Introduction
Periostin, also known as osteoblast-specific factor
2 (OSF-2), is an adhesion molecule that was initially identified in
mouse osteoblastic cells as a secreted extracellular matrix (ECM)
protein (1). Periostin may
interact with other ECM proteins and induce cell adhesion and
motility by binding to integrins (2). Periostin is highly expressed in many
normal connective tissues, including periodontal ligaments, the
fascia of muscles and joint ligaments, and may be involved in
structural maintenance and tissue development (3).
Abnormally high expression levels of periostin
protein in a variety of human tumors, including thyroid cancer,
oral cancer, gastric cancer and breast cancer, have been reported
(4–7). Furthermore, periostin overexpression
correlates with the angiogenesis, invasion and metastasis of these
tumors. Periostin also stimulates tumor cell growth by promoting
cell survival and angiogenesis through the Akt/PKB pathway
(7,8). As a mesenchymal-specific protein,
periostin is expressed and secreted by tumor mesenchymal cells.
Overexpression of the periostin gene in 293T cells has been
reported to cause epithelial-mesenchymal transition (EMT) as well
as cell migration, invasion and adhesion (9). Moreover, these
periostin-overexpressing recombinant cells quickly formed
metastatic loci when transplanted into immune-deficient mice
(9).
Lung cancer is a malignant tumor with high
occurrence that originates from normal bronchial epithelial cells.
The majority of lung cancers are non-small cell lung cancer
(NSCLC), and patients with NSCLC are most commonly detected at an
advanced stage, leading to a low 5-year survival rate (~15%).
Cigarette smoking has been implicated in the pathogenesis and
increased metastasis of lung cancer (10). Nicotine is the most active
carcinogen in cigarettes and may induce cell proliferation,
invasion and EMT in breast and lung cancer cells (11).
Although the stimulation by nicotine of the
proliferation, invasion and EMT of NSCLC has been confirmed by a
number of studies, the relationship between nicotine and periostin
has not been reported. Furthermore, the overexpression of periostin
has been found in the cells and serum of NSCLC and is related to
the proliferation and migration of tumor cells and the poor
survival of patients (12,13). Therefore, we propose periostin to
be a novel therapeutic target for NSCLC. We investigated the
effects on the proliferation, drug resistance, invasion and EMT of
NSCLC cells of silencing periostin with small interfering RNA
(siRNA). Our study indicated that periostin may be a downstream
target gene regulated by nicotine in the NSCLC cells.
Materials and methods
Cell culture
The human lung cancer cell line A549 was purchased
from the Institute of Biochemistry and Cell Biology, Shanghai
Institutes for Biological Sciences, Chinese Academy of Sciences.
The cells were cultured in DMEM media (Invitrogen-Gibco, Carlsbad,
CA, USA) supplemented with 10% fetal bovine serum (FBS; Sijichun
Bioengineering Materials Inc., Hangzhou, Zhejiang, China), 100 U/ml
penicillin and 100 μg/ml streptomycin, in a humidified incubator at
37°C with 5% CO2. The cells were seeded in 24-well
culture plates at a density of 1×106/ml and treated with
1 μM nicotine (Sigma Chemical Co., St. Louis, MO, USA). In studies
using the generalized nicotinic acetylcholine receptor (nAChR)
antagonist hexamethonium, quiescence of the cells was induced by
serum starvation for 24 h, followed by treatment with nicotine.
Construction of and transfection with
periostin siRNA plasmid
The complementary oligonucleotides for periostin
siRNA were obtained from the GenBank (accession number NM_006475.2)
and were designed according to the general designing principles of
siRNA. Two-step PCR was performed to amplify siRNA expression
cassettes using forward primers for U6 promoter amplification and
reverse primers (at 136, 246, 268 bp) for periostin at 94°C for 30
sec and 72°C for 90 sec for 40 cycles. The PCR products were
ligated with plasmid pRNAT-U6.1 using T4 DNA ligase at 16°C
overnight, followed by transformation of competent Escherichia
coli DH5α. The transformed DH5α was selected by blue-white
screening, and confirmed by enzyme digestion and gene sequencing
(Shen You Inc., Shanghai, China). The pRNAT-U6.1-periostin plasmid
with the correct sequence was amplified and purified in large
quantities.
