 12:8115782022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bonkhoff H: Estrogen receptor signaling in
prostate cancer: Implications for carcinogenesis and tumor
progression. Prostate. 78:2–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ryu WS, Kim JH, Jang YJ, Park SS, Um JW,
Park SH, Kim SJ, Mok YJ and Kim CS: Expression of estrogen
receptors in gastric cancer and their clinical significance. J Surg
Oncol. 106:456–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Deng H, Huang X, Fan J, Wang L, Xia Q,
Yang X, Wang Z and Liu L: A variant of estrogen receptor-alpha,
ER-alpha36 is expressed in human gastric cancer and is highly
correlated with lymph node metastasis. Oncol Rep. 24:171–176.
2010.PubMed/NCBI
|
|
49
|
Xu CY, Guo JL, Jiang ZN, Xie SD, Shen JG,
Shen JY and Wang LB: Prognostic role of estrogen receptor alpha and
estrogen receptor beta in gastric cancer. Ann Surg Oncol.
17:2503–2509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jacenik D and Krajewska WM: Significance
of G protein-coupled estrogen receptor in the pathophysiology of
irritable bowel syndrome, inflammatory bowel diseases and
colorectal cancer. Front Endocrinol (Lausanne). 11:3902020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Baldissera VD, Alves AF, Almeida S,
Porawski M and Giovenardi M: Hepatocellular carcinoma and estrogen
receptors: Polymorphisms and isoforms relations and implications.
Med Hypotheses. 86:67–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wei T, Chen W, Wen L, Zhang J, Zhang Q,
Yang J, Liu H, Chen BW, Zhou Y, Feng X, et al: G protein-coupled
estrogen receptor deficiency accelerates liver tumorigenesis by
enhancing inflammation and fibrosis. Cancer Lett. 382:195–202.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gupta VK, Banerjee S and Saluja AK:
Learning from gender disparity: Role of estrogen receptor
activation in coping with pancreatic cancer. Cell Mol Gastroenterol
Hepatol. 10:862–863. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dika E, Patrizi A, Lambertini M,
Manuelpillai N, Fiorentino M, Altimari A, Ferracin M, Lauriola M,
Fabbri E, Campione E, et al: Estrogen receptors and melanoma: A
review. Cells. 8:14632019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
He M, Yu W, Chang C, Miyamoto H, Liu X,
Jiang K and Yeh S: Estrogen receptor α promotes lung cancer cell
invasion via increase of and cross-talk with infiltrated
macrophages through the CCL2/CCR2/MMP9 and CXCL12/CXCR4 signaling
pathways. Mol Oncol. 14:1779–1799. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu S, Hu C, Li M, An J, Zhou W, Guo J and
Xiao Y: Estrogen receptor beta promotes lung cancer invasion via
increasing CXCR4 expression. Cell Death Dis. 13:702022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu C, Liao Y, Fan S, Tang H, Jiang Z,
Zhou B, Xiong J, Zhou S, Zou M and Wang J: G protein-coupled
estrogen receptor (GPER) mediates NSCLC progression induced by
17β-estradiol (E2) and selective agonist G1. Med Oncol. 32:1042015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Heer E, Harper A, Escandor N, Sung H,
McCormack V and Fidler-Benaoudia MM: Global burden and trends in
premenopausal and postmenopausal breast cancer: A population-based
study. Lancet Glob Health. 8:e1027–e1037. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cserni G: Histological type and typing of
breast carcinomas and the WHO classification changes over time.
Pathologica. 112:25–41. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen P, Li B and Ou-Yang L: Role of
estrogen receptors in health and disease. Front Endocrinol
(Lausanne). 13:8390052022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Huang B, Omoto Y, Iwase H, Yamashita H,
Toyama T, Coombes RC, Filipovic A, Warner M and Gustafsson JÅ:
Differential expression of estrogen receptor α, β1 and β2 in
lobular and ductal breast cancer. Proc Natl Acad Sci USA.
