Introduction
Immune and inflammatory responses in the central
nervous system (CNS) are principally mediated by microglia. These
responses are activated during neuropathological conditions and
restore CNS homeostasis (1). The
activation of microglia involves proliferation, migration to the
injury site, increased expression of immunomodulators and
transformation into phagocytes (1,2).
Activated microglia also promote neuronal injury through the
release of proinflammatory and cytotoxic factors, including
cytokines, nitric oxide (NO) and reactive oxygen species (ROS)
(2). Chronic microglial activation
has been implicated in neuronal destruction associated with various
neurodegenerative diseases, including Alzheimer’s and Parkinson’s
(3). Therefore, downregulation of
negative-regulatory mechanisms to reduce the activation of
microglial cells is essential to avoid excessive CNS inflammatory
processes (4). The identification
of agents that target over-activated microglial cells is essential
for the reduction of neuronal destruction associated with
neurodegenerative diseases.
Uncaria rhynchophylla is a traditional
oriental herb that has been used for treatment of disorders of the
cardiovascular and central nervous systems (5). Hirsutine (HS) is a major indole
alkaloid of U. rhynchophylla. HS has been reported to have
antihypertensive and antiarrhythmic activities through its effects
on intracellular Ca2+ levels in rat aorta and the action
potential in cardiac muscle (6,7). In
a previous study, HS was demonstrated as effective for the
protection of rat cardiomyocytes from hypoxia-induced cell death
(8). Studies using animal models
have revealed that extracts isolated from U. rhynchophylla
demonstrate neuroprotective potential against diverse neuronal
injuries associated with excitotoxicity, amnesia, epileptic
seizures and Parkinson’s and Alzheimer’s disease (9–13).
In vitro studies on the neuroprotective roles of HS have
demonstrated that the compound attenuates glutamate-induced cell
death in PC12 and cerebellar granule cells (14,15).
Based on these studies, components of U. rhynchophylla have
been proposed to act as neuroprotective agents. However, the
efficacy of the compounds on neuroinflammation control has been
largely unexplored. The purpose of the present study was to examine
the ability of HS in the control of inflammatory responses of the
brain microglia and the protective potential of HS for reducing
inflammation-induced neurotoxicity.
Materials and methods
Drug, chemicals and reagents
All cell and tissue culture products were purchased
from Invitrogen (Carlsbad, CA, USA). HS (product no. 082–0461) was
purchased from Wako Pure Chemical Industries (Osaka, Japan).
Escherichia coli lipopolysaccharide (LPS) and other
chemicals were purchased from Sigma (St. Louis, MO, USA).
Antibodies against phospho-p44/42 MAPK, p44/42 MAPK,
phospho-SAPK/JNK, SAPK/JNK, phospho-p38, p38, phospho-Akt and Akt
were purchased from Cell Signaling Technology (Beverly, MA,
USA).
Experimental animals
Rats were maintained in accordance with the
Institutional Animal Care and Use Committee guidelines of Kyung Hee
University. All animal protocols were approved by the Animal Ethics
Committee of Kyung Hee University in accordance with the 14th
article of the Korean Animal Protection Law.
Organotypic hippocampal slice
culture
Organotypic hippocampal slice cultures were prepared
from male Sprague-Dawley rats (seven days old; Orient, Kyunggido,
Korea) using the methods previously described by Stoppini and
others (16,17). Briefly, the hippocampus was
isolated and cut transversely at a thickness of 350 μm with a
McIlwain Tissue Chopper (Mickle Laboratory Engineering, Surrey,
UK). The slices were placed on membrane inserts (Millicell-CM;
Millipore, Bedford, MA, USA) in six-well plates. Each well
contained 1 ml of culture medium composed of 50% MEM, 25% Hank’s
Balanced Salt Solution and 25% horse serum. The slices were
cultured at 36°C in an incubator in the presence of 5%
CO2 for 12–14 days and the medium was changed every 2–3
days.
LPS treatment and assessment of neuronal
damage
Neurotoxicity was evaluated by the uptake of the
fluorescent dye propidium iodide (PI) as previously described
(17,18). Briefly, LPS (10 μg/ml) was applied
to hippocampal cultures with or without pretreatment with HS.
