Introduction
Colorectal cancer is a disease that is detrimental
to human health. The rate of colorectal cancer incidence in China
has increased year by year. The mortality rate for colorectal
cancer has risen to rank fourth among deaths caused by malignant
tumors in China. Previous studies have shown that the initiation
and development of colorectal cancer is closely associated with the
malignant transformation of colorectal polyps, particularly in
colorectal hamartomatous polyposis (hereditary hamartomatous
polyposis syndrome, HHPS) (1). An
investigation by Morson et al revealed that 50–70% of
colorectal cancer originates from adenomas and that the rate of
malignant transformation from multiple familial polyps is 100%
(2). A high degree of villous
composition in adenomas is associated with a high rate of malignant
transformation (3). Colorectal
polyps are a common gastrointestinal disease in children. According
to pathological histology, colon polyps are divided into several
subtypes, including colorectal juvenile polyps and colorectal
hamartomatous polyposis (including juvenile polyposis,
Peutz-Jeghers polyps and familial adenomatous polyposis syndrome).
The malignant transformation of colorectal polyps may be induced by
multiple factors, involves multiple genes and is a complex
pathological process that proceeds through multiple stages. We
utilized an immunohistochemical approach to examine the expression
profiles of the molecular markers toll-like receptor 3 (TLR3) and
TLR4 (inflammation response factors), bone morphogenetic protein 2
(BMP2; a cell differentiation and proliferation factor) and
cyclooxygenase-2 (COX2; an enzymatic response factor) in colorectal
juvenile polyp, hamartomatous polyposis, adenoma and adenocarcinoma
tissues and identified the molecular markers that indicate the
early malignant transformation of colorectal polyps.
Materials and methods
Clinical data of patients and details of
the tissue specimens
Twenty children diagnosed with colorectal juvenile
polyp and 15 children diagnosed with colorectal hamartomatous
polyposis (including 9 juvenile polyposis cases; 2 Pentz-Jeghers
syndrome cases and 4 familial adenomatous polyposis syndrome cases)
were treated at the Children’s Hospital of Chongqing Medical
University between June 2006 and June 2011. Twenty adult patients
diagnosed pathologically with colorectal adenoma and 20 adult
patients diagnosed pathologically with colorectal adenocarcinoma
were selected at random from patients who were treated at The
Second Affiliated Hospital of Chongqing Medical University between
June 2006 and June 2011. All diagnoses were confirmed by
pathological examination and all patients and the parents of the
children provided informed consent for tumor preservation and
biological analysis prior to surgery. The following patient
characteristics were recorded: i) the children comprised 25 boys
and 10 girls of which 6 were aged 1–3 years, 20 were aged 3–6 years
and 9 were aged 6–12 years; ii) the adult patients were 27 men and
13 women, of which 2 were 20–30 years old, 2 were 30–40 years old,
11 were 40–50 years old, 15 were 50–60 years old, 5 were 60–70
years old and 5 were 70–80 years old. The tissue specimens were
obtained from the rectum (45 cases), the sigmoid colon (11 cases),
the descending colon (3 cases), the transverse colon (11 cases) and
the ascending colon (5 cases). Consent was obtained either from the
patient or the patient’s family.
Immunohistochemistry
An immunohistochemical streptavidin-peroxidase (SP)
method was used. Staining was performed by strictly following the
manufacturer’s instructions for the staining kit. A positive biopsy
provided in the kit was used as the positive control. Phosphate
buffer was used instead of the primary antibody as the negative
control. The tissue sections were dewaxed and rehydrated and the
sections were treated with 3% hydrogen peroxide for 5 min to
inactivate endogenous peroxidase. The antigens were recovered by
heating in a microwave and the appropriate primary antibody was
then added [BMP2 (1:300), TLR3 (1:500), TLR4 (1:200) and COX2
(1:300)]. The slides were incubated at 4°C in a humidified chamber
overnight, then incubated with a biotin-conjugated secondary
antibody at 37°C for 30 min. Horseradish peroxidase-linked avidin
was then added and the slides were incubated at 37°C for 30 min.
The substrate diaminobenzidine (DAB) was added to develop the color
and the slides were dehydrated and mounted. The image analysis
software Image Pro Plus was used to semi-quantitatively analyze the
images. Five high-magnification (x400) areas of high-quality
staining were randomly selected for every sample. An integrated
optical density (IOD) value was calculated for the positively
stained cells. An average of 5 viewing areas was used to determine
the OD value for the positive cells of the sample.
