Introduction
Pulmonary hypertension (PH) is induced under various
conditions, including hypoxia and is defined as a hemodynamic
abnormality caused by a number of structural and functional
alterations. Exposure to hypoxia leads to sustained vascular
contraction, persistent elevation in pulmonary artery pressure,
vascular remodeling of the pulmonary arteries and eventually to
right-side heart failure. Recent studies have confirmed that
endothelial apoptosis is the initial and triggering event for PH
(1). When exposed to various
environmental triggers, including hypoxia, excessive apoptosis of
pulmonary arterial endothelial cells (PAECs) leads to loss of
pre-capillary arteriolar continuity, endothelial dysfunction and
abnormal overgrowth of pulmonary arterial smooth muscle cells.
These events may lead to increased pulmonary vascular resistance
and appearance of abnormal apoptosis-resistant, hyperproliferative
PAECs which form occlusive intimal and plexiform lesions (1). A number of studies have also reported
the importance of endothelial nitric oxide synthase (eNOS) in the
endothelial system. Endothelial dysfunction is characterized by
reduced synthesis of NO (2–4) and
hypoxia decreases expression of eNOS (5), which further jeopardizes endothelial
function. Therefore, new treatments for PH should be directed
towards antagonizing PAEC apoptosis and restoring eNOS
expression.
p38 mitogen-activated protein kinases (p38 MAPKs)
are a class of evolutionarily conserved serine/threonine protein
kinases (6). Recent studies
(7,8) have focused on the response of p38
MAPK towards hypoxia and its role in the pathogenesis of hypoxic
PH. Analysis of the cardiovascular and pulmonary vascular systems
indicates that p38 MAPK is closely associated with endothelial
function, particularly the regulation of endothelial cell
proliferation/apoptosis and expression of eNOS (7,9–11).
Nicorandil is a mitochondrial ATP-sensitive
potassium (mitoKATP) channel activator with a nitrate-like action.
The molecule dilates peripheral and coronary arterioles and
systemic veins by opening mitoKATP channels in vascular smooth
muscle cells. Previous studies indicated that KATP channels may
also affect the endothelin system. Nicorandil inhibits endothelial
apoptosis, enhances eNOS expression and preserves endothelial
function via activation of the mitoKATP channels in the endothelial
cell (12–16). In addition, KATP channels have been
reported to interact with various MAPK signals (3,17–19).
Therefore, we hypothesized that nicorandil
antagonizes apoptosis induced by hypoxia and upregulates eNOS
expression via activation of the mitoKATP channels in PAEC and this
endothelial protective effect may involve the inhibition of p38
MAPK phosphorylation. To test this hypothesis, the effect of
nicorandil on hypoxia-induced apoptosis in human PAEC (HPAEC), eNOS
expression and phosphorylation of p38 MAPK was investigated.
Materials and methods
Materials
Cell culture medium components were purchased from
Hyclone Laboratories (Logan, UT, USA). HPAECs were obtained from
(ScienCell Research Laboratories, Carlsbad, CA, USA). SB203580,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
5-hydroxydecanoate (5-HD), Hoechst 33342, annexin
V-fluoresceinisothocyanate (FITC) and propidium iodide (PI) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Nicorandil was
purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
Antibodies against phosphorylated (phospho)-p38 MAPK, p38 MAPK,
Bcl-2-associated X protein (Bax), B-cell lymphoma 2, (Bcl-2),
caspase-8 and −9, the cleaved form of caspase-3 and eNOS were
purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA)
and β-actin was obtained from Santa Cruz Biotechnology Inc., (Santa
Cruz, CA, USA). AnaeroPack-Anaero was purchased from Mitsubishi Gas
Chemical Co. (Tokyo, Japan).
Cell culture and treatment
HPAECs were plated in 100-mm plastic tissue culture
dishes and cultured in growth medium composed of 10% (v/v) FBS, 1%
(v/v) VEGF and 100 U/ml penicillin and streptomycin in a humidified
incubator containing 5% CO2 at 37°C. Drugs and hypoxia
treatments were performed when cells reached 70–80% confluency.
HPAECs were divided into 5 groups: i) control, cultured in normoxia
(20% O2, 5% CO2); ii) hypoxia alone, exposed
to hypoxia for 24 h; iii) hypoxia + nicorandil, treated with
nicorandil (10 μM, 100 and 1,000 μM for MTT or 100 μM for other
methods) prior to 24 h hypoxia; iv) hypoxia + nicorandil + 5-HD,
pretreated with 5-HD (500 μM) 30 min prior to nicorandil followed
by hypoxia treatment; and v) hypoxia + SB203580, pretreated with
SB203580 (10 μM) prior to 24 h hypoxia. AnaeroPack-Anaero was used
to create the hypoxic environment.
