Introduction
Paclitaxel, which was isolated from Taxus
brevifolia in the early 1970s and approved by the FDA in 1993,
is one of the most active antineoplastic agents against a wide
spectrum of malignancies, including ovarian, breast, lung, head and
neck cancers, and Kaposi’s sarcoma (1,2). Due
to its poor solubility, conventional preparations of paclitaxel
(Taxol) were formulated in a special vehicle, which contained
polyethoxylated castor oil (PCO) and anhydrous ethanol (1:1 V/V)
(3–5). Despite the extensive clinical
utilization and success of Taxol, serious toxic effects, such as
serious hypersensitivity reactions and neurotoxicity, are
associated with PCO (4–6). To prevent and manage these serious
problems, premedication with high doses of corticosteroids and
antihistamines must routinely be administrated (6), which increases the possibility of
drug interactions. Therefore, an improved paclitaxel formulation,
which could eliminate the adverse effects associated with PCO while
retaining similar anticancer activity, would greatly benefit cancer
patients and their caregivers.
Surfactant-free paclitaxel formulations, which could
replace Taxol, have been the object of considerable investigation
(7). To date, promising
alternative formulations involved the use of albumin nanoparticles,
polyglutamates, taxane analogs, prodrugs, emulsions and liposomes
(8,9). Among those, only one paclitaxel
protein-bound particle for injection (Abraxane) has been approved
by the FDA for the treatment of breast cancer (10), and several novel formulations with
different vehicles are at different stages of preclinical studies
or clinical trials (11,12).
Among all drug carrier systems, liposomes represent
a mature, versatile technology with considerable potential for
encapsulation of lipophilic and hydrophilic drugs (8), and have been used for the treatment
of neoplastic and infectious diseases, such as injectable
doxorubicin hydrochloride liposome (Doxil) (13) and amphotericin B liposome
(Ambisome) (14). Currently,
several liposomal PTX formulas are in various stages of clinical
trials. A liposome-entrapped paclitaxel formulation (LEP-ETU)
(15) and an ionically charged
paclitaxel-lipid complex (EndoTAG-1) (16) are in phase II clinical trials. As
part of the continuing effort to improve the utilization of
paclitaxel-based chemotherapy, we developed a polyethoxylated
castor oil-free, liposome-based alternative paclitaxel formulation
known as Lipusu, which was launched in China (8). In the current study, we performed
in vitro and in vivo strategies to compare the safety
profiles of Taxol and Lipusu, with special regard to the induction
of the hypersensitivity reaction.
Materials and methods
Materials
Taxol, in which paclitaxel was formulated in PCO and
anhydrous ethanol, was purchased from Haikou Pharm (Cat. no.:
111006; Haikou, China). Lipusu, in which paclitaxel was formulated
into liposomes, was provided by Luye Pharm (Cat. no.: 20120102;
Nanjing, China). In in vitro and in vivo experiments,
Taxol and Lipusu were diluted into the required concentration using
5% glucose according to the manufacturer’s instructions.
Cell lines and cell culture
The human oral carcinoma cell line KB was purchased
from Cell Culture Center of Institute of Basic Medical Sciences,
Chinese Academy of Medical Sciences (Beijing, China). The cells
were cultured in DMEM media supplemented with 10% fetal calf serum,
penicillin (100 U/ml) and streptomycin (10 μg/ml; Gibco BRL, NY,
USA), and incubated at 37°C in a humidified air atmosphere
containing 5% CO2. All cells were harvested in their
exponential growth phase.
Animals
Swiss mice (18–22 g) were obtained from Shandong
Luye Pharmaceutical Company (Yantai, China). The animals were
housed in a light- and temperature-controlled room (20–24°C,
humidity 40–65%) and kept on a standard diet and provided with
water. All experiments were performed according to the Guidelines
for Care and Use of Experimental Animals of the Experimental Animal
Research Committee of Yantai University (China).
Acute toxicity study
The mice were randomly divided into 10 groups
(5/sex/group). Different concentrations of Taxol and Lipusu
solution were obtained using geometric dilution with 5% glucose
injection at a dose-ratio of 1:0.85. Five doses, 96.8, 82.3, 69.9,
59.4 or 50.5 mg/kg, for Lipusu, and five doses, 44.1, 37.5, 31.8,
27.0 or 23.0 mg/kg, for Taxol, were administered intravenously
(i.v.) in a 10 ml/kg injection volume. General behavior was
observed continuously for 1 h following treatment; animals were
further observed for up to 14 days following treatment for signs of
toxicity, mortality and latent time to mortality. The median lethal
dose (LD50) and 95% confidence limit were determined
using the Bliss method (17).