Following LPS treatment, the culture medium was collected and
subjected to the nitrite assay prior to being replaced with fresh
serum-free medium containing 5 μg/ml PI. Neuronal death was
observed within 30–60 min of PI addition. PI-stained images were
captured using a laser scanning microscope (LSM 510; Carl Zeiss,
Cambridge, UK) and the observed PI-uptake areas were measured using
confocal microscopy with LSM 510 software (release 3.2; Carl
Zeiss). All the data were background subtracted using the
fluorescence emission originated from a region on the insert
containing no tissue. For immunofluorescent staining of neurons,
hippocampal slices were fixed in 4% paraformaldehyde and stained
with Alexa fluor 488-conjugated mouse anti-NeuN monoclonal antibody
(Chemicon International, Temecula, CA, USA). The immunostained
images were observed under a Carl Zeiss LSM 510 microscope.
Primary microglia culture
Primary microglial cells were prepared from cerebral
cortices of one-day-old rat pups (Orient) as described previously
(19,20). Cells reached confluence at 12–14
days and flasks were agitated to remove the microglia. The detached
cells were incubated for 1 h and the non-adherent cells were
removed. The adherent microglial cells were cultured for 24 h and
the purity of the cultures was routinely >95%, as judged by
immunostaining with an anti-OX-42 antibody (Chemicon). The cells
were pretreated with HS in fresh medium containing 0.1% fetal
bovine serum for 30 min prior to the addition of LPS.
Nitrite assay
Nitrite in culture supernatants was measured as an
indicator of NO production. An aliquot of the culture supernatant
was mixed with a volume of Griess reagent (Molecular Probes,
Eugene, OR, USA) and the absorbance at 570 nm was determined using
a microplate reader. Sodium nitrite (0–100 μM) was used as a
standard to assess nitrite concentrations.
Cell viability assay
For the cell viability assay, cultures were
incubated in MTT solution (1 mg/ml; Sigma) in two volumes of
culture medium for 1 h at 37°C. The MTT solution was then removed,
the cells were dissolved in dimethyl sulfoxide (150 μl) and optical
density of the samples was measured at 570 nm using a microplate
reader.
IL-1β and prostaglandin (PG)
E2 assays
Following each treatment, culture medium was
collected in microcentrifuge tubes and centrifuged at 10,000 × g
for 10 min. The supernatants were assayed for secreted mediators
using PGE2 and rat IL-1β immunoassay kits (R&D
Systems, Minneapolis, MN, USA), according to the manufacturer’s
instructions.
Intracellular ROS assay
Presence of intracellular ROS was measured using a
non-fluorescent 2′,7′-dichlorofluorescein (DCFH-DA; Molecular
Probes) dye as described previously (21). DCF fluorescence was measured using
a Wallac 1420 fluorometer (Perkin Elmer, Waltham, MA, USA) at 485
nm for excitation and 530 nm for emission.
Western blot analysis
Cells were lysed on ice in lysis buffer [50 mM
Tris-HCl (pH 8.0), 150 mM NaCl, 1% Triton X-100, 0.5% sodium
deoxycholate, 0.1% SDS, 1 mM EDTA, 1% protease inhibitor cocktail
and 1% phosphatase inhibitor cocktail; Sigma]. Following
centrifugation, the supernatant was collected and assayed for
protein concentration using a DC Protein Assay kit (Bio-Rad,
Hercules, CA, USA). Lysate samples containing 30 μg of protein were
fractionated by SDS-10% polyacrylamide gel electrophoresis and then
electroblotted onto nitrocellulose membranes. The membranes were
probed with primary antibodies and immunoreactivity was detected
with ECL Reagent (Amersham Biosciences, Piscataway, NJ, USA).