111:1933–1938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Beral V; Million Women Study
Collaborators, : Breast cancer and hormone-replacement therapy in
the Million Women Study. Lancet. 362:419–427. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Salagame U, Canfell K and Banks E: An
epidemiological overview of the relationship between hormone
replacement therapy and breast cancer. Expert Rev Endocrinol Metab.
6:397–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Meng YG, Han WD, Zhao YL, Huang K, Si YL,
Wu ZQ and Mu YM: Induction of the LRP16 gene by estrogen promotes
the invasive growth of Ishikawa human endometrial cancer cells
through the downregulation of E-cadherin. Cell Res. 17:869–880.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xu X, Zhang M, Xu F and Jiang S: Wnt
signaling in breast cancer: Biological mechanisms, challenges and
opportunities. Mol Cancer. 19:1652020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Anbalagan M and Rowan BG: Estrogen
receptor alpha phosphorylation and its functional impact in human
breast cancer. Mol Cell Endocrinol. 418:Pt 3:264–272. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tsai EM, Wang SC, Lee JN and Hung MC: Akt
activation by estrogen in estrogen receptor-negative breast cancer
cells. Cancer Res. 61:8390–8392. 2001.PubMed/NCBI
|
|
68
|
Dustin D, Gu G and Fuqua SAW: ESR1
mutations in breast cancer. Cancer. 125:3714–3728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Toy W, Weir H, Razavi P, Lawson M,
Goeppert AU, Mazzola AM, Smith A, Wilson J, Morrow C, Wong WL, et
al: Activating ESR1 mutations differentially affect the efficacy of
ER antagonists. Cancer Discov. 7:277–287. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Klinge CM, Riggs KA, Wickramasinghe NS,
Emberts CG, McConda DB, Barry PN and Magnusen JE: Estrogen receptor
alpha 46 is reduced in tamoxifen resistant breast cancer cells and
re-expression inhibits cell proliferation and estrogen receptor
alpha 66-regulated target gene transcription. Mol Cell Endocrinol.
323:268–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wang ZY and Yin L: Estrogen receptor
alpha-36 (ER-α36): A new player in human breast cancer. Mol Cell
Endocrinol. 418:Pt 3:193–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Giacinti L, Claudio PP, Lopez M and
Giordano A: Epigenetic information and estrogen receptor alpha
expression in breast cancer. Oncologist. 11:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhao C, Matthews J, Tujague M, Wan J,
Ström A, Toresson G, Lam EW, Cheng G, Gustafsson JA and
Dahlman-Wright K: Estrogen receptor beta2 negatively regulates the
transactivation of estrogen receptor alpha in human breast cancer
cells. Cancer Res. 67:3955–3962. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hopp TA, Weiss HL, Parra IS, Cui Y,
Osborne CK and Fuqua SA: Low levels of estrogen receptor b protein
predict resistance to tamoxifen therapy in breast cancer. Clin
Cancer Res. 10:7490–7499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Markey GC, Cullen R, Diggin P, Hill AD, Mc
Dermott EW, O'Higgins NJ and Duffy MJ: Estrogen receptor-beta mRNA
is associated with adverse outcome in patients with breast cancer.
Tumour Biol. 30:171–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Esslimani-Sahla M, Simony-Lafontaine J,
Kramar A, Lavaill R, Mollevi C, Warner M, Gustafsson JA and
Rochefort H: Estrogen receptor beta (ER beta) level but not its ER
beta cx variant helps to predict tamoxifen resistance in breast
cancer. Clin Cancer Res. 10:5769–5776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shaaban AM, Green AR, Karthik S, Alizadeh
Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders
PT, et al: Nuclear and cytoplasmic expression of ERbeta1, ERbeta2
and ERbeta5 identifies distinct prognostic outcome for breast
cancer patients. Clin Cancer Res. 14:5228–5235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lee MT, Ho SM, Tarapore P, Chung I and
Leung YK: Estrogen receptor β isoform 5 confers sensitivity of
breast cancer cell lines to chemotherapeutic agent-induced
apoptosis through interaction with Bcl2L12. Neoplasia.