Anaphylaxis study
Taxol and Lipusu were freshly prepared according to
the manufacturer’s instructions and diluted with 5% glucose
injection prior to use. The vehicle used as control (5% glucose
injection) or the drugs being evaluated (30 mg/kg) were
administered i.v. in a 10 ml/kg injection volume to the male mice
(8/group) with or without dexamethasone (8 mg/kg, gavage) and
cimetidine (40 mg/kg, i.v.) treatment (18). Allergic signs were observed and
scored according to Table I. Blood
was sampled under anesthesia by inhaling ether 90 min after the
single dose, and the animals were then sacrificed by cervical
dislocation. Serum was prepared by centrifugation and the content
of serum complement split product SC5b-9 was detected using
commercial ELISA kits (Jingke Bio, Shanghai, China), according to
the manufacturer’s instructions. After the mice were sacrificed,
lungs were removed quickly and weighed for the calculation of
organ/body ratio. Half of the tissues were cooled, homogenized, and
tested for histamine using commercial ELISA kits (Jingke Bio). The
remaining lung tissue was used for histological examination, in
which all specimens were analyzed and photographed by two
pathologists in a blind investigation.
 | Table IThe severity of hypersensitivity
reactions. |
Table I
The severity of hypersensitivity
reactions.
| Grade | Clinical signs |
|---|
| 0/− | Normal |
| 1/+ | Disturbance, head
shaking |
| 2/++ | Shortness of breath,
drowsiness |
| 3/+++ | Dyspnea, syncope,
gatism |
| 4/++++ | Mortality |
In vitro complement activation study
Human blood was drawn from 10 healthy volunteers
according to protocols approved by the Human Use Committee of
Yantai University, and all subjects gave written informed consent
to use their blood for research purposes. Specimens were incubated
with the drugs being evaluated at the volume ratio of 3:1, as
previously reported with minor modifications (5). Briefly, 5 μl of drugs being evaluated
with a concentration of 1 mg/ml were mixed with 15 μl serum in
Eppendorf tubes and incubated in a shaking table (80 rpm cycle) at
37°C for 60 min. The reaction was stopped by adding 980 μl PBS with
10 mM EDTA (pH 7.4), and the SC5b-9 content was determined with
commercial ELISA kits (Jingke Bio).
In vitro cytotoxicity assay
The cytotoxicity of Taxol and Lipusu were determined
by MTT assay, as described previously with minor modifications
(19). Briefly, KB cells were
seeded into a 96-well plate at 4,000 cells per well. Culture medium
was then replaced with 200 μl medium containing serial dilutions of
the drugs. After 72 h of incubation at 37°C, MTT stock solution
(500 μg/ml) was added into each well, and the plate was incubated
for 2 h. Medium was then removed and DMSO was added to dissolve
formazan crystals converted from MTT. Cell viability was assessed
by absorbance at 570 nm measured using a microplate reader
(Wellscan MK3, Helsinki, Finland).
Data analysis and statistics
Results are presented as the means ± SD. Comparisons
between more than two groups used analysis of variance (one way
ANOVA), followed by the Student’s t-test. P≤0.05 was considered to
indicate a statistically significant difference.
Results
Lipusu exhibited a greater safety margin
than Taxol
Single dose acute toxicity assays were performed on
Swiss mice for Taxol or Lipusu, and the mortalities and clinical
signs were observed. The majority of the Taxol-injected animals
were subsequently demonstrated anaphylactic responses such as
piloerection, anhelation and syncope, which were not observed in
the Lipusu-injected animals. The mortality of these animals was
recorded from the dosing day (Day 1) until the end of observation
(Day 14), and the mortalities for Taxol and Lipusu are shown in
Table II. Based on these results,
the LD50 values (95% confidence limits) for Lipusu and
Taxol were calculated to be 69.82 mg/kg (58.9–82.7) and 33.0 mg/kg
(30.2–36.1), respectively.
 | Table IIThe mortality and clinical signs of
animals following injection with Taxol and Lipusu in the acute
toxicity study. |
Table II
The mortality and clinical signs of
animals following injection with Taxol and Lipusu in the acute
toxicity study.