Statistical analysis
For statistical analysis, data were expressed as the
mean ± SEM from three independent experiments. The Student’s paired
t-test was used for statistical analyses which were performed using
SPSS software (version 13.0, SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Protection against LPS-mediated neuronal
damage
In an effort to develop neuroprotective drugs,
strategies to ameliorate the inflammatory microenvironment, which
indirectly damages neurons via glial cell mediators, are promising
(4). We previously established an
experimental condition to determine the effect of LPS exposure on
neuronal damage in organotypic hippocampal slice cultures (20). In the present study, slice cultures
exposed to LPS for 72 h exhibited marked PI uptake in the
hippocampus in comparison to untreated control slices (Fig. 1A). The increased PI uptake was
markedly blocked by treatment with HS (Fig. 1A and B), in parallel with the
inhibition of LPS-induced production of various proinflammatory
mediators, including NO, PGE2 and IL-1β (Fig. 1D-F). Reduced immunoreactivity of
NeuN, a neuronal-specific marker, accompanied with elevated PI
fluorescence indicated that loss of neurons resulted from the LPS
insult (Fig. 1C). Treatment with
HS restored immunoreactivity of NeuN and simultaneously decreased
PI fluorescence (Fig. 1C).
Together, these results indicate that HS has protective effects
against inflammation-induced neurotoxicity.
 | Figure 1Effect of HS on LPS-induced
hippocampal cell death. Organotypic hippocampal slice cultures were
pretreated with HS at the indicated concentrations for 30 min prior
to the addition of 10 μg/ml LPS. Following stimulation with LPS for
72 h, the culture medium was replaced with fresh serum-free medium
containing PI. (A) PI fluorescence images. Scale bar, 500 μm. (B)
Quantification of PI images. Data are expressed as percentage of
the LPS value (mean ± SEM, n=10–15 each). (C) The same slices were
immunostained with NeuN that marked neuronal nuclei (NeuN, green;
PI uptake, red). A magnified image from an outlined area in the
region of the LPS-treated hippocampal slices is demonstrated in the
right upper panel to reveal the substantial colocalization of NeuN
immunoreactivity and PI fluorescence. Scale bar, 500 μm.
Determinations of (D) nitrite, (E) PGE2 and (F) IL-1β in
culture supernatants of hippocampal slices. ##P<0.001
vs. control group; **P<0.001, *P<0.05
vs. LPS-only treated group. HS, hirsutine; LPS, lipopolysaccharide;
PI, propidium iodide; PGE2, prostaglandin E2;
IL-1β, interleukin-1β. |
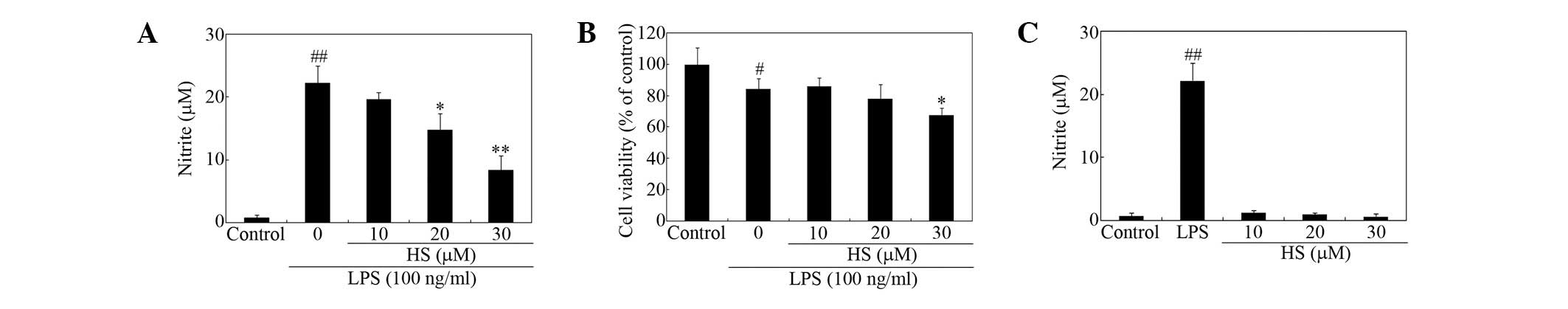
Suppression of LPS-stimulated microglial
inflammatory responses
To determine the mechanisms of the anti-inflammatory
effects of HS in more detail, various mediators of microglial
activation were measured. The effects of treatment with HS on
secretion of proinflammatory mediators from microglial cells were
tested. HS suppressed LPS-induced nitrite release from microglial
cells in a dose-dependent manner (Fig.
2A). Cell viability, as measured using the MTT assay, was not
significantly affected at ≤20 μM (Fig.
2B). HS appeared to decrease cell viability of activated
microglia to a degree at 30 μM (Fig.