Cell growth assay
Cell viability of HPAEC was determined by measuring
the MTT dye absorbance in cells. Cells were seeded in 96-well
microtiter plates with 3×104 cells/well. Following drug
treatment, the cells were exposed to hypoxia and MTT solution (5
mg/ml in PBS) was added to the plates (20 μl/well) and incubated
for an additional 4 h at 37°C. MTT solution was discarded and DMSO
(100 μl) was added to each well to solubilize formazan crystals
formed in viable cells. A microplate reader was used to measure
optical density at 570 nm.
Flow cytometry
Cell apoptosis was measured by flow cytometry.
HPAECs were seeded in 6-well plates (2×105 cells/well).
Cells were treated as described above. Trypsin was added to each
well following hypoxia and detached cells were collected and double
stained for 10 min with FITC-coupled Annexin V protein and PI. Flow
cytometry was performed with a 488-nm laser coupled to a cell
sorter. Cells stained with Annexin V and PI or Annexin V only were
considered necrotic and apoptotic, respectively.
Fluorescent staining of cells with
Hoechst 33342
Cells were cultured in 24-well plates and treated at
~80% confluency, as described above. Following hypoxia, the cells
were washed with PBS prior to 20 min staining with Hoechst 33342
(10 mg/ml). Images of stained cells were captured by fluorescence
microscopy and the percentage of apoptosis was calculated by
counting (>500 cells were scored/group).
Western blot analysis
Expression of phospho-p38 MAPK/p38 MAPK, Bax, Bcl-2,
caspase-8 and −9, the cleaved form of caspase-3 and eNOS was
detected to study the mechanisms of nicorandil on apoptosis of
HPAEC at the molecular level. Cell lysates contained 90% RIPA
buffer and 10% PMSF and protease and phosphatase inhibitors were
added to the cell culture dishes when the cells were 80% confluent.
Lysates were collected and centrifuged at 14,000 × g for 15 min and
supernatants were boiled with SDS loading buffer for 10 min. Equal
amounts of sample were loaded onto 12/15% SDS-polyacrylamide gels
and transferred to PVDF membranes (Millipore, Billerica, MA, USA).
Membranes were blocked for 1 h in 5% non-fat milk in PBS and then
incubated with primary antibodies overnight at 4°C. This process
was followed by 3 TBST washes for 5 min and incubation with
peroxidase-conjugated goat anti-rabbit IgG (1:10,000; Pierce
Biotechnology, Inc., Rockford, IL, USA) for 1 h at room
temperature. Membranes were again washed 3 times with TBST and
developed using the ECL chemiluminescence detection system.
Statistical analysis
Data were expressed as the mean ± SE and analyzed by
one-way analysis of variance ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of nicorandil on cell growth and
death in hypoxia-treated HPAECs
HPAECs were treated with nicorandil, 5-HD or
SB203580 in the presence or absence of hypoxia, as described.
Following this, an MTT assay was performed to examine the effect of
nicorandil on cell viability. Hypoxia treatment alone was found to
significantly decrease cell growth (Fig. 1A), which was antagonized by
nicorandil in a dose-dependent manner (Fig. 1B). The effect of nicorandil was
completely blocked by the mitoKATP inhibitor, 5-HD (Fig. 1B). The p38 inhibitor SB203580 also
had a similar effect to nicorandil on viability of HPAEC.
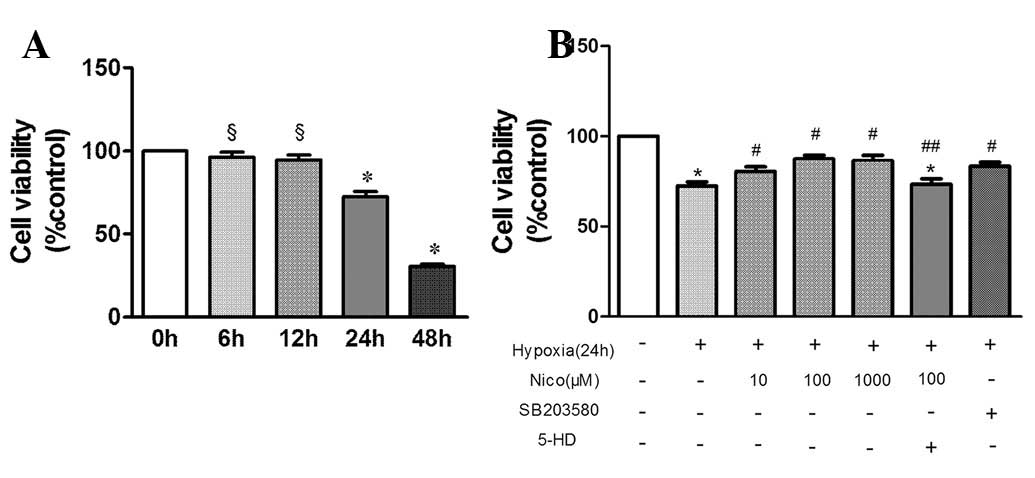 | Figure 1Cell viability using MTT assay. (A)
Exposure to various durations of hypoxia. (B) Effect of various
doses of nicorandil and SB203580 on HPAEC cell viability following
exposure to hypoxia for 24 h. §P>0.05, vs. control;
*P<0.05, vs. control; #P<0.05, vs.