| Drug | Formulation
(paclitaxel, mg/kg) | Animals/group |
Mortalities/group | Mortality ratio
(%) | Clinical signs |
|---|
| Lipusu | 96.8 | 10 | 8 | 80 | Asthenia,
anorexia |
| 82.3 | 10 | 6 | 60 | Asthenia,
anorexia |
| 69.9 | 10 | 5 | 50 | Asthenia,
anorexia |
| 59.4 | 10 | 3 | 30 | None |
| 50.5 | 10 | 3 | 30 | None |
| Taxol | 44.1 | 10 | 9 | 90 | Asthenia, anorexia,
syncope, dyspnea |
| 37.5 | 10 | 7 | 70 | Asthenia, anorexia,
syncope, dyspnea |
| 31.8 | 10 | 5 | 50 | Asthenia, anorexia,
syncope, dyspnea |
| 27.0 | 10 | 2 | 20 | Asthenia, anorexia,
syncope, dyspnea |
| 23.0 | 10 | 0 | 0 | Asthenia, anorexia,
syncope, dyspnea |
Lipusu induced much milder
hypersensitivity reactions in mice than Taxol
Taxol or Lipusu, at a dosage of 30 mg/kg, were
intravenously injected into mice. Behaviors were observed and
hypersensitivity reactions were ranked according to Table I. The animals in the Taxol group
were all observed to have acute hypersensitivity reactions at 2–5
min after injection, and recovered 17–30 min later. All the animals
in the Lipusu group, however, showed much milder reactions. The
severity of the hypersensitivity reactions induced by paclitaxel
injection, particularly for Taxol, could be attenuated by
pretreatment with dexamethasone and cimetidine. The individual
response for each animal was summarized in Table III.
 | Table IIIThe rank of hypersensitivity
reactions for the animals after injection with Taxol or Lipusu at
the therapeutic dosage. |