A549 cells were seeded in 6-well plates at a density
of 4×105/ml. After culturing for 24 h, the cells were
transfected with either pRNAT-U6.1-periostin siRNA plasmid or
pRNAT-U6.1 control plasmid. Reverse transcription-polymerase chain
reaction (RT-PCR) and real-time PCR were performed to detect
periostin mRNA expression 48 h after transfection. The A549 cells
with successful periostin silencing were selected for further G418
screening (400 μg/ml).
RT-PCR for detecting periostin mRNA
expression following transfection with periostin siRNA
Total RNA was isolated from the A549 cells 48 h
after transfection and RT-PCR was performed to quantify the
periostin mRNA expression levels normalized to β-actin. cDNA was
synthesized from RNA (5 μg) by reverse transcription using random
primers (SuperScript III First-Strand Synthesis SuperMix kit;
Invitrogen, Carlsbad, CA, USA). The forward and reverse primers
were synthesized by Yingjun Biotechnology, Inc. (Shanghai, China)
and are as follows: periostin forward,
5′-AGGCAAACAGCTCAGAGTCTTCGT-3′ and reverse,
5′-TGCAGCTTCAAGTAGGCTGAGGAA-3′; and β-actin forward,
5′-CTGGCACCACACCTTCTACAATGA-3′ and reverse,
5′-TTAATGTCACGCACGATTTCCCGC-3′. The conditions for the PCR of
periostin and β-actin mRNA are 94°C for 4 min (1 cycle); 94°C for
30 sec, 57°C for 30 sec and 72°C for 1 min (33 cycles); and 72°C
for 7 min (1 cycle). The PCR products were subjected to
electrophoresis on a 1.5% agarose gel containing ethidium bromide
and then visualized under ultraviolet light.
Real-time PCR analysis
Periostin mRNA expression levels were further
analyzed to confirm the results of RT-PCR using an RT-Cycler™
Real-Time PCR Detection System (CapitalBio, Ltd., Beijing, China)
with SYBR-Green (Molecular Probes, Eugene, OR, USA). Following RNA
extraction and the reverse transcription of RNA to cDNA, 1 μl cDNA
was added to a 20-μl reaction mixture containing 0.5X SYBR-Green,
1X TransStart Green qPCR SuperMix (TransGen Biotech Co., Ltd.,
Beijing, China) and 0.5 μmol/l primer sets. The cycling conditions
for the two genes were as follows: 95°C for 5 min for 1 cycle,
followed by 95°C for 45 sec, 57°C for 30 sec and 72°C for 20 sec
for 40 cycles. The expression levels of the cDNA of the two genes
were internally normalized to those of β-actin. The
2−ΔΔCT method was used to calculate the relative
quantitative levels of periostin mRNA. Each experiment was
performed in duplicate and repeated three times.
MTT assay
The cell growth rate was determined by MTT assay
(Sigma Chemical Co.). Briefly, 100 μl cells at the logarithmic
growth phase were seeded into 96-well culture plates at a density
of 1×103 cells/well. Then, cells transfected with
control or periostin siRNA plasmids were cultured with DMEM media
supplemented with 10% FBS and incubated with 1 μM nicotine (Sigma)
for 1, 2, 3, 4 and 5 days. An MTT solution (5 mg/ml, 10 μl) was
added to each well and the plates were incubated at 37°C for 4 h.
After centrifugation at 3,000 rpm for 10 min, the supernatant was
removed and the remaining formazan pellet was dissolved completely
in 100 μl DMSO. An ELISA plate reader was used to measure the
absorbance at 570 nm to determine the amount of pellet.
Cell apoptosis assay
A549 cells were cultured in vitro and
randomly assigned to one of four groups: normal control (siRNA-C),
periostin-silenced (siRNA-P), normal treated with nicotine
(siRNA-C+N) and periostin-silenced treated with nicotine
(siRNA-P+N). After 72 h, 2×105 cells from each group
were harvested for centrifugation at 2,000 rpm for 5 min. After
washing with PBS buffer, the pellet was resuspended in 100 μl 1X
binding buffer and incubated with 2.5 μl Annexin V and 5 μl
propidium iodine (PI) (final concentration: 10 μg/ml) for 15 min in
the dark. Apoptosis was determined by flow cytometry and analyzed
using Lysis software. At least 10,000 events were analyzed for each
sample.
Cell migration assay
The in vitro migration of the A549 cells was
evaluated using a Boyden chamber invasion assay. A Transwell filter
membrane was used for experiments in 24-well tissue culture plates.