Statistical analysis
The data were analyzed with the statistical software
SPSS 13.0 and are presented as the mean ± standard deviation (±s).
The groups were compared using the Student’s t-test and p≤0.05 was
considered to indicate a statistically significant result.
Results
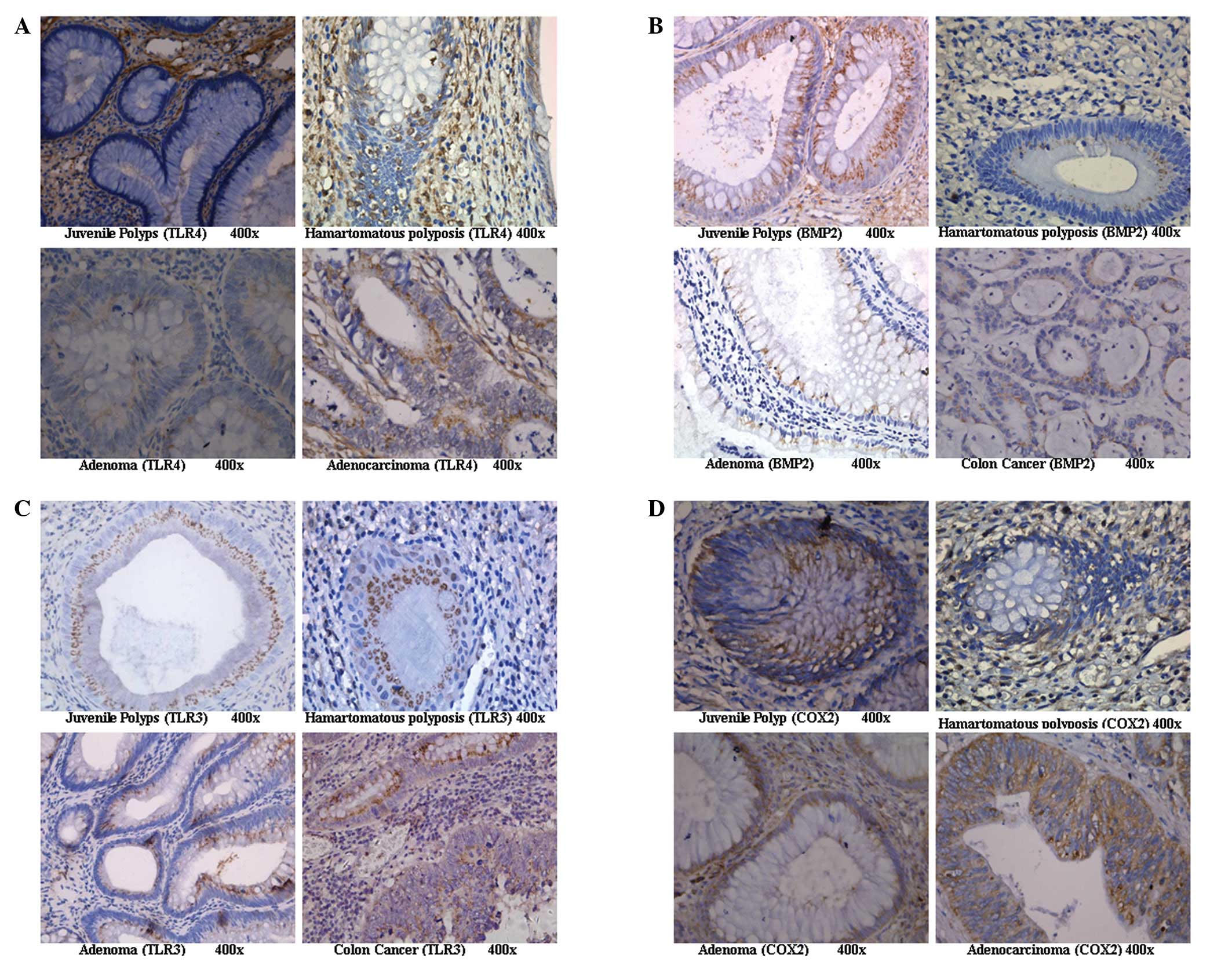
Expression of TLR4 in the colorectal
juvenile polyp, hamartomatous polyposis, adenoma and adenocarcinoma
groups
In the glandular tissue of colorectal juvenile
polyps, TLR4 was weakly expressed or was not expressed at all.