Table III
The rank of hypersensitivity
reactions for the animals after injection with Taxol or Lipusu at
the therapeutic dosage.
| No. | 5% glucose | Taxol | Lipusu | 5% glucose
premedication | Taxol
premedication | Lipusu
premedication |
|---|
| 1 | − | +++ | + | − | + | + |
| 2 | − | +++ | + | − | + | − |
| 3 | − | +++ | + | − | ++ | + |
| 4 | − | +++ | + | − | + | + |
| 5 | − | +++ | ++ | − | + | + |
| 6 | − | +++ | + | − | + | − |
| 7 | − | +++ | + | − | ++ | + |
| 8 | − | +++ | + | − | ++ | + |
Lipusu did not induce pulmonary
edema
Lungs were harvested and weighed 90 min after
injection of the drugs or the control vehicle solution, after which
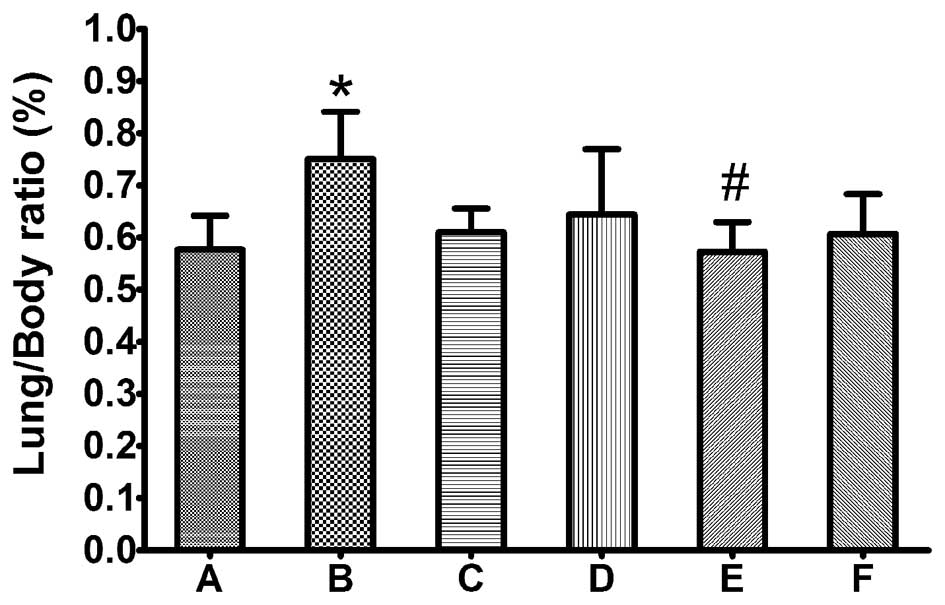
the lung weight/body weight ratio (%) was calculated. As shown in
Fig. 1, the lung/body ratio was
significantly increased in animals treated with Taxol compared with
the control group (P≤0.05), whereas there was no significant
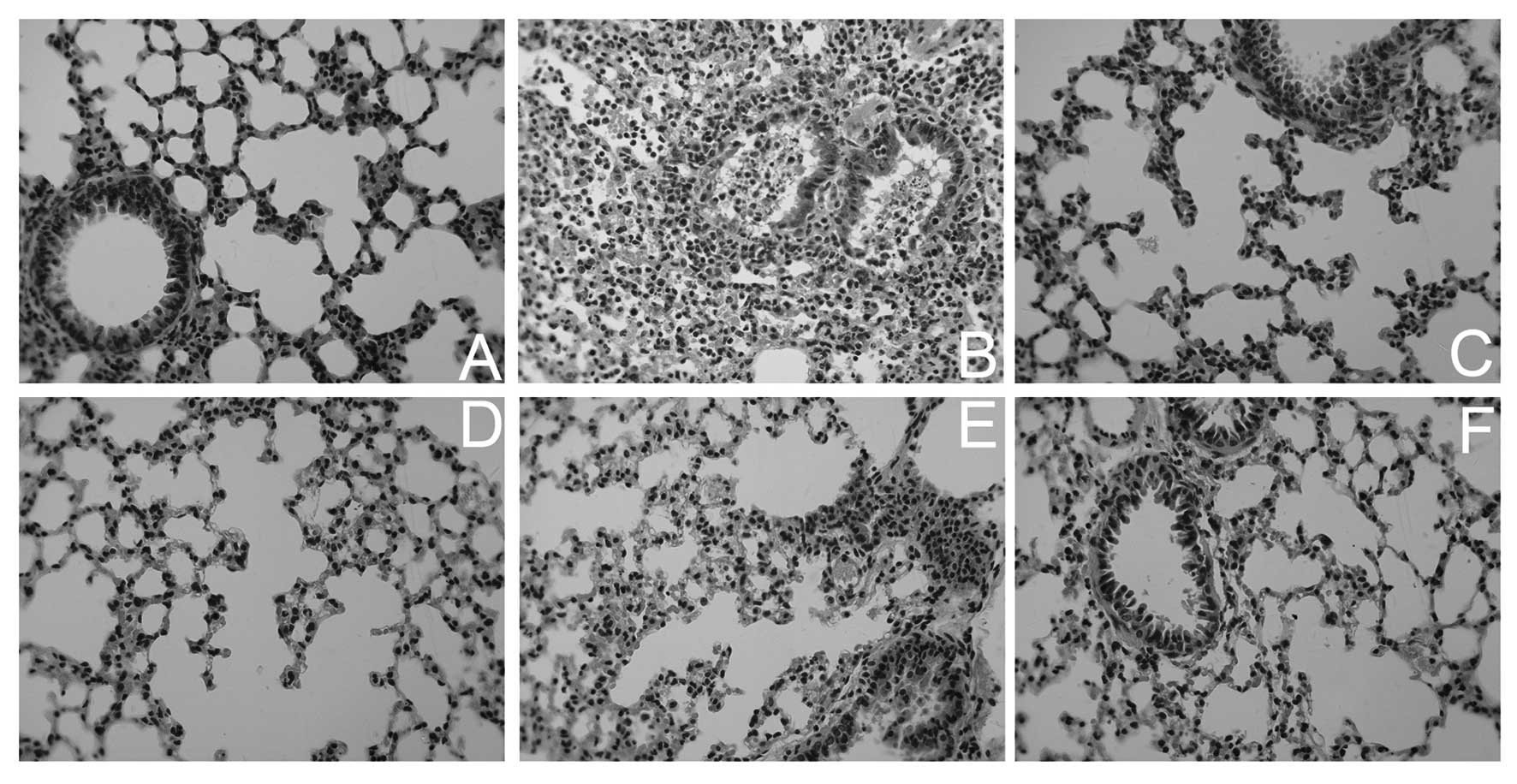
difference between the control and Lipusu groups. By histological
examination, more severe lung injuries were observed in
Taxol-treated animals, including pulmonary edema, infiltration of
tissue, alveoli with inflammatory cells and signs of tissue injury,
and thickening of alveolar walls (Fig.
2). The histological appearance of lungs in Lipusu-treated
animals, however, was relatively normal. Pretreatment with
dexamethasone and cimetidine greatly attenuated the lung damage in
Taxol-treated mice.