2B), however, it alone had no effect on basal NO release at
<30 μM (Fig. 2C).
Effect of HS on secretion of PGE2 and
production of ROS
HS was found to reduce LPS-induced production of
PGE2 (Fig. 3A).
Intracellular ROS act as second messengers in the regulation of the
LPS-stimulated production of neurotoxic factors in microglia
(22). The ROS levels measured
using DCFH-DA revealed that pretreatment with HS decreased
LPS-induced ROS production in microglia (Fig. 3B).
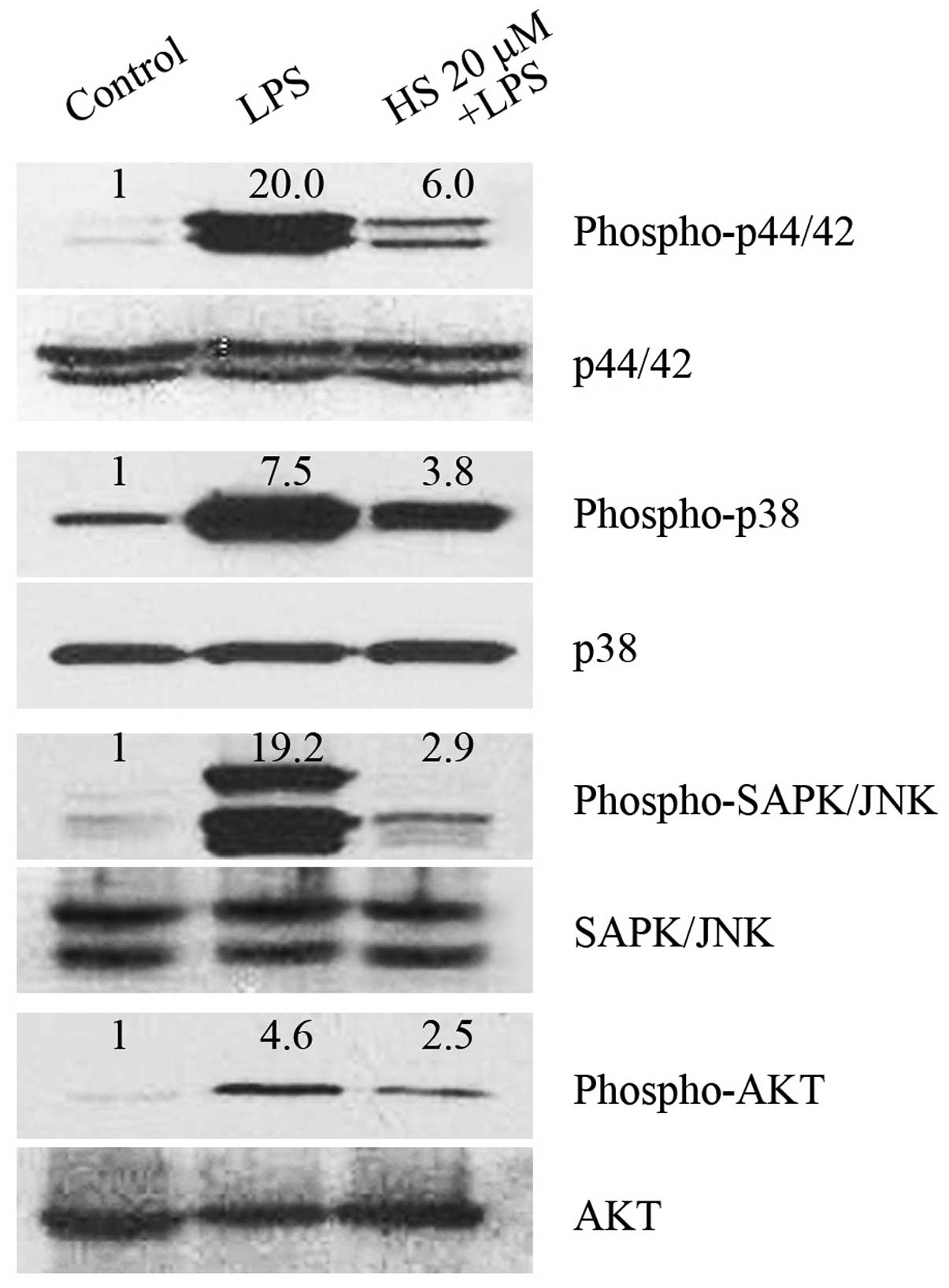
HS inhibits multiple signaling
pathways
Multiple signaling pathways, including those
involving mitogen-activated protein kinases (MAPKs) and Akt, have
been reported to be involved in LPS-induced signal transduction,
which results in the induction of proinflammatory gene expression
(23–26). The present study demonstrates that
HS markedly inhibited the LPS-enhanced phosphorylation of p44/42
MAPK, p38, SAPK/JNK (Fig. 4).