hypoxia treatment alone; ##P<0.05 vs. hypoxia +
nicorandil. Results are shown as the mean ± SE (n=6). MTT,
3-(4,5-dimethylthiazol-2yl-)-2,5-diphenyltetrazolium bromide;
HPAEC, human pulmonary arterial endothelial cells, Nico,
nicorandil; 5-HD, 5-hydroxydecanoate. |
Nicorandil hypoxia-induced apoptosis in
HPAEC
Apoptosis in HPAEC was detected by the Annexin
V-FITC/PI double staining method (Fig.
2) to confirm the protective role of nicorandil against
hypoxia-induced apoptosis. Frequency of apoptosis increased from
1.917±0.184 in control to 19.435±2.342% in the hypoxia group
(P<0.05). However, treatment with nicorandil and SB203580
decreased apoptosis to 7.341±0.763 and 8.312±0.851%, respectively.
No significant difference was found between pretreatment with 5-HD
and hypoxia alone (P<0.05). Nicorandil was observed to protect
HPAEC from hypoxia-induced apoptosis and this effect was blocked
completely by 5-HD. The p38 MAPK inhibitor SB203580 demonstrated a
similar protective effect.
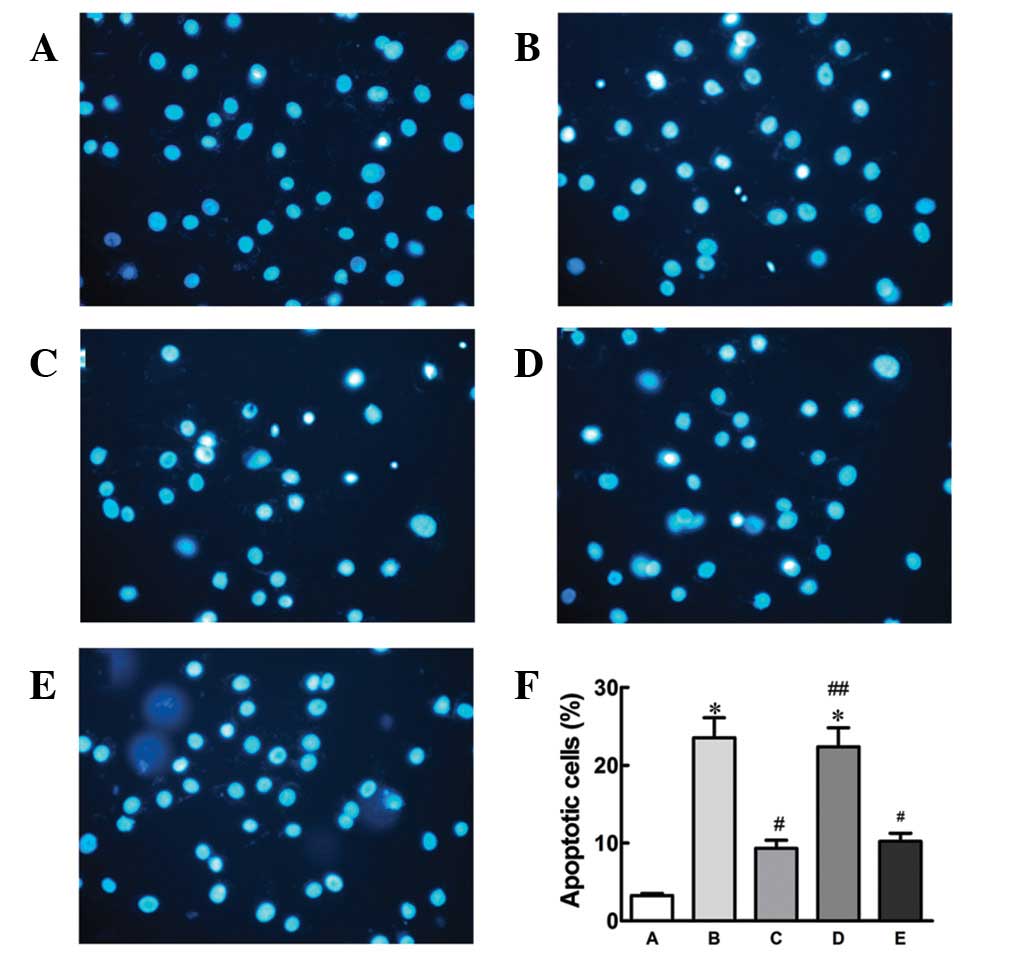
To confirm the protective effect of nicorandil
against hypoxia-induced apoptosis, Hoechst 33342 staining was
performed to detect apoptosis (Fig.