Lipusu did not induce complement
activation or increase histamine accumulation
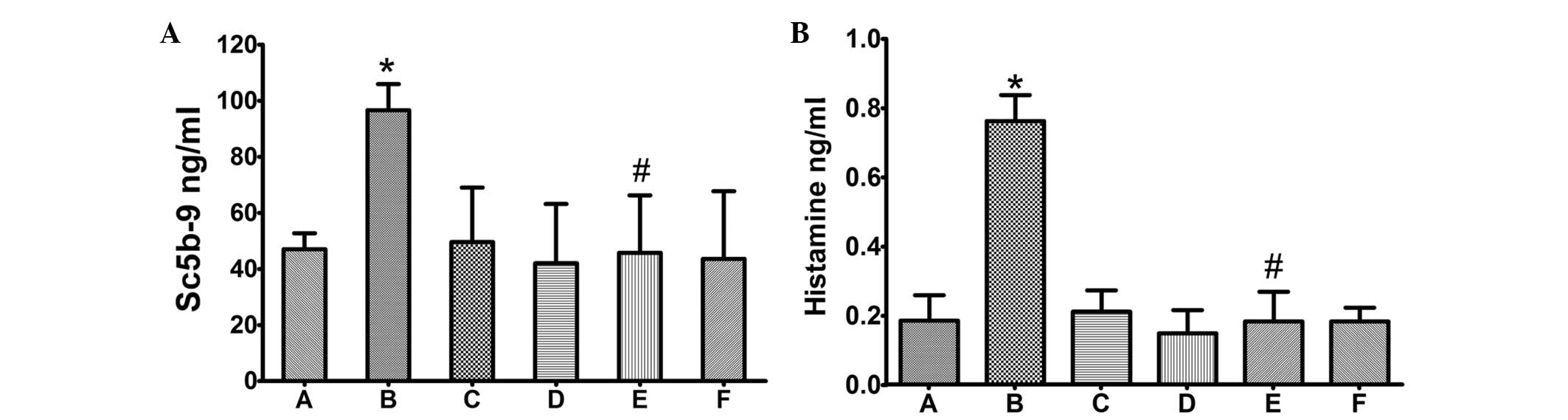
Serum SC5b-9 content was measured by ELISA. As shown
in Fig. 3, SC5b-9 was
significantly induced in animals administered Taxol (P≤0.05,
compared with control group), but not in animals administered the
same dosage of Lipusu. Notably, the increased serum SC5b-9 was
markedly ameliorated by pretreatment with dexamethasone and
cimetidine (P≤0.05, compared with the Taxol group), which are
glucocorticoid and histamine H2-receptor antagonists, respectively.
Similar results were observed for lung histamine (detected by
ELISA); administration of Taxol, but not Lipusu, increased the
content of histamine in lung tissue (Fig. 3), which could also be blocked
significantly by premedication.
Lipusu did not induce complement
activation in vitro in healthy human serum
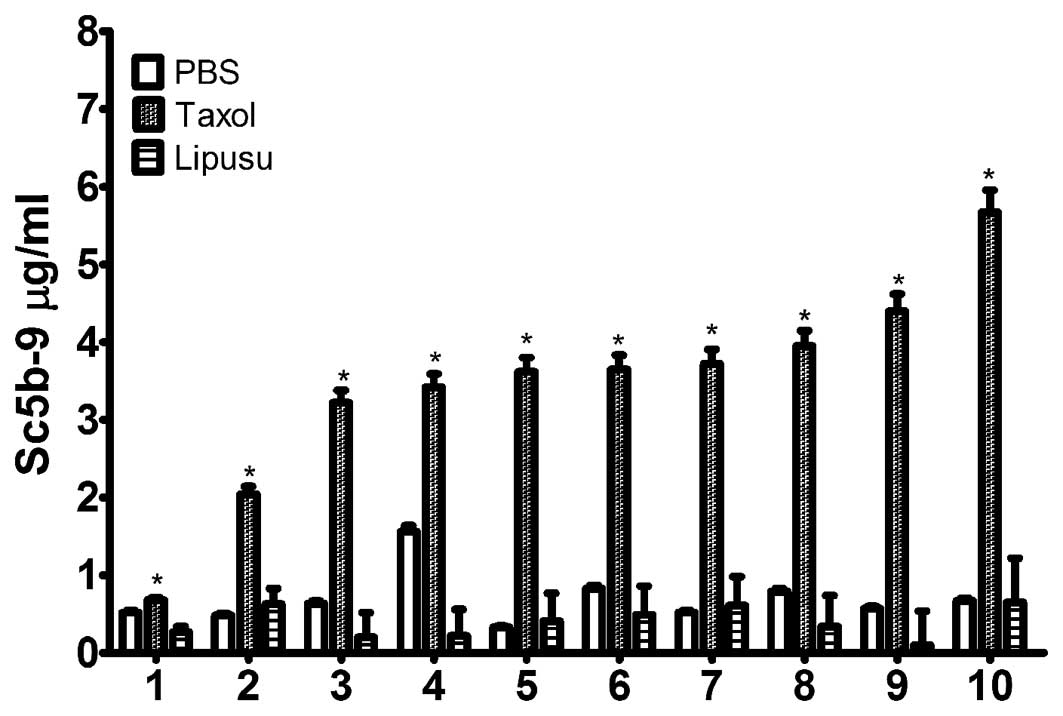
The effects of Taxol and Lipusu on complement
activation were determined in vitro using sera from healthy
subjects. Following incubation with the drugs, serum SC5b-9 content