15:1262–1271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ignatov T, Weißenborn C, Poehlmann A,
Lemke A, Semczuk A, Roessner A, Costa SD, Kalinski T and Ignatov A:
GPER-1 expression decreases during breast cancer tumorigenesis.
Cancer Invest. 31:309–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Fan X, Xu H, Warner M and Gustafsson JA:
ERbeta in CNS: New roles in development and function. Prog Brain
Res. 181:233–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zuloaga DG, Puts DA, Jordan CL and
Breedlove SM: The role of androgen receptors in the masculinization
of brain and behavior: What we've learned from the testicular
feminization mutation. Horm Behav. 53:613–626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Biegon A: In vivo visualization of
aromatase in animals and humans. Front Neuroendocrinol. 40:42–51.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kudwa AE, Bodo C, Gustafsson JA and
Rissman EF: A previously uncharacterized role for estrogen receptor
beta: Defeminization of male brain and behavior. Proc Natl Acad Sci
USA. 102:4608–4612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Skordis N, Kyriakou A, Dror S, Mushailov A
and Nicolaides NC: Gender dysphoria in children and adolescents: An
overview. Hormones (Athens). 19:267–276. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Henningsson S, Westberg L, Nilsson S,
Lundström B, Ekselius L, Bodlund O, Lindström E, Hellstrand M,
Rosmond R, Eriksson E and Landén M: Sex steroid-related genes and
male-to-female transsexualism. Psychoneuroendocrinology.
30:657–664. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hare L, Bernard P, Sánchez FJ, Baird PN,
Vilain E, Kennedy T and Harley VR: Androgen receptor repeat length
polymorphism associated with male-to-female transsexualism. Biol
Psychiatry. 65:93–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ujike H, Otani K, Nakatsuka M, Ishii K,
Sasaki A, Oishi T, Sato T, Okahisa Y, Matsumoto Y, Namba Y, et al:
Association study of gender identity disorder and sex
hormone-related genes. Prog Neuropsychopharmacol Biol Psychiatry.
33:1241–1244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fernández R, Esteva I, Gómez-Gil E, Rumbo
T, Almaraz MC, Roda E, Haro-Mora JJ, Guillamón A and Pásaro E:
Association study of ERβ, AR and CYP19A1 genes and MtF
transsexualism. J Sex Med. 11:2986–2994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Cortés-Cortés J, Fernández R, Teijeiro N,
Gómez-Gil E, Esteva I, Almaraz MC, Guillamón A and Pásaro E:
Genotypes and Haplotypes of the Estrogen Receptor α Gene (ESR1) Are
Associated With Female-to-Male Gender Dysphoria. J Sex Med.
14:464–472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Fernández R, Guillamon A, Cortés-Cortés J,
Gómez-Gil E, Jácome A, Esteva I, Almaraz M, Mora M, Aranda G and
Pásaro E: Molecular basis of Gender Dysphoria: Androgen and
estrogen receptor interaction. Psychoneuroendocrinology.
98:161–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Foreman M, Hare L, York K, Balakrishnan K,
Sánchez FJ, Harte F, Erasmus J, Vilain E and Harley VR: Genetic
link between gender dysphoria and sex hormone signaling. J Clin
Endocrinol Metab. 104:390–396. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ramírez KDV, Fernández R, Delgado-Zayas E,
Gómez-Gil E, Esteva I, Guillamon A and Pásaro E: Implications of
the Estrogen Receptor Coactivators SRC1 and SRC2 in the Biological
Basis of Gender Incongruence. Sex Med. 9:1003682021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Nilsson S, Mäkelä S, Treuter E, Tujague M,
Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M and
Gustafsson JA: Mechanisms of estrogen action. Physiol Rev.