3). Compared with the control, hypoxia treatment alone
increased apoptosis in cells, which was antagonized by nicorandil
or SB203580. No significant difference was found between treatment
with 5-HD prior to nicorandil and hypoxia alone, indicating that
the protective effects of nicorandil against apoptosis were
completely blocked by 5-HD.
Nicorandil inhibited phosphorylation of
p38 induced by hypoxia
Results demonstrated that nicorandil protects HPEAC
from hypoxia-induced apoptosis by opening mitoKATP channels. To
investigate the effects of nicorandil on the phosphorylation of p38
MAPK induced by hypoxia, western blot analysis was used to
determine expression levels of phospho- and total-p38 MAPK
(Fig. 4A). The results
demonstrated that hypoxia treatment alone increases phosphorylation
of p38 MAPK. However, a significant decrease was observed in the
nicorandil and SB20380 treatment groups, while 5-HD blocked the
antagonistic effect of nicorandil against phosphorylation of p38
MAPK.
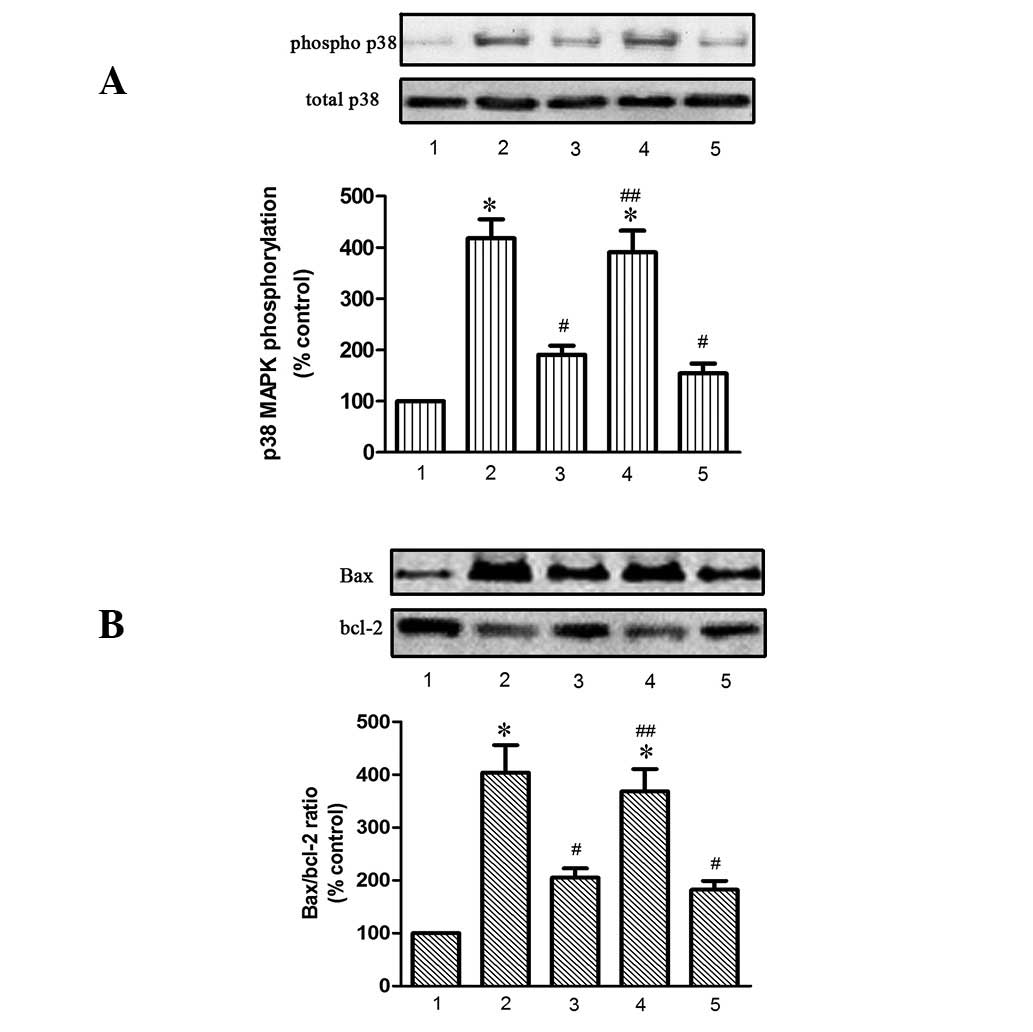 | Figure 4Nicorandil and SB203580 (A) inhibit
phosphorylation of p38 MAPK and (B) increase the Bax/Bcl-2 ratio
induced in HPAEC following induction of hypoxia for 24 h. 1,
control; 2, hypoxia alone; 3, hypoxia + nicorandil; 4, hypoxia +
nicorandil + 5-HD; and 5, hypoxia + SB203580.