However, TLR4 expression was significantly enhanced in cancerous
tissue and displayed a diffuse or granular distribution on the cell
membrane and in the cytoplasm but was absent from the nucleus
(Fig. 1A). The expression levels
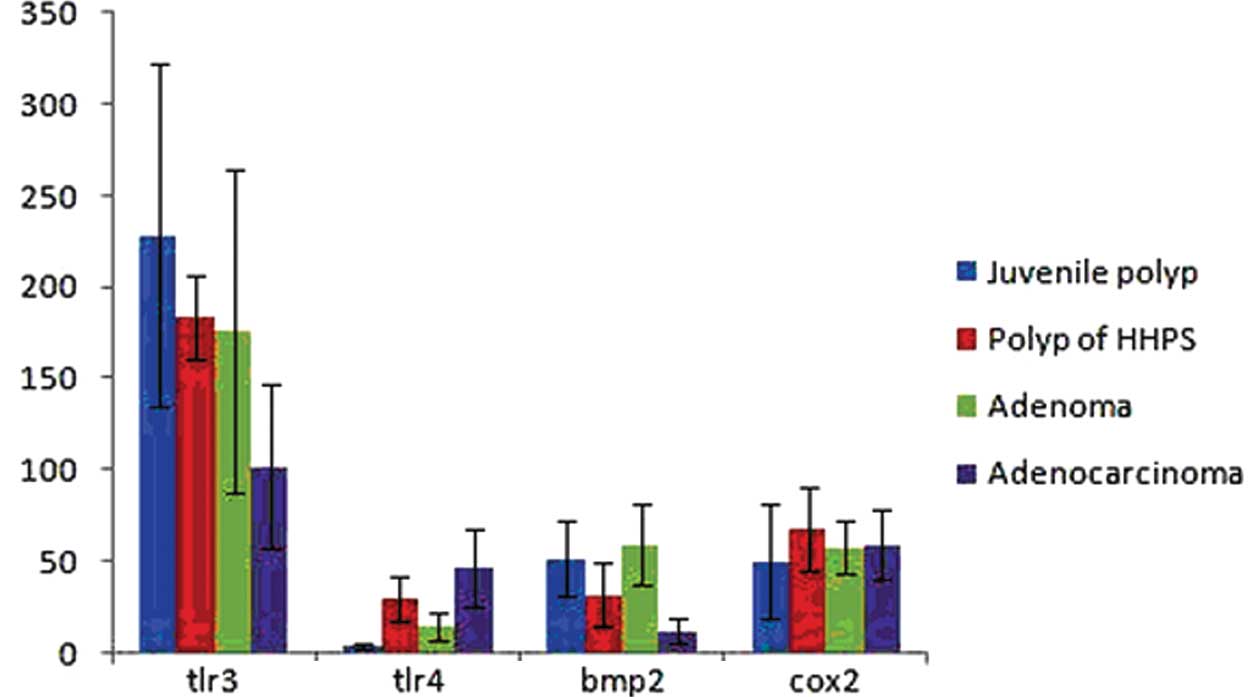
of TLR4 in the colorectal juvenile polyp and colorectal adenoma
groups were significantly lower than those in the colorectal
hamartomatous polyposis and colorectal adenocarcinoma groups. We
observed no significant difference between the juvenile polyp and
adenoma groups (p>0.05) and no significant difference between
the hamartomatous polyposis and adenocarcinoma groups (p>0.05).
However, significant differences between the juvenile polyp and
hamartomatous polyposis groups (p<0.05) and between the adenoma
and adenocarcinoma groups (p<0.05) were observed (Fig. 2).
Expression of BMP2 in the colorectal
juvenile polyp, hamartomatous polyposis, adenoma and adenocarcinoma
groups
BMP2 was expressed in the glandular polyp tissue of
colorectal juvenile polyps and adenomas as uniform diffuse brown
positive staining in the cytoplasm (Fig. 1B). The expression levels of BMP2 in
the colorectal juvenile polyp and adenoma groups were significantly
higher than those in the colorectal hamartomatous polyposis and
adenocarcinoma groups. We observed no significant differences among
the colorectal juvenile polyp, hamartomatous polyposis and adenoma
groups (p>0.05). However, the expression levels of BMP2 were
significantly lower in the colorectal adenocarcinoma group
(p<0.05; Fig. 2).