Among the kinases, phosphorylation of SAPK/JNK was reduced to the
greatest extent by HS (Fig. 4).
Taken together, the present data indicate that the
anti-inflammatory action of HS in microglia is, at least in part,
mediated by the inhibition of these signaling pathways.
Discussion
In the present study, HS, one of the major alkaloids
of U. rhynchophylla, effectively repressed diverse
inflammatory mediators induced by LPS, including NO,
PGE2, intracellular ROS and phosphorylation of MAPKs and
Akt in primary microglial cell culture. Beyond the control of
microglial activation, more direct efficacy of HS against
inflammation-induced neurotoxicity was observed in hippocampal
slice cultures. As such, our results demonstrate that HS may be
useful in ameliorating brain disorders associated with uncontrolled
microglia-mediated inflammatory responses. Taken together with
previous studies on anti-inflammatory actions of rhynchophylline
and isorhynchophylline (27,28),
the present study supports a pharmacological potential for U.
rhynchophylla and its active components in manipulating
neuroinflammation associated with diverse neuropathologies.
Among the diverse neuroprotective activities of
U. rhynchophylla, antioxidant properties are well
established (12,14,29).
In previous studies, U. rhynchophylla exhibited the ability
to reduce levels of free radicals in rat brain and increase
glutathione levels in PC12 cells (12,29).
U. rhynchophylla inhibits the NMDA receptor-activated ion
current in hippocampal neurons (10). Additionally, U.
rhynchophylla inhibits the aggregation of amyloid β protein, a
pathological hallmark of Alzheimer’s disease (9). Neuroprotective potential conferred by
HS has also been explained with its inhibitory capacities on
oxidative stress, ion channels and Ca2+ influx (14,15,30).
However, little is known regarding the effects of U.
rhynchophylla and its active compounds on microglia.
According to a previous study, U.
rhynchophylla reduces microglial activation in the region of
neuronal damage caused by kainic acid administration (13). In the present study, U.
rhynchophylla reduced ED1- and inducible NO
synthase-immunoreactive cell counts in rat brain, demonstrating
that U. rhynchophylla may suppress microglia activation
in vivo. Numerous studies have demonstrated an inhibitory
function of rhynchophylline-type alkaloids of U.
rhynchophylla in microglial activation in vitro(27,28).
Rhynchophylline and isorhynchophylline have been shown to suppress
the release of NO and proinflammatory cytokines and the
phosphorylation of p44/42 and p38 MAPKs in LPS-activated N9
microglial cell lines (28).
A detailed study on alkaloids from U.
rhynchophylla previously identified that geissoschizine methyl
ether, a corynanthean-type indole alkaloid, is a potent
acetylcholinesterase inhibitor and may be a suitable candidate for
Alzheimer’s disease (31).
Matsumoto et al demonstrated that isorhynchophylline
regulates neurotransmission by suppressing the serotonin
5-hydroxytryptamine 2A receptor function in the brain by
competitive antagonism (32).
Studies of alkaloids, including the present study, may broaden
understanding of the pharmacological value of these compounds of
U. rhynchophylla in the treatment of neuropathologies, by
highlighting various beneficial roles in neuronal survival,
synaptic plasticity and microglial activation in the CNS. In this
sense, the present results may stimulate further investigation to
confirm novel neuropharmacological roles of each individual
compound of U. rhynchophylla.
References
|
1
|
Hanisch UK and Kettenmann H: Microglia:
active sensor and versatile effector cells in the normal and
pathologic brain. Nat Neurosci. 10:1387–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walter L and Neumann H: Role of microglia
in neuronal degeneration and regeneration. Semin Immunopathol.