81:1535–1565. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
McKenna NJ, Lanz RB and O'Malley BW:
Nuclear receptor coregulators: Cellular and molecular biology.
Endocr Rev. 20:321–344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yi P, Wang Z, Feng Q, Pintilie GD, Foulds
CE, Lanz RB, Ludtke SJ, Schmid MF, Chiu W and O'Malley BW:
Structure of a biologically active estrogen receptor-coactivator
complex on DNA. Mol Cell. 57:1047–1058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Maioli S, Leander K, Nilsson P and
Nalvarte I: Estrogen receptors and the aging brain. Essays Biochem.
65:913–925. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Osterlund MK and Hurd YL: Estrogen
receptors in the human forebrain and the relation to
neuropsychiatric disorders. Prog Neurobiol. 64:251–267. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Mehra RD, Sharma K, Nyakas C and Vij U:
Estrogen receptor alpha and beta immunoreactive neurons in normal
adult and aged female rat hippocampus: A qualitative and
quantitative study. Brain Res. 1056:22–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
González M, Cabrera-Socorro A,
Pérez-García CG, Fraser JD, López FJ, Alonso R and Meyer G:
Distribution patterns of estrogen receptor alpha and beta in the
human cortex and hippocampus during development and adulthood. J
Comp Neurol. 503:790–802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Toffoletto S, Lanzenberger R, Gingnell M,
Sundström-Poromaa I and Comasco E: Emotional and cognitive
functional imaging of estrogen and progesterone effects in the
female human brain: A systematic review. Psychoneuroendocrinology.
50:28–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Waters EM, Yildirim M, Janssen WG, Lou WY,
McEwen BS, Morrison JH and Milner TA: Estrogen and aging affect the
synaptic distribution of estrogen receptor β-immunoreactivity in
the CA1 region of female rat hippocampus. Brain Res. 1379:86–97.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Morrison JH, Brinton RD, Schmidt PJ and
Gore AC: Estrogen, menopause and the aging brain: How basic
neuroscience can inform hormone therapy in women. J Neurosci.
26:10332–10348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Dwyer JB, Aftab A, Radhakrishnan R, Widge
A, Rodriguez CI, Carpenter LL, Nemeroff CB, McDonald WM and Kalin
NH; APA Council of Research Task Force on Novel Biomarkers and
Treatments, : Hormonal treatments for major depressive disorder:
State of the Art. Am J Psychiatry. 177:686–705. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Goveas JS, Espeland MA, Woods NF,
Wassertheil-Smoller S and Kotchen JM: Depressive symptoms and
incidence of mild cognitive impairment and probable dementia in
elderly women: The Women's Health Initiative Memory Study. J Am
Geriatr Soc. 59:57–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kilpi F, Soares ALG, Fraser A, Nelson SM,
Sattar N, Fallon SJ, Tilling K and Lawlor DA: Changes in six
domains of cognitive function with reproductive and chronological
ageing and sex hormones: A longitudinal study in 2411 UK mid-life
women. BMC Womens Health. 20:1772020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wood Alexander M, Wu CY, Coughlan GT, Puri
T, Buckley RF, Palta P, Swardfager W, Masellis M, Galea LAM,
Einstein G, et al: Associations between age at menopause, vascular
risk and 3-year cognitive change in the canadian longitudinal study
on aging. Neurology. 102:e2092982024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Georgakis MK, Kalogirou EI, Diamantaras
AA, Daskalopoulou SS, Munro CA, Lyketsos CG, Skalkidou A and
Petridou ET: Age at menopause and duration of reproductive period
in association with dementia and cognitive function: A systematic
review and meta-analysis. Psychoneuroendocrinology. 73:224–243.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Song YJ, Li SR, Li XW, Chen X, Wei ZX, Liu
QS and Cheng Y: The effect of estrogen replacement therapy on
Alzheimer's disease and parkinson's disease in postmenopausal
women: A meta-analysis. Front Neurosci. 14:1572020. View Article : Google Scholar : PubMed/NCBI
|