Expression of TLR3 in the colorectal
juvenile polyp, hamartomatous polyposis, adenoma and adenocarcinoma
groups
The expression of TLR3 on the cell membrane and in
the cytoplasm in the colorectal juvenile polyps was significantly
higher than that in the other groups (Fig. 1C). The expression levels of TLR3
revealed a gradual downward trend from the colorectal juvenile
polyp group to the colorectal hamartomatous polyposis, adenoma and
adenocarcinoma groups, respectively. We observed no significant
differences among the colorectal juvenile polyp, hamartomatous
polyposis and adenoma groups (p>0.05). However, the expression
level of TLR3 was significantly lower in the colorectal
adenocarcinoma group (p<0.05; Fig.
2).
Expression of COX2 in the colorectal
juvenile polyp, hamartomatous polyposis, adenoma and adenocarcinoma
groups
No significant differences in COX2 expression levels
were observed among the 4 treatment groups (p>0.05; Fig. 1D and Fig. 2).
Discussion
TLRs are important membrane receptors that not only
are involved in tissue development and maintenance but also have
critical functions in the innate immune response. TLRs are able to
recognize the highly conserved molecular structure known as the
pathogen-associated molecular pattern (PAMP), which commonly exists
in one or multiple specific types of microbial pathogens and their
metabolic products. The recognition of PAMPs by TLRs initiates an
early response to target the invading pathogen and subsequently
induce the acquired immune response. The ligands of TLRs are
primarily specific metabolic products from microorganisms. TLR3
predominantly recognizes dsDNA from viruses and parasites while
TLR4 particularly recognizes LPS of Gram-negative bacteria
(4). The same PAMP may trigger
different TLRs to produce different cytokines, which suggests that
invasion by the same pathogen may stimulate multiple TLRs and
diversify the TLR-induced innate immune response. The immune system
uses this mechanism to powerfully and effectively remove pathogens.
Chronic infection and inflammation may lead to tumorigenesis and
certain pathogens induce tumorigenesis through TLRs (5). Previous studies have shown that TLR3
stimulated by its ligand participates in tumor cell migration.
Polyinosinic:polycytidylic acid (poly I:C) is recognized by TLR3
and induces the maturation of dendritic cells (DCs), which
effectively stimulates the immune response (6). Upregulation of the expression of
TLR4-induced signal transduction factors in intestinal carcinoma
cells may stimulate the production of immunosuppressive factors,
including TGF-β and VEGF, and modify the local microenvironment of
the tumor to facilitate carcinoma cell escape, proliferation,
invasion and metastasis (7). Our
investigation revealed that in the glandular tissue of juvenile
polyps, TLR4 was weakly expressed or was not expressed at all.
However, TLR4 expression was significantly enhanced in cancer
tissue and displayed a diffuse or granular distribution on the cell
membrane and in the cytoplasm but was absent from the nucleus. The
increased expression of TLR4 in the colorectal hamartomatous
polyposis and adenocarcinoma groups was significant (p<0.05).
The expression of TLR3 on the cell membrane and in the cytoplasm in
the juvenile polyp group was significantly higher than that in the
colorectal hamartomatous polyposis, adenoma and adenocarcinoma
groups (p<0.05). We suggest that TLR3 and TLR4 are important
markers to indicate the early malignant transformation of
colorectal polyps. The difference between the two TLRs suggests
that TLR-mediated mechanisms, in addition to the
inflammation-induced response, may contribute to tumor initiation.
Bendelac et al proposed a “danger model” theory for tumor
tissue (8). The ligands for TLRs
on the cell surface of antigen-presenting cells represent a subset
of the danger signals. Following the initiation of a specific
antitumor immune response, tumor cells are constantly killed by
cytotoxicity, which subsequently causes the continuous production
of the danger signals. Therefore, TLRs are able to continuously
stimulate the antibody-tumor immune response. However, some
researchers consider that toll receptors participate in the
mechanism by which tumor cells escape immune surveillance (9). Tumor cells use TLRs expressed on the
cell surface to generate a tumor-promoting microenvironment and
escape the immune attack.