was quantified using an ELISA kit. As shown in Fig. 4, Taxol significantly increased the
amount of terminal complement activation products in all 10 normal
subjects following incubation for 30 min at 37°C, which was not
observed in sera after incubation with either 5% glucose injection
or with Lipusu. Our results also showed differences in complement
response to Taxol in different subjects, as changes in SC5b-9
levels ranged from 2-fold to 6-fold in various individuals.
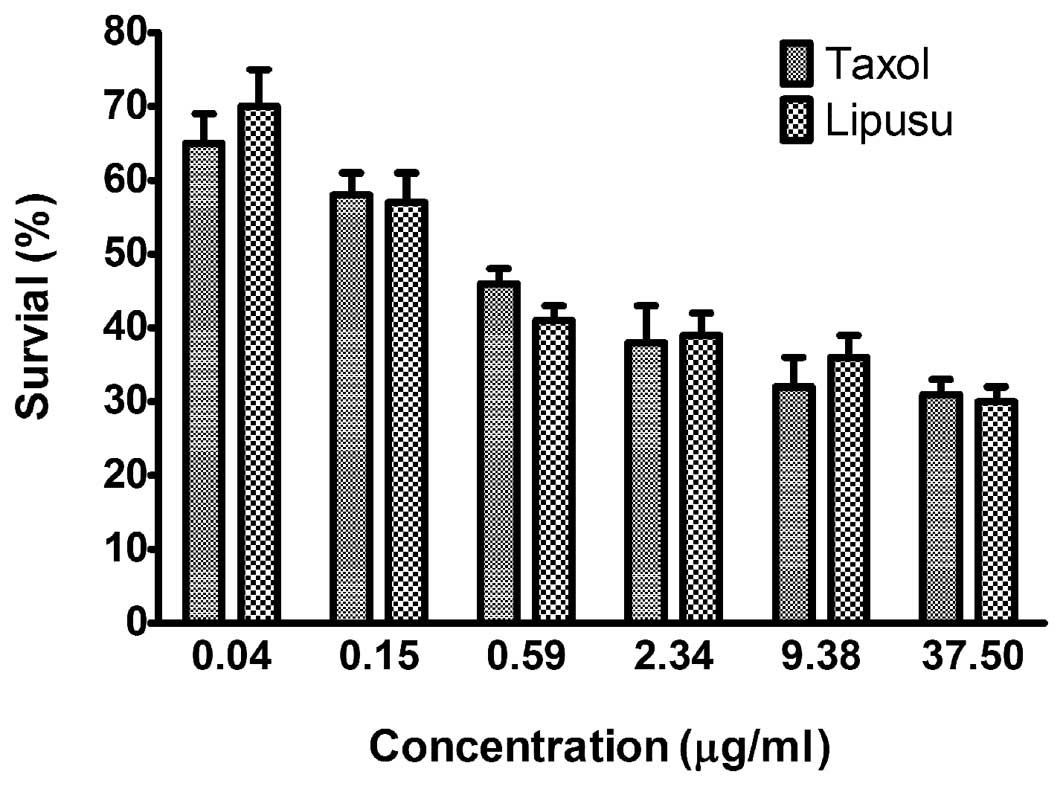
Lipusu and Taxol showed similar cytotoxic
activity against KB cancer cells in vitro
In vitro cytotoxicity of Lipusu against KB
oral carcinoma cells was compared with that of Taxol using an MTT
assay. To achieve different doses of paclitaxel, standard
formulations of Taxol and Lipusu were diluted with the culture
media, resulting in the final concentrations (0.04–37.5 μg/ml). As
shown in Fig. 5, Taxol and Lipusu
displayed robust anti-proliferation activity against KB cancer
cells in a dose-dependent manner, with no significant difference
between these two formulations.