*P<0.05, vs. control; #P<0.05, vs.
hypoxia treatment alone; ##P<0.05, vs. hypoxia +
nicorandil. Results are shown as the mean ± SE (n=4). HPAEC, human
pulmonary arterial endothelial cells; Bax, Bcl-2-associated X
protein; Bcl-2, B-cell lymphoma 2; 5-HD, 5-hydroxydecanoate;
phospho, phosphorylated. |
Nicorandil inhibited expression of
apoptosis-related proteins induced by hypoxia
To further investigate the potential cell signaling
mechanisms involved, apoptosis-related proteins Bax, Bcl2,
caspase-8 and −9 and cleaved caspase-3 were assessed (Figs. 4B and 5). Expression of eNOS (Fig. 5) was also assayed. As revealed in
Figs. 4 and 5, hypoxia alone increased the expression
of these apoptotic proteins but reduced synthesis of eNOS. This
effect was significantly antagonized by nicorandil and SB203580,
while 5-HD completely blocked the beneficial effect of
nicorandil.
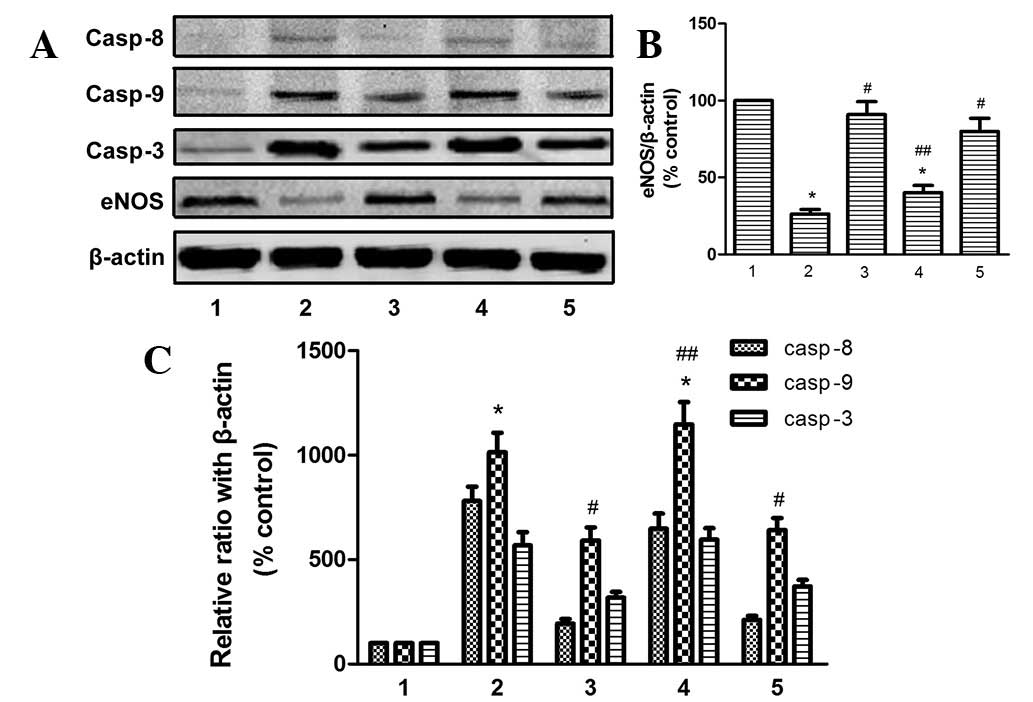 | Figure 5Effect of nicorandil and SB203580 on
casp-8, −9 and −3 and eNOS in HPAEC following exposure to hypoxia
for 24 h. 1, control; 2, hypoxia alone; 3, hypoxia + nicorandil; 4,
hypoxia + nicorandil + 5-HD; 5, hypoxia + SB203580.