The lower side of the filter was coated with type I collagen (0.5
mg/ml) and contained low-serum medium in the presence or absence of
nicotine. In the upper part of the Transwell plate,
5×104 cells transfected with control or periostin siRNA
plasmid were resuspended in 100 μl DMEM medium and plated. The
cells were fixed with methanol and stained with hematoxylin and
eosin (Sigma). After 24 h, the cells on the upper surface of the
filter were removed. The cells that had migrated to the lower part
were counted by light microscopy (magnification ×200) as the number
of migrated cells. Each sample was assayed in triplicate and
repeated twice
Western blot analysis
Proteins were extracted for the determination of
their concentrations using a bicinchoninic acid protein
concentration assay kit (Beijing Biosea Biotechnology Co., Ltd.,
Beijing, China). Following separation on sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels
(polyacrylamide concentration 100 g/l), the proteins (50 μg) were
electrophoretically transferred to a PVDF membrane. The PVDF
membrane was blocked with 3% BSA at 37°C for 1 h and probed with
the mouse monoclonal antibodies against Snail (1:1,000) or
periostin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The
second antibody was horseradish peroxidase-conjugated rabbit
anti-mouse IgG and used at a dilution of 1:1,000 for 2 h at room
temperature. A chemiluminescence method was used to visualize the
density of the targeted bands. β-actin was used as an internal
control.
Statistical analysis
Commercially available software (SPSS version 14.0)
was used to perform the statistical analysis. The data are
expressed as the mean ± SD. The Student's t-test (unpaired,
two-tailed) was performed to compare the means between the two
groups. P<0.05 was considered to indicate a statistically
significant result.
Results
Efficacy of periostin siRNA
transfection