Discussion
Paclitaxel is a powerful anti-cancer drug and is
used widely against several types of malignant tumors (12). Due to its poor solubility,
commercial paclitaxel is prepared with a PCO-ethanol solvent, which
introduces serious side effects, such as hypersensitivity and
neurotoxicity (8). To overcome
these problems, several strategies have been used to improve
paclitaxel formulations without PCO (7). In this study, we reported for the
first time a novel paclitaxel-liposome formulation designated as
Lipusu, which was proven to eliminate the hypersensitivity
reactions while retaining robust anti-proliferative activity
similar to Taxol.
The LD50 for the two formulations was
first determined in Swiss mice. Lipusu, with a higher
LD50, was shown to have a greater safety margin than
Taxol. As the PCO and ethanol vehicle in Taxol is considered to be
the toxic agent and main cause of hypersensitivity (15), Lipusu, which is devoid of PCO and
ethanol, was observed to have greatly decreased overall toxicity.
Above all, the anaphylaxis-like symptoms associated with PCO, such
as syncope and dyspnea, were only observed in animals injected with
Taxol, but not in those injected with Lipusu.
An anaphylaxis study was then performed using
bioequivalent dosages to those used in the clinic. Almost all of
the Taxol-injected mice were observed to have different degrees of
hypersensitivity reactions, which were not observed in
Lipusu-injected animals. Based on the asthma-like findings in the
animals with hypersensitivity reactions, the lungs were weighed and
examined. Significantly increased lung/body ratios and accumulation
of inflammatory exudate were common in animals injected with Taxol
instead of Lipusu, which may be responsible for the respiratory
symptoms in animals. Based on our unpublished data, both Taxol and
Lipusu were quickly redistributed to the lung post-injection, in
which the inflammatory cells and exudate accumulated, resulting in
the symptoms described above.
Unwanted complement activation played an important
role in Taxol-induced hypersensitivity and tissue lesions (5,6).
After systemic exposure to Taxol rather than Lipusu, the animals
were observed to have increased serum SC5b-9, which may be
initiated by PCO binding with C3 (20). As the terminal product of
complement activation, it could directly bind with membranes and
cause osmatic lysis of target cells (20,22).
The active molecules produced during complement activation, such as
C3a and C5a, could bind to mast cells to trigger histamine release
(6), which consequently increased
microvascular permeability and promoted the exudation of
inflammation cells (21). The
pathophysiologic changes resulting from increased SC5b-9 and
aggregated histamine contributed to the clinical signs as well as
the hypersensitivity reactions. Notably, the hypersensitivity
reactions and the lung lesions, as well as the increased content of
serum SC5b and lung histamine, were alleviated significantly by
pretreatment with corticosteroids and antihistamines, as used in
clinics.
To further confirm the anaphylaxis findings in
animal experiments, sera from healthy volunteers were used to
perform the in vitro complement activation assay. Consistent
with the literature (5,6), Taxol was observed to activate
complement in vitro, which was indicated by the increased
SC5b-9 content. Lipusu incubated at same concentration, however,
could not activate the complement pathway. These findings, with
regard to the activity of Lipusu on complement activation, were not
completely consistent with certain reports (20), in which it was shown that
liposome-based formulations induced hypersensitivity reactions.
Other researchers, however, published similar findings to ours, in
which liposome-based paclitaxel did not induce hypersensitivity
reactions (23,24). The exact circumstances and
mechanisms in which hypersensitivity reactions were induced by
liposome formulations need to be further explored.
The last question addressed in this study called for
comparison of the antitumor activities of these two formulations.
From the in vitro data, no significant difference in
cytotoxic activity was observed between these two formulations by
MTT assay in KB cells. Together with the results mentioned above,
Lipusu did not induce hypersensitivity reactions in vitro
and in vivo, and retained robust anti-proliferative
activity, which would improve the compliance of cancer
patients.
In conclusion, Lipusu, a novel liposome-based
paclitaxel formulation, was proven to eliminate the
hypersensitivity reactions associated with PCO, while showing
anti-proliferative activity similar to Taxol. Therefore, the
improved formulation of paclitaxel will bring a number of benefits
to cancer patients.
Acknowledgements
This study was supported by the Taishan Scholar
Project and the Technology Development Program Projects of Shandong
Province (2011YD18075).