*P<0.05, vs. control; #P<0.05, vs.
hypoxia treatment alone; ##P<0.05, vs. hypoxia +
nicorandil. Results are thrown as the mean ± SE (n=4). Casp,
caspase; eNOS, endothelial nitric oxide synthase; HPAEC, human
pulmonary arterial endothelial cells; 5-HD, 5-hydroxydecanoate. |
Discussion
Results of the present study support our hypothesis
that nicorandil protects endothelial cell function by opening
mitoKATP channels and reversing activation of p38 MAPK. A number of
studies have reported a protective role of nicorandil against cell
apoptosis in various cell types. In the cardiovascular system,
nicorandil appears to reduce mitochondrial impairment and inhibit
apoptosis in cardiomyocytes by various mechanisms (12,13,19,20).
Similar results were also found in studies involving neurons
(21). In vascular endothelial
cells, nicorandil inhibits serum starvation-induced apoptosis
(22). In the present study, the
effects of nicorandil on hypoxia-induced apoptosis in HPAEC were
evaluated. Results demonstrate that nicorandil maintained cell
viability in a dose-dependent manner (Fig. 1B) by antagonizing hypoxia-induced
apoptosis (Figs. 2 and 3). This maintenance was completely
abrogated by treatment with 5-HD, a mitoKATP channel inhibitor,
indicating that this effect was mediated by mitoKATP channels in
HPAEC.
Several studies have revealed a correlation between
nicorandil and the MAPK signaling pathway (17–19,23),
particularly p38 MAPK, a kinase associated with apoptosis (11,24,25).
Previously, hypoxia was demonstrated to induce apoptosis of HUVECs
in an in vitro capillary model (24) by activating p38 MAPK. In an
additional study (11), inhibition
of p38 MAPK reduced pyrogallol-induced apoptosis. In this study,
the p38 MAPK inhibitor, SB203580, markedly reduced hypoxia-induced
apoptosis in HPAEC (Figs. 2 and
3). Results indicate that p38 MAPK
is a major mediator of hypoxia-induced apoptosis. Therefore, we
suggest that the effect of nicorandil against endothelial apoptosis
may involve the regulation of p38 MAPK. To test this hypothesis,
the effect of nicorandil on phosphorylated p38 MAPK was analyzed.
Hypoxia increased expression of phospho-p38, consistent with
previous studies (7,8). Nicorandil and SB203580 markedly
decreased phosphorylation of p38 MAPK, while this effect was
reversed by 5-HD. Results indicate that nicorandil inhibits
phosphorylation of p38 MAPK via activation of the mitoKATP
channels. The association between mitoKATP channels and p38 MAPK
remains to be adequately studied, particularly in PAEC. In an in
vivo cardiac model, activation of mitoKATP channels restored
protection of preconditioning via p38 MAPK (17), while an additional study using
microglia cells revealed that activation of microglial KATP
channels inhibits rotenone-induced neuroinflammation by
deactivating p38 MAPK (18).
Results of the present study confirm this interaction between
mitoKATP channels and p38 MAPK in PAEC when exposed to a hypoxic
environment.
The cell death signals involved in the protective
effect of nicorandil against hypoxia-induced apoptosis were also
explored. We found that nicorandil and SB203580 reduced the
Bax/Bcl2 ratio and downregulated the expression of caspase-8, −9
and −3, compared with hypoxia alone. The effect of nicorandil on
these proteins was completely eliminated by 5-HD. Cell apoptosis is
induced by the mitochondrial and death receptor signaling pathways.
The former involves translocation of Bax, leading to caspase-9
activation via the release of cytochrome c. The latter is triggered
by members of the death receptor family followed by recruitment of
caspase-8. Caspase-8 and −9 activation also activates caspase-3,
eventually leading to cell apoptosis. Early studies involving
various cell types demonstrated that nicorandil is a mitoKATP
channel activator and largely affects the mitochondrial pathway by
reducing the Bax/Bcl2 ratio and caspase-3 activation (13). Present results indicate that
nicorandil also affects the death receptor pathway. It is well
known that activation of p38 MAPK is a critical event leading to
induction of the cell death pathway (26,27).