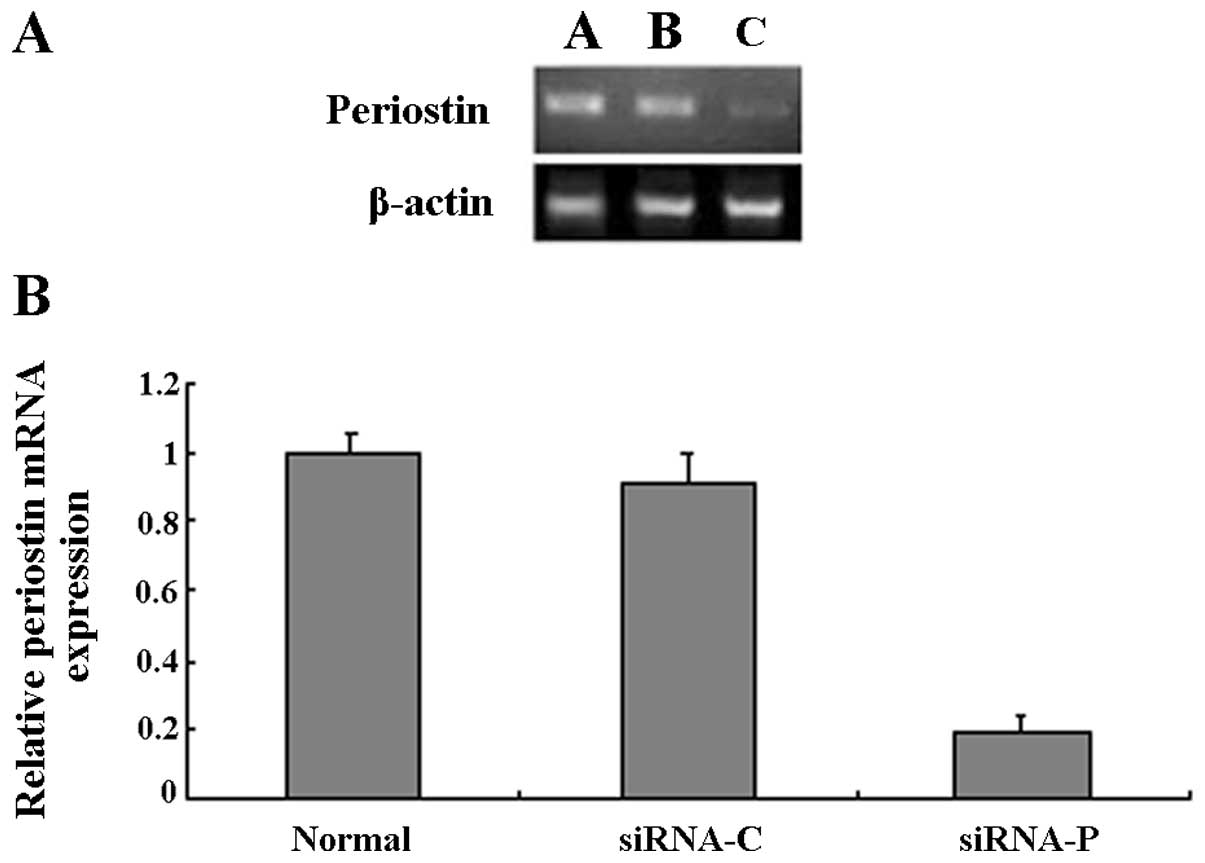
RT-PCR was used to detect the levels of periostin
mRNA following the transfection of the A549 cells with siRNA. The
cells transfected with the siRNA for periostin showed clearly
decreased expression of periostin mRNA. However, no difference in
periostin mRNA levels was observed between the control
plasmid-transfected cells and the untransfected cells (Fig. 1A). To further confirm the results
of RT-PCR quantitatively, real-time RT-PCR was carried out. The
periostin mRNA levels of the A549 cells which stably expressed
periostin siRNA were decreased by ~80% compared with those of the
untransfected A549 cells, whereas the control plasmid had no
influence on the periostin mRNA levels in the A549 cells (Fig. 1B). The periostin mRNA expression
levels were significantly reduced by transfection with periostin
siRNA. The A549 cells which had been successfully transfected with
periostin siRNA were further screened in culture media supplemented
with G418 (400 μg/ml).
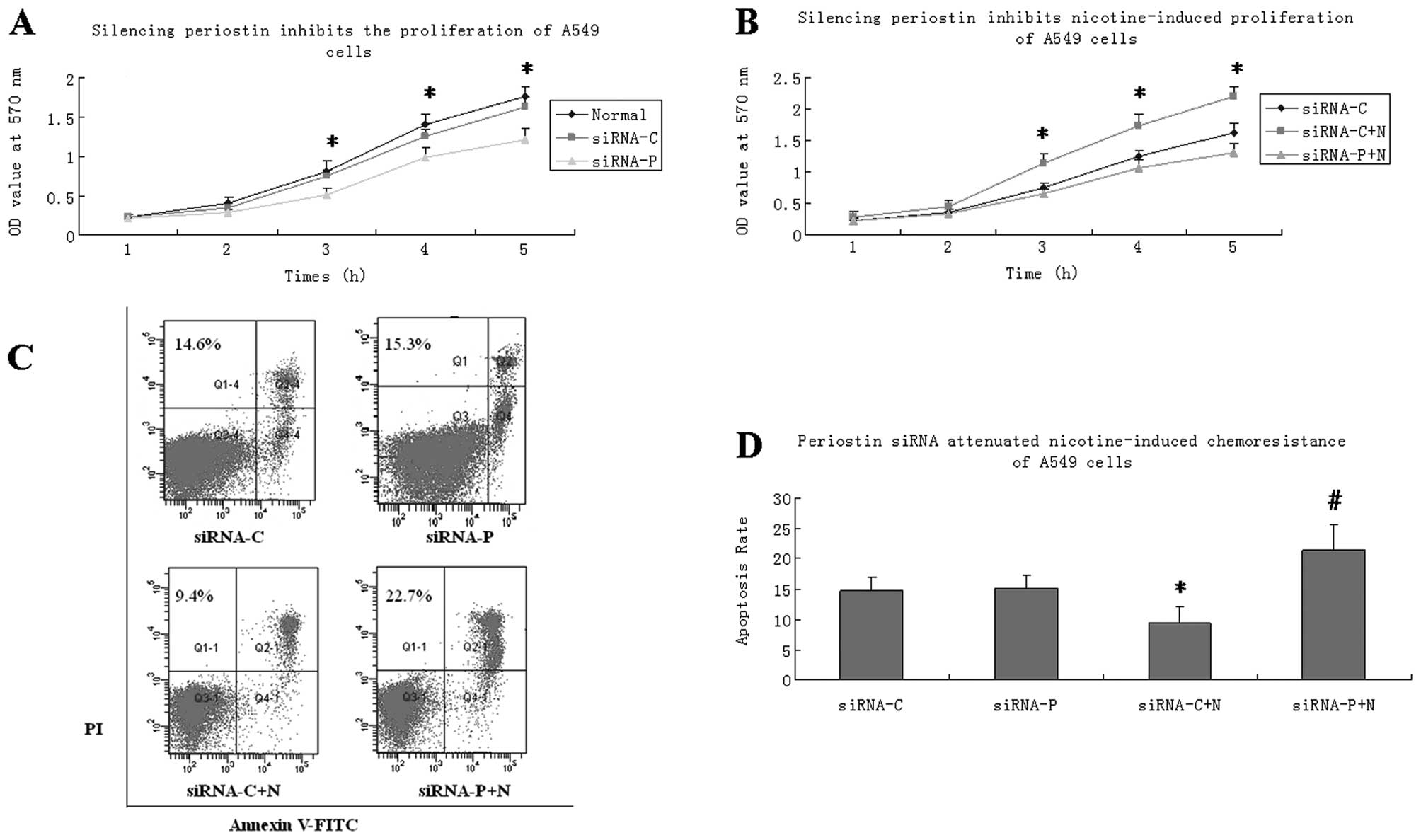
Periostin siRNA inhibited
nicotine-induced lung cancer cell proliferation
To investigate the effect of silencing periostin on
cell proliferation, we performed an MTT assay to evaluate the
growth of the A549 cells. The results indicated that the A549 cells
stably expressing periostin siRNA showed significantly slower
growth on days 3, 4 and 5 than the normal A549 cells (Fig. 2A). We observed no difference in
cell growth between the cells transfected with control plasmid and
the normal A549 cells. Nicotine treatment increased the growth of
the A549 cells transfected with control plasmid. The effect of
nicotine was significantly reduced by transfection with periostin
siRNA (Fig. 2B).
Periostin siRNA attenuated the
nicotine-induced chemoresistance of lung cancer cells
A549 cells were incubated with cisplatin (5 μg/ml)
throughout this experiment. The cells were randomly divided into