31:513–525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugama S, Takenouchi T, Cho BP, Joh TH,
Hashimoto M and Kitani H: Possible roles of microglial cells for
neurotoxicity in clinical neurodegenerative diseases and
experimental animal models. Inflamm Allergy Drug Targets.
8:277–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Skaper SD: The brain as a target for
inflammatory processes and neuroprotective strategies. Ann NY Acad
Sci. 1122:23–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ehrman TM, Barlow DJ and Hylands PJ:
Phytochemical informatics of traditional Chinese medicine and
therapeutic relevance. J Chem Inf Model. 47:2316–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Horie S, Yano S, Aimi N, Sakai S and
Watanabe K: Effects of hirsutine, an antihypertensive indole
alkaloid from Uncaria rhynchophylla, on intracellular
calcium in rat thoracic aorta. Life Sci. 50:491–498. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masumiya H, Saitoh T, Tanaka Y, Horie S,
Aimi N, Takayama H, Tanaka H and Shigenobu K: Effects of hirsutine
and dihydrocorynantheine on the action potentials of sino-atrial
node, atrium and ventricle. Life Sci. 65:2333–2341. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu LX, Gu XF, Zhu YC and Zhu YZ:
Protective effects of novel single compound, hirsutine on hypoxic
neonatal rat cardiomyocytes. Eur J Pharmacol. 650:290–297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujiwara H, Takayama S, Iwasaki K, Tabuchi
M, Yamaguchi T, Sekiguchi K, Ikarashi Y, Kudo Y, Kase Y, Arai H and
Yaegashi N: Yokukansan, a traditional Japanese medicine,
ameliorates memory disturbance and abnormal social interaction with
anti-aggregation effect of cerebral amyoid β proteins in amyloid
precursor protein transgenic mice. Neuroscience. 180:305–313.
2011.PubMed/NCBI
|
|
10
|
Lee J, Son D, Lee P, Kim DK, Shin MC, Jang
MH, Kim CJ, Kim YS, Kim SY and Kim H: Protective effect of methanol
extract Uncaria rhynchophylla against excitotoxicity induced
by N-methyl-d-aspartate in rat hippocampus. J Pharmacol Sci.
92:70–73. 2003.
|
|
11
|
Lee SC, Linh PT, Jing Z, Ryu SY, Myung CS,
Kim YH and Kang JS: Effects of repeated administration of
Uncaria hooks on the acquisition and central neuronal
activities in ethanol-treated mice. J Ethnopharmacol. 94:123–128.
2004.
|
|
12
|
Shim JS, Kim HG, Ju MS, Choi JG, Jeong SY
and Oh MS: Effects of the hook of Uncaria rhynchophylla on
neurotoxiciy in the 6-hydroxydopamin model of Parkinson’s disease.
J Ethnopharmacol. 126:361–365. 2009.
|
|
13
|
Tang NY, Liu CH, Su SY, Jan YM, Hsieh CT,
Cheng CY, Shyu WC and Hsieh CL: Uncaria rhynchophylla (Miq)
Jack plays a role in neuronal protection in kainic acid-treated
rats. Am J Chin Med. 38:251–263. 2010. View Article : Google Scholar
|
|
14
|
Kawakami Z, Kanno H, Ikarashi Y and Kase
Y: Yokukansan, a kampo medicine, protects against glutamate
cytotoxicity due to oxidative stress in PC12 cells. J
Ethnopharmacol. 134:74–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimada Y, Goto H, Itoh T, Sakakibara I,
Kubo M, Sasaki H and Terasawa K: Evaluation of the protective
effects of alkaloids isolated from the hooks and stems of
Uncaria sinensis on glutamate-induced neuronal death in
cultured cerebellar granule cells from rats. J Pharm Pharmacol.