Consistent with these observations, the present study confirmed
that nicorandil downregulates the two distinct cell death signaling
pathways via deactivation of p38 MAPK (Fig. 5).
A number of studies (14–16)
have demonstrated the critical role of eNOS in PH, particularly in
the maintenance of endothelial function. Findings of studies on
cardiovascular disease have shown that nicorandil protects
endothelial function (14) and
enhances eNOS expression (16). In
a monocrotaline-induced pulmonary arterial hypertension model,
similar effects of nicorandil were identified (15). Therefore, in the present study,
expression of eNOS was determined and regulatory mechanisms were
explored. Results indicate that nicorandil induced significant
upregulation of eNOS, which was suppressed by hypoxia via
deactivation of p38 MAPK (Figs. 4
and 5). These results are in
accordance with a previous study (9) on the effects of p38 MAPK on eNOS,
which indicated that activation of p38 MAPK is responsible for the
downregulation of eNOS.
In previous years, various cell signaling pathways
involved in hypoxia-induced apoptosis have been extensively studied
in various cell types (22,28,29).
Additional studies are required to determine whether the beneficial
effect of nicorandil against apoptosis and endothelial dysfunction
induced by hypoxia also involves other signaling pathways. Future
studies are likely to focus on the mechanism by which nicorandil
inhibits phosphorylation of p38 MAPK and also on the involvement of
additional cell signaling pathways, including phosphoinositide
3-kinase/AKT, c-Jun N-terminal kinase, extracellular
signal-regulated kinase 1/2 and nuclear factor-κB and the
cross-talk between them.
In conclusion, results of this study have
demonstrated that nicorandil protects HPAEC from hypoxia-induced
apoptosis by inhibiting the mitochondrial and death receptor
pathways and aids maintenance of endothelial function by restoring
eNOS expression. This protective effect is hypothesized to be
associated with deactivation of p38 MAPK via mitoKATP channels.
Acknowledgements
The present study was supported by NSFC (no.
81273571).
References
|
1
|
Jurasz P, Courtman D, Babaie S and Stewart
DJ: Role of apoptosis in pulmonary hypertension: from experimental
models to clinical trials. Pharmacol Ther. 126:1–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Linke A, Recchia F, Zhang X and Hintze TH:
Acute and chronic endothelial dysfunction: implications for the
development of heart failure. Heart Fail Rev. 8:87–97. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawashima S and Yokoyama M: Dysfunction of
endothelial nitric oxide synthase and atherosclerosis. Arterioscler
Thromb Vasc Biol. 24:998–1005. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Triggle CR, Hollenberg M, Anderson TJ, et
al: The endothelium in health and disease - a target for
therapeutic intervention. J Smooth Muscle Res. 39:249–267. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Steudel W, Scherrer-Crosbie M, Bloch KD,
et al: Sustained pulmonary hypertension and right ventricular
hypertrophy after chronic hypoxia in mice with congenital
deficiency of nitric oxide synthase 3. J Clin Invest.
101:2468–2477. 1998. View
Article : Google Scholar
|
|
6
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weerackody RP, Welsh DJ, Wadsworth RM and
Peacock AJ: Inhibition of p38 MAPK reverses hypoxia-induced
pulmonary artery endothelial dysfunction. Am J Physiol Heart Circ
Physiol. 296:H1312–H1320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang CL, Song F, Zhang J and Song QH:
Hypoxia-induced Bcl-2 expression in endothelial cells via p38 MAPK
pathway. Biochem Biophys Res Commun. 394:976–980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xing F, Jiang Y, Liu J, et al:
Downregulation of human endothelial nitric oxide synthase promoter
activity by p38 mitogen-activated protein kinase activation.
Biochem Cell Biol. 84:780–788. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grossini E, Molinari C, Caimmi PP, Uberti
F and Vacca G: Levosimendan induces NO production through p38 MAPK,
ERK and Akt in porcine coronary endothelial cells: role for
mitochondrial K(ATP) channel. Br J Pharmacol. 156:250–261. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han YH, Moon HJ, You BR, Kim SZ, Kim SH
and Park WH: JNK and p38 inhibitors increase and decrease
apoptosis, respectively, in pyrogallol-treated calf pulmonary
arterial endothelial cells. Int J Mol Med. 24:717–722.
2009.PubMed/NCBI
|
|
12
|
Sanbe A, Marunouchi T, Yamauchi J,
Tanonaka K, Nishigori H and Tanoue A: Cardioprotective effect of
nicorandil, a mitochondrial ATP-sensitive potassium channel opener,
prolongs survival in HSPB5 R120G transgenic mice. PLoS One.