four groups: siRNA-C, siRNA-P, siRNA-C+N and siRNA-P+N. Treatment
with nicotine for 72 h significantly decreased the apoptotic rate
of the A549 cells treated with cisplatin, as shown by flow
cytometry. However, this chemoresistance was reversed in the
periostin siRNA-transfected cells which had a significantly higher
apoptotic rate than the cells transfected with the control plasmid.
In the cells that did not undergo nicotine treatment, periostin
siRNA transfection alone did not increase the rate of apoptosis
(Fig. 2C and D).
Periostin siRNA inhibited invasion and
the expression of EMT marker protein Snail
Periostin siRNA-transfected cells showed a slightly
lower index of invasion than cells transfected with control
plasmid. Following nicotine treatment, the A549 cells showed a
significantly higher index of invasion. However, in the
nicotine-treated A549 cells, siRNA-periostin transfection
significantly decreased the index of invasion compared with that of
cells transfected with the control plasmid (Fig. 3A).
The expression of the EMT marker Snail was
determined by western blot analysis. In cells tranfected with the
control plasmid (siRNA-C), the Snail protein levels were
significantly increased by nicotine treatment. However,
transfection with periostin siRNA significantly decreased the Snail
protein expression levels in the A549 cells treated with nicotine
(Fig. 3B and C).
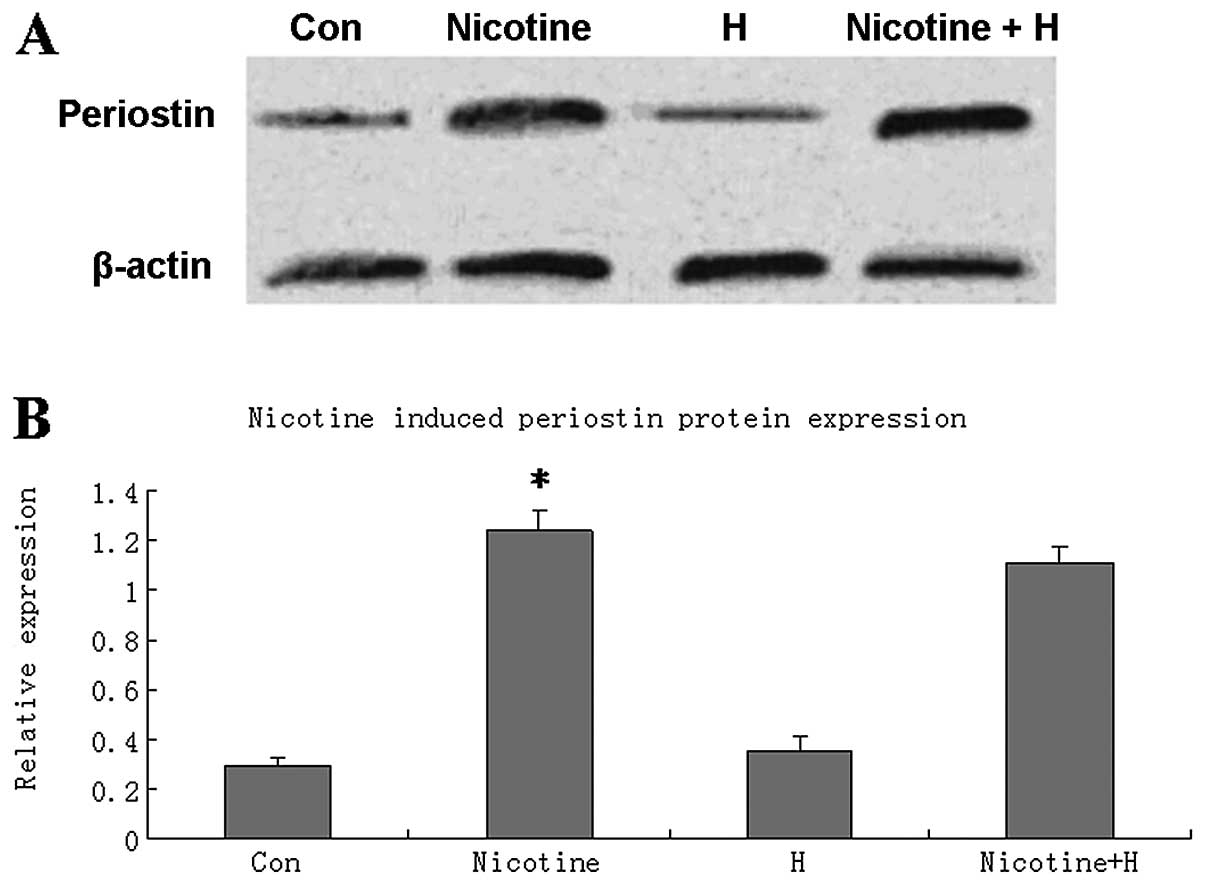
Nicotine induced periostin upregulation
in lung cancer cells in a nAChR-independent manner
Western blot analysis revealed that treatment with 1
μM nicotine for 24 h significantly upregulated periostin protein
expression levels in the A549 cells, suggesting that periostin may
be a downstream target of nicotine in lung cancer cells.
Pretreatment with a generalized nAChR antagonist, hexamethonium,
did not influence the expression of periostin in the