BMPs were originally discovered as proteins that
induce bone and cartilage formation. However, subsequent studies
have shown that BMPs are widely distributed in multiple tissues and
cell types and have multiple functions, including participation in
embryo formation and development and cell differentiation and
proliferation. BMP signal transduction may be significant in
intestinal cell differentiation (10). Hardwick et al found that
BMP2 inhibits colon epithelial cell growth in vitro, induces
apoptosis and inhibits cell proliferation (11). BMP2 expression was detected in the
epithelial cells of normal adults and of mice; however, BMP2
expression was lost in the micro adenomas of patients with familial
adenomatous polyps. In the current study, BMP2 expression in the
glandular tissue of the polyps was observed as uniformly diffuse
brown positive staining in the cytoplasm and was weakly stained
positive in cancer tissue. The trend towards the significant
decrease in the expression of BMP2 from the colorectal juvenile
polyp group to the colorectal hamartomatous polyposis, adenoma and
adenocarcinoma groups, respectively, suggests that the loss of BMP2
causes hyperplasia of intestinal epithelial cells and
tumorigenesis.
COX2 is a 604-amino acid, rate-limiting enzyme that
converts arachidonic acid into prostaglandins (PGs). Under normal
conditions, COX2 is not expressed in the majority of tissues but is
stimulated when cells are triggered by endogenous and exogenous
signals. Therefore, COX2 is recognized as a “rapid response gene”
(12). COX2 gene overexpression
may enhance the adhesion of intestinal epithelial cells to the
extracellular matrix, extend the G1 phase of the cell cycle 3-fold,
inhibit apoptosis, trigger a series of gene mutations, and
eventually lead to tumor growth. Previous studies have demonstrated
that COX2 expression is closely correlated with the malignant
transformation of intestinal polyps and that COX2 may be used as a
molecular marker for the early malignant transformation of
intestinal polyps (13). Our study
shows that COX2 expression does not significantly differ among the
various groups. We propose that COX2 expression does not change
significantly during the transition from polyps to adenoma and
adenocarcinoma.
In summary, our current study demonstrates that
there are significant differences among the expression levels of
TLR3, TLR4 and BMP2 in colorectal juvenile polyps, hamartomatous
polyposis, adenomas and adenocarcinomas. These three proteins may
be significant in the development and the malignant transformation
of colorectal polyps.
References
|
1
|
Half E, Gldberg Y, Kariv R, et al:
Guidelines for diagnosis, treatment, surveillance and prevention of
cancer in patients with familiar non-adenomatous polyposis.
Harefuah. 150:602011.PubMed/NCBI
|
|
2
|
Morson BG: Genesis of colorectal cancer.
Clin Gastroenterol. 5:505–507. 1976.
|
|
3
|
Winawer SJ, Zauber AG, Fletcher RH, et al:
Guidelines for colonoscopy surveillance after polypectomy. A
consensus update by the US Multi-Society Task Force on Colorectal
Cancer and the American Cancer Society. Gastroenterol.
130:1872–1885. 2006. View Article : Google Scholar
|
|
4
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–367. 2003. View Article : Google Scholar
|
|
5
|
He X and Bai H: Current progress on the
investigation of Toll-like receptor. Northwest National Defense
Medical Journal. 31:445–446. 2010.(In Chinese).
|
|
6
|
Salaun B, Coste I, Rissoan MC, et al: TLR3
can directly trigger apoptosis in human cancer cells. J Immunol.
176:4894–4901. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin H and Meng Q: Measurement of TLR4
expression in human colon cancer by semi quantitative RT-PCR.
Journal of Modern Applied Medicine. 21:682–684. 2009.(In
Chinese).
|
|
8
|
Bendelac A and Medzhitov R: Adjuvants of
immunity: harnessing innate immunity to promote adaptive immunity.
J Exp Med. 195:F19–F23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He L, Zhang L, Li Z and Zhang Q: The roles
of toll-like receptors in carcinogenesis and cancer immunotherapy.
Chin Ger Journal Clin Oncol. 9:118–120. 2010. View Article : Google Scholar
|
|
10
|
Fiocchi C: TGF-beta/Smad signaling defects
in inflammatory bowel disease: mechanisms and possible novel
therapies for chronic inflammation. J Clin Invest. 108:523–526.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hardwick JC, Van Den Brink GR, Bleuming
SA, et al: Bone morphogenetic protein 2 is expressed by, and acts
upon, mature epithelial cells in the colon. Gastroenterology.
126:111–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang F, Warskulat U, Wettstein M, et al:
Hyperosmolarity stimulates prostaglandin synthesis and
cyclooxygenase-2 expression in activated rat liver macrophages.
Biochem J. 312:135–143. 1995.PubMed/NCBI
|
|
13
|
Wasilewicz MP, Kołodziej B, Bojułko T, et
al: Expression of cyclooxygenase-2 in colonic polyps. Pol Arch Med
Wewn. 120:313–320. 2010.PubMed/NCBI
|