6:e189222011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishikawa S, Tatsumi T, Shiraishi J, et
al: Nicorandil regulates Bcl-2 family proteins and protects cardiac
myocytes against hypoxia-induced apoptosis. J Mol Cell Cardiol.
40:510–519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao JL, Yang YJ, Chen JL, Kang LM, Wu Y
and Gao RL: Nicorandil reduces myocardial no-reflow by protection
of endothelial function via the activation of KATP channel. Clin
Chim Acta. 374:100–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hongo M, Mawatari E, Sakai A, et al:
Effects of nicorandil on monocrotaline-induced pulmonary arterial
hypertension in rats. J Cardiovasc Pharmacol. 46:452–458. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horinaka S, Kobayashi N, Higashi T, Hara
K, Hara S and Matsuoka H: Nicorandil enhances cardiac endothelial
nitric oxide synthase expression via activation of adenosine
triphosphate-sensitive K channel in rat. J Cardiovasc Pharmacol.
38:200–210. 2001. View Article : Google Scholar
|
|
17
|
Iliodromitis EK, Aggeli IK, Gaitanaki C,
et al: p38-MAPK is involved in restoration of the lost protection
of preconditioning by nicorandil in vivo. Eur J Pharmacol.
579:289–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou F, Yao HH, Wu JY, Ding JH, Sun T and
Hu G: Opening of microglial K(ATP) channels inhibits
rotenone-induced neuroinflammation. J Cell Mol Med. 12:1559–1570.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagata K, Obata K, Odashima M, et al:
Nicorandil inhibits oxidative stress-induced apoptosis in cardiac
myocytes through activation of mitochondrial ATP-sensitive
potassium channels and a nitrate-like effect. J Mol Cell Cardiol.
35:1505–1512. 2003. View Article : Google Scholar
|
|
20
|
Akao M, Teshima Y and Marban E:
Antiapoptotic effect of nicorandil mediated by mitochondrial
atp-sensitive potassium channels in cultured cardiac myocytes. J Am
Coll Cardiol. 40:803–810. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teshima Y, Akao M, Baumgartner WA and
Marban E: Nicorandil prevents oxidative stress-induced apoptosis in
neurons by activating mitochondrial ATP-sensitive potassium
channels. Brain Res. 990:45–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Date T, Taniguchi I, Inada K, et al:
Nicorandil inhibits serum starvation-induced apoptosis in vascular
endothelial cells. J Cardiovasc Pharmacol. 46:721–726. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chao HH, Hong HJ, Sung LC, Chen JJ, Cheng
TH and Liu JC: Nicorandil attenuates cyclic strain-induced
endothelin-1 expression via the induction of activating
transcription factor 3 in human umbilical vein endothelial cells.
Eur J Pharmacol. 667:292–297. 2011. View Article : Google Scholar
|
|
24
|
Eguchi R, Suzuki A, Miyakaze S, Kaji K and
Ohta T: Hypoxia induces apoptosis of HUVECs in an in vitro
capillary model by activating proapoptotic signal p38 through
suppression of ERK1/2. Cell Signal. 19:1121–1131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hartel FV, Holl M, Arshad M, et al:
Transient hypoxia induces ERK-dependent anti-apoptotic cell
survival in endothelial cells. Am J Physiol Cell Physiol.
298:C1501–C1509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park MT, Choi JA, Kim MJ, et al:
Suppression of extracellular signal-related kinase and activation
of p38 MAPK are two critical events leading to caspase-8- and
mitochondria-mediated cell death in phytosphingosine-treated human
cancer cells. J Biol Chem. 278:50624–50634. 2003. View Article : Google Scholar
|
|
27
|
Lin FL, Hsu JL, Chou CH, Wu WJ, Chang CI
and Liu HJ: Activation of p38 MAPK by damnacanthal mediates
apoptosis in SKHep 1 cells through the DR5/TRAIL and
TNFR1/TNF-alpha and p53 pathways. Eur J Pharmacol. 650:120–129.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ikeda R, Che XF, Ushiyama M, et al:
2-Deoxy-D-ribose inhibits hypoxia-induced apoptosis by suppressing
the phosphorylation of p38 MAPK. Biochem Biophys Res Commun.
342:280–285. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liou SF, Ke HJ, Hsu JH, et al:
San-huang-xie-xin-tang prevents rat hearts from
ischemia/reperfusion-induced apoptosis through eNOS and MAPK
pathways. Evid Based Complement Alternat Med. 9150512011.PubMed/NCBI
|