nicotine-treated or nicotine-untreated A549 cells (Fig. 4).
Discussion
In this study, we investigated the effect of
periostin on the proliferation, drug resistance, tumor invasion and
EMT of NSCLC cells by silencing periostin with RNAi. A stably
periostin-silenced cell line was acquired by G418 screening. The
periostin mRNA levels of the NSCLC cells with stably expressed
periostin siRNA were decreased by ~80% compared with those of the
NSCLC cells without treatment. The periostin-silenced NSCLC cells
exhibited reduced cell proliferation, elevated sensitivity to
chemotherapy with cisplatin, decreased cell invasion and Snail
expression levels. Nicotine upregulated periostin protein in the
NSCLC cells, and this effect was not blocked by the generalized
nAChR antagonist, hexamethonium.
Periostin overexpression in human tumors may promote
tumor growth and survival by inducing the Akt/PKB pathway (14). However, periostin overexpression is
not always associated with increased proliferation, as shown in
several human cancer cell lines (15). In our study, we found that
periostin mRNA was expressed in the A549 NSCLC cell line and may be
fully silenced by RNAi (Fig. 1).
In order to explore the effects of silencing periostin, we used an
MTT assay to evaluate the proliferation of the NSCLC cells. The
results revealed that the stably periostin siRNA-expressing NSCLC
cells grew more slowly than the cells transfected with control
plasmid (Fig. 2A). Furthermore,
nicotine treatment increased the proliferation of the NSCLC cells
and this effect was reversed by periostin silencing (Fig. 2B).
Our study demonstrated that periostin silencing may
significantly enhance the sensitivity of NSCLC cells to the
chemotherapy agent cisplatin. Cisplatin treatment induced a
moderate degree of apoptosis in the NSCLC cells and nicotine
increased the cell survival rate and reduced the apoptotic rate.