51:715–722. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stoppini L, Buchs PA and Muller D: A
simple method for organotypic cultures of nervous tissue. J
Neurosci Methods. 37:173–182. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
You JM, Yun SJ, Nam KN, Kang C, Won R and
Lee EH: Mechanism of glucocorticoid-induced oxidative stress in rat
hippocampal slice cultures. Can J Physiol Pharmacol. 87:440–447.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vornov JJ, Tasker RC and Park J:
Neurotoxicity of acute glutamate transport blockade depends on
coactivation of both NMDA and AMPA/kainate receptors in organotypic
hippocampal cultures. Exp Neurol. 133:7–17. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCarthy KD and de Vellis J: Preparation
of separate astroglial and oligodendroglial cell cultures from rat
cerebral tissue. J Cell Biol. 85:890–902. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nam KN, Son MS, Park JH and Lee EH:
Shikonins attenuate microglial inflammatory responses by inhibition
of ERK, Akt and NF-kappaB: neuroprotective implications.
Neuropharmacology. 55:819–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nam KN, Choi YS, Jung HJ, Park GH, Park
JM, Moon SK, Cho KH, Kang C, Kang I, Oh MS and Lee EH: Genipin
inhibits the inflammatory response of rat brain microglial cells.
Int Immunopharmacol. 10:493–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin L, Liu Y, Wang T, Wei SJ, Block ML,
Wilson B, Liu B and Hong JS: NADPH oxidase mediates
lipopolysaccharide-induced neurotoxicity and proinflammatory gene
expression in activated microglia. J Biol Chem. 279:1415–1421.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhat NR, Zhang P, Lee JC and Hogan EL:
Extracellular signal-regulated kinase and p38 subgroups of
mitogen-activated protein kinases regulate inducible nitric oxide
synthase and tumor necrosis factor-alpha gene expression in
endotoxin-stimulated primary glial cultures. J Neurosci.
18:1633–1641. 1998.
|
|
24
|
Jones BW, Heldwein KA, Means TK, Saukkonen
JJ and Fenton MJ: Differential roles of toll-like receptors in the
elicitation of proinflammatory responses by macrophages. Ann Rheum
Dis. 60:6–12. 2001.PubMed/NCBI
|
|
25
|
Koistinaho M and Koistinaho J: Role of p38
and p44/42 mitogen-activated protein kinases in microglia. Glia.
40:175–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YG, Lee J, Byeon SE, Yoo DS, Kim MH,
Lee SY and Cho JY: Functional role of Akt in macrophage-mediated
innate immunity. Front Biosci. 16:517–530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan D, Ma B, Wu C, Yang J, Zhang L, Liu
S, Wu L and Kano Y: Alkaloids from the leaves of Uncaria
rhynchophylla and their inhibitory activity and NO production
in lipopolysaccharide-activated microglia. J Nat Prod.
71:1271–1274. 2008.
|
|
28
|
Yuan D, Ma B, Yang J, Xie Y, Wang L, Zhang
L, Kano Y and Wu C: Anti-inflammatory effects of rhynchophylline
and isorhynchophylline in mouse N9 microglial cells and the
molecular mechanism. Int Immunopharmacol. 9:1549–1554. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsieh CL, Tang NY, Chiang SY, Hsieh CT and
Lin JG: Anticonvulsive and free radical scavenging actions of two
herbs, Uncaria rhynchophylla (Miq) Jack and Gastrodia
elata Bl, in kainic acid-treated rats. Life Sci. 65:2071–2082.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakazawa K, Watano T, Ohara-Imaizumi M,
Inoue K, Fujimori K, Ozaki Y, Harada M and Takanaka A: Inhibition
of ion channels by hirsutine in rat pheochromocytoma cells. Jpn J
Pharmacol. 57:507–515. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang ZD, Duan DZ, Du J, Yang MJ, Li S and
Yao XJ: Geissoschizine methyl ether, a corynanthean-type indole
alkaloid from Uncaria rhynchophylla as a potential
acetylcholinesterase inhibitor. Nat Prod Res. 26:22–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Matsumoto K, Morishige R, Murakami Y,
Tohda M, Takayama H, Sakakibara I and Watanabe H: Suppressive
effects of isorhynchophylline on 5-HT2A receptor function in the
brain: behavioural and electrophysiological studies. Eur J
Pharmacol. 517:191–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee P, Lee J, Kim S, Lee MS, Yagita H, Kim
SY, Kim H and Suk K: NO as an autocrine mediator in the apoptosis
of activated microglial cells: correlation between activation and
apoptosis of microglial cells. Brain Res. 892:380–385. 2001.
View Article : Google Scholar : PubMed/NCBI
|