References
|
1
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kavallaris M: Microtubules and resistance
to tubulin-binding agents. Nat Rev Cancer. 10:194–204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adams JD, Flora KP, Goldspiel BR, Wilson
JW, Arbuck SG and Finley R: Taxol: a history of pharmaceutical
development and current pharmaceutical concerns. J Natl Cancer Inst
Monogr. 141–147. 1993.PubMed/NCBI
|
|
4
|
Szebeni J, Muggia FM and Alving CR:
Complement activation by Cremophor EL as a possible contributor to
hypersensitivity to paclitaxel: an in vitro study. J Natl Cancer
Inst. 90:300–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rowinsky EK, Eisenhauer EA, Chaudhry V,
Arbuck SG and Donehower RC: Clinical toxicities encountered with
paclitaxel (Taxol). Semin Oncol. 20:1–15. 1993.PubMed/NCBI
|
|
6
|
Weiszhar Z, Czucz J, Revesz C, Rosivall L,
Szebeni J and Rozsnyay Z: Complement activation by polyethoxylated
pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20.
Eur J Pharm Sci. 45:492–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hennenfent KL and Govindan R: Novel
formulations of taxanes: a review. Old wine in a new bottle? Ann
Oncol. 17:735–749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koudelka S and Turanek J: Liposomal
paclitaxel formulations. J Control Release. 163:322–334. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo C, Wang Y, Chen Q, et al: Advances of
paclitaxel formulations based on nanosystem delivery technology.
Mini Rev Med Chem. 12:434–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elsadek B and Kratz F: Impact of albumin
on drug delivery - new applications on the horizon. J Control
Release. 157:4–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ribeiro JT, Macedo LT, Curigliano G, et
al: Cytotoxic drugs for patients with breast cancer in the era of
targeted treatment: back to the future? Ann Oncol. 23:547–555.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferlini C, Gallo D and Scambia G: New
taxanes in development. Expert Opin Investig Drugs. 17:335–347.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Porche DJ: Liposomal doxorubicin (Doxil).
J Assoc Nurses AIDS Care. 7:55–59. 1996. View Article : Google Scholar
|
|
14
|
Petrikkos GL: Lipid formulations of
amphotericin B as first-line treatment of zygomycosis. Clin
Microbiol Infect. 15(Suppl 5): 87–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang JA, Anyarambhatla G, Ma L, et al:
Development and characterization of a novel Cremophor EL free
liposome-based paclitaxel (LEP-ETU) formulation. Eur J Pharm
Biopharm. 59:177–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fasol U, Frost A, Buchert M, et al:
Vascular and pharmacokinetic effects of EndoTAG-1 in patients with
advanced cancer and liver metastasis. Ann Oncol. 23:1030–1036.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosiello AP, Essignmann JM and Wogan GN:
Rapid and accurate determination of the median lethal dose (LD50)
and its error with a small computer. J Toxicol Environ Health.
3:797–809. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rose WC: Taxol-based combination
chemotherapy and other in vivo preclinical antitumor studies. J
Natl Cancer Inst Monogr. 47–53. 1993.PubMed/NCBI
|
|
19
|
Wang H, Li H, Zuo M, et al: Lx2-32c, a
novel taxane and its antitumor activities in vitro and in vivo.
Cancer Lett. 268:89–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szebeni J: Complement activation-related
pseudoallergy: a new class of drug-induced acute immune toxicity.
Toxicology. 216:106–121. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karasuyama H, Tsujimura Y, Obata K and
Mukai K: Role for basophils in systemic anaphylaxis. Chem Immunol
Allergy. 95:85–97. 2010. View Article : Google Scholar
|
|
22
|
Bossi F, Fischetti F, Pellis V, et al:
Platelet-activating factor and kinin-dependent vascular leakage as
a novel functional activity of the soluble terminal complement
complex. J Immunol. 173:6921–6927. 2004. View Article : Google Scholar
|
|
23
|
Cabanes A, Briggs KE, Gokhale PC, Treat JA
and Rahman A: Comparative in vivo studies with paclitaxel and
liposome-encapsulated paclitaxel. Int J Oncol. 12:1035–1040.
1998.PubMed/NCBI
|
|
24
|
Xia XJ, Guo RF, Liu YL, et al:
Formulation, characterization and hypersensitivity evaluation of an
intravenous emulsion loaded with a paclitaxel-cholesterol complex.
Chem Pharm Bull (Tokyo). 59:321–326. 2011. View Article : Google Scholar
|