However, periostin silencing significantly increased apoptosis in
the cells treated with cisplatin and nicotine. By contrast, with
nicotine treatment, periostin silencing showed little influence on
the apoptotic rate of the NSCLC cells. This phenomenon may be
caused by nicotine and periostin inducing common survival pathways.
The activation of survival pathways by nicotine may enhance the
dependence of the NSCLC cells on these pathways and increase their
sensitivity to periostin silencing. Nicotine stimulates
proliferation and inhibits apoptosis in colon cancer cells by the
activation of survival pathways, for example, by significantly
increasing the expression of PI3K and the P-Akt/Akt ratio (16). The Akt/PKB pathway also
participates in the enhanced proliferation and survival promoted by
periostin in several human cancer cell lines (14). The possible mechanisms underlying
the shared survival pathways of nicotine and periostin require
further investigation. It has been reported that nicotine may
induce chemoresistance by activating anti-apoptotic pathways in
several cancer cell lines (17–19),
therefore the chemosensitizing effect due to shared survival
pathways is a promising therapeutic approach in periostin-targeting
therapy.
Tumor invasion is a complex, multi-step process that
involves the alteration of cell adhesion to extracellular matrix
protein interactions. Nicotine enhances the phosphorylation of
calpains and increases the expression levels of COX2, VEGF and
VEGFR2, which are molecules affecting the invasive process
(20–22). Our findings show that the
pro-invasive effects of nicotine may be reversed by periostin
silencing. Given the correlation between periostin overexpression
and tumor proliferation and migration, our results indicate that
periostin is not only a promising biomarker for NSCLC prognosis but
also a potential target for therapeutical intervention.
EMT is a vital step occurring in epithelial cells
for the acquisition of a malignant phenotype, including the
capabilities of migration, invasion and metastasis to a new
location (23). Our results
revealed that after nicotine treatment, the periostin gene-silenced
NSCLC cells had significantly downregulated expression levels of
Snail, which is an EMT-inducing gene. This suggests that periostin
expression is required for EMT to occur in NSCLC cells.
Our study revealed that periostin expression was
regulated by nicotine in NSCLC cells, suggesting that periostin is
a downstream target of nicotine. The pathophysiological effects of
nicotine are mediated by nAChRs, which are mainly expressed on
neurons and neuromuscular junctions (24). nAChRs have also been detected in
primary endothelial cells as well as the A549 human NSCLC cell
line. Nicotine has been shown to induce the invasion and migration
of NSCLC cells in a α7-receptor-dependent manner (11). In our study, we pretreated A549
cells with a generalized nAChR antagonist hexamethonium, followed
by nicotine treatment. In the nicotine-induced A549 cells, the
elevated expression of periostin was not affected by hexamethonium.
This indicates that nicotine may upregulate periostin expression
through nAChR-independent pathways. It also suggests that periostin
overexpression is essential but not sufficient for nicotine-induced
proliferation, drug resistance, invasion and EMT in NSCLC
cells.
In conclusion, we identified that periostin is a
nicotine-regulated gene and contributes to nicotine-induced cell
growth, drug resistance, tumor invasion and EMT in NSCLC cells.
Silencing periostin expression using siRNA may inhibit the
proliferation, chemoresistance, invasion and EMT of NSCLC cells
induced by nicotine. Periostin is a nicotine-regulated gene in
NSCLC cells. Therefore, periostin may be a promising therapeutical
target for NSCLC intervention in the future.
References
|
1
|
Takeshita S, Kikuno R, Tezuka K and Amann
E: Osteoblast specific factor 2: cloning of a putative bone
adhesion protein with homology with the insect protein fasciclin I.
Biochem J. 294:271–278. 1993.PubMed/NCBI
|
|
2
|
Gillan L, Matei D, Fishman DA, Gerbin CS,
Karlan BY and Chang DD: Periostin secreted by epithelial ovarian
carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5)
integrins and promotes cell motility. Cancer Res. 62:5358–5364.
2002.PubMed/NCBI
|
|
3
|
Hamilton DW: Functional role of periostin
in development and wound repair: implications for connective tissue
disease. J Cell Commun Signal. 2:9–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Puppin C, Fabbro D, Dima M, Di Loreto C,
Puxeddu E, Filetti S, Russo D and Damante G: High periostin
expression correlates with aggressiveness in papillary thyroid
carcinomas. J Endocrinol. 197:401–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Zhang G, Li J, Tao Q and Tang W:
The expression analysis of periostin in human breast cancer. J Surg
Res. 160:102–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li JS, Sun GW, Wei XY and Tang WH:
Expression of periostin and its clinicopathological relevance in
gastric cancer. World J Gastroenterol. 13:5261–5266. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siriwardena BS, Kudo Y, Ogawa I, Kitagawa
M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M and Takata T:
Periostin is frequently overexpressed and enhances invasion and
angiogenesis in oral cancer. Br J Cancer. 95:1396–1403. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shao R, Bao S, Bai X, Blanchette C,
Anderson RM, Dang T, Gishizky ML, Marks JR and Wang XF: Acquired
expression of Periostin by human breast cancers promotes tumor
angiogenesis through up-regulation of vascular endothelial growth
factor receptor 2 expression. Mol Cell Biol. 24:3992–4003. 2004.
View Article : Google Scholar
|
|
9
|
Yan W and Shao R: Transduction of a
mesenchyme-specific gene periostin into 293T cells induces cell
invasive activity through epithelial-mesenchymal transformation. J
Biol Chem. 281:19700–19708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hecht SS: Cigarette smoking and lung
cancer: chemical mechanisms and approaches to prevention. Lancet
Oncol. 3:461–469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dasgupta P, Rizwani W, Pillai S, Kinkade
R, Kovacs M, Rastogi S, Banerjee S, Carless M, Kim E, Coppola D, et
al: Nicotine induces cell proliferation, invasion and
epithelial-mesenchymal transition in a variety of human cancer cell
lines. Int J Cancer. 124:36–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong L, Sun H, Lv X, Yang D, Zhang J and
Shi Y: Expression of periostin in the serum of NSCLC and its
function on proliferation and migration of human lung
adenocarcinoma cell line (A549) in vitro. Mol Biol Rep.
37:2285–2293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sasaki H, Dai M, Auclair D, Fukai I,
Kiriyama M, Yamakawa Y, Fujii Y and Chen LB: Serum level of the
periostin, a homologue of an insect cell adhesion molecule, as a
prognostic marker in nonsmall cell lung carcinomas. Cancer.
92:843–848. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasaki H, Lo KM, Chen LB, Auclair D,
Nakashima Y, Moriyama S, Fukai I, Tam C, Loda M and Fujii Y:
Expression of Periostin, homologous with an insect cell adhesion
molecule, as a prognostic marker in non-small cell lung cancers.
Jpn J Cancer Res. 92:869–873. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruan K, Bao S and Ouyang G: The
multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci.
66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cucina A, Dinicola S, Coluccia P, Proietti
S, D'Anselmi F, Pasqualato A and Bizzarri M: Nicotine stimulates
proliferation and inhibits apoptosis in colon cancer cell lines
through activation of survival pathways. J Surg Res. Mar
10–2012.(Epub ahead of print).
|
|
17
|
Do NY and Lim SC: A low level of
nicotine-induced chemoresistance in a KB cell line. Mol Med Report.
1:55–60. 2008.PubMed/NCBI
|
|
18
|
Chen RJ, Ho YS, Guo HR and Wang YJ:
Long-term nicotine exposure-induced chemoresistance is mediated by
activation of Stat3 and downregulation of ERK1/2 via nAChR and
beta-adrenoceptors in human bladder cancer cells. Toxicol Sci.
115:118–130. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martínez-García E, Irigoyen M,
González-Moreno O, Corrales L, Teijeira A, Salvo E and Rouzaut A:
Repetitive nicotine exposure leads to a more malignant and
metastasis-prone phenotype of SCLC: a molecular insight into the
importance of quitting smoking during treatment. Toxicol Sci.
116:467–476. 2010.PubMed/NCBI
|
|
20
|
Xu L and Deng X: Protein kinase Ciota
promotes nicotine-induced migration and invasion of cancer cells
via phosphorylation of micro- and m-calpains. J Biol Chem.
281:4457–4466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cooke JP: Angiogenesis and the role of the
endothelial nicotinic acetylcholine receptor. Life Sci.
80:2347–2351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanda Y and Watanabe Y: Nicotine-induced
vascular endothelial growth factor release via the EGFR-ERK pathway
in rat vascular smooth muscle cells. Life Sci. 80:1409–1414. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gotti C and Clementi F: Neuronal nicotinic
receptors: from structure to pathology. Prog Neurobiol. 74:363–396.
2004. View Article : Google Scholar : PubMed/NCBI
|