Introduction
Hepatocellular carcinoma (HCC) is one of the most
common and aggressive malignant tumor types worldwide. The
incidence of HCC has markedly increased to >600,000 cases
annually, and HCC has become the second most common cause of
mortality in China (1,2). Numerous risk factors, including
chronic infection with hepatitis B and C viruses, alcohol-related
cirrhosis and non-alcoholic fatty liver diseases, are closely
correlated with the occurrence and development of HCC (3–5).
Furthermore, a large percentage of patients with advanced HCC
require systemic or selective chemotherapy to achieve a longer life
expectancy. Unfortunately, the overall survival rate remains poor
with the use of traditional chemotherapy, as HCC is insensitive to
conventional cytotoxic agents (6,7).
Therefore, there is a significant requirement for the development
of innovative treatment approaches to improve patient outcome.
AMP-activated protein kinase (AMPK) is a metabolic
sensing protein kinase that acts as an important energy sensor,
mainly in ATP-deprived conditions (8). Therefore, AMPK is known to play a
major protective role under conditions of metabolic stress. In the
activated state, AMPK downregulates several anabolic enzymes and
thus turns off ATP-consuming metabolic pathways (9,10).
Several studies have observed the strong pro-apoptotic potential of
AMPK in activated conditions, including AMPK activator
(AICAR)-treated cells or constitutively active AMPK mutants
(11). Collectively, these studies
highlight the importance of AMPK as a direct therapeutic target in
all stages of cancer.
Berberine, an alkaloid purified from the
Berberis species (Fig. 1),
has been extensively studied and is known to exhibit multiple
pharmacological activities, including antiprotozoal,
antihypertensive, antibacterial, anti-inflammatory, anticholinergic
and anti-arrhythmic effects (12–14).
Previous studies have demonstrated that berberine has anticancer
activities against several types of cancer, to include liver cancer
(15,16). Berberine has been demonstrated to
induce apoptosis in numerous cultured cancer cell lines (17,18);
however, the molecular mechanisms underlying berberine-induced
apoptosis and the pathways involved have not been fully elucidated
to date. In the present study, we aimed to investigate the
inhibitory effect of berberine on HCC cell lines and determine
whether berberine-induced apoptosis was correlated with the
AMPK-mediated caspase-dependent mitochondrial pathway.
Materials and methods
Reagents
Berberine (purity, >99%), sulforhodamine B
(SRB)and protease inhibitors were purchased from Sigma-Aldrich (St.
Louis, MO, USA). The high-glucose medium, Dulbecco’s Modified
Eagle’s Medium (DMEM), was obtained from Gibco-BRL (Carlsbad, CA,
USA). The Bradford Protein Assay kit, RIPA lysis buffer and the
Annexin V-FITC Apoptosis Detection kit were purchased from Beyotime
Institute of Biotechnology (Jiangsu, China). Activated signaling
pathways were identified using primary antibodies, including
anti-AMPK, anti-phosphospecific AMPK, anti-Akt,
anti-phosphospecific Akt, anti-Bax, anti-Bcl-2, anti-cytochrome c,
anti-caspase-3 and anti-caspase-9, purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The internal control
antibody (anti-β-actin) was also obtained from Santa Cruz
Biotechnology, Inc.
Cell culture
The human hepatocelluar carcinoma cell lines (HepG2,
SMMC-7721 and Bel-7402) and the normal liver cell line (HL-7702)
were purchased from Hsiang-Ya Medical College (Hunan, China). Cells
were cultured at 37°C and 5% CO2 in DMEM supplemented
with 10% fetal bovine serum (FBS), penicillin (100 U/ml) and
streptomycin (100 mg/ml). The study was approved by the ethics
committee of Sichuan University.
Cell treatment and cell viability
assay
The in vitro cytotoxicity of berberine
against HepG2, SMMC-7721, Bel-7402 and HL-7702 cells was assessed
using the traditional SRB assay. The SRB assay is routinely used
for cytotoxicity determination, based on the measurement of live
cell protein content (19). In
brief, cells were seeded into 96-well plates at a density of 7,000
cells/well. Following 24 h of culture in DMEM supplemented with 10%
FBS, the medium was replaced with DMEM without FBS for 24 h.
Subsequently, the cells were treated with berberine at final
concentrations of 3.125, 6.25, 12.5, 25, 50 and 100 μM for 24 or 48
h prior to the SRB assay. All experiments were conducted in
parallel with controls; at 24 or 48 h, 100 μl of 10%
trichloroacetic acid was also added to each well when staining for
30 min, and then the excess dye was removed by washing repeatedly
with 1% acetic acid. The protein-bound dye was dissolved in 10 mM
Tris Base solution (Santa Cruz Biotechnology, Inc.) for optical
density (OD) determination at 570 nm using a microculture reader
(Bio-Rad, Richmond, CA, USA). The percentage of viable cells was
calculated as follows: (A of experimental group/A of control group)
×100, where A represents the absorbance.
Apoptosis assays by flow cytometry
(FCM)
Apoptosis was analyzed by FCM using the Annexin
V-FITC Apoptosis Detection kit (Beyotime Institute of
Biotechnology), according to the manufacturer’s instructions.
Briefly, following treatment with 0, 12.5, 50 and 100 μM berberine
for 24 h, HepG2 cells were detached and resuspended in 100 μl
binding buffer containing fluorescein isothiocyanate-conjugated
annexin V and propidium iodide (PI). Following incubation for 15
min at room temperature in the dark, the cells were analyzed by FCM
(BD Biosciences, Franklin Lakes, NJ, USA).
Western blot assay
The cells were washed twice with cold
phosphate-buffered saline (PBS) and then suspended in RIPA buffer
for 30 min on ice. The supernatant was collected as the total
protein extract. The protein concentration was estimated by the
Bradford Protein Assay kit (Beyotime Institute of Biotechnology),
according to the manufacturer’s instructions. Equal amounts of
protein (40 μg) were analyzed by sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 5 or
12% separation gel, and molecular weight markers were run
simultaneously. The proteins were transferred to nitrocellulose
membranes and then blocked for 2 h at room temperature in a
solution of 5% milk or bovine serum albumin (BSA). Incubation with
the appropriate primary antibodies was performed overnight at 4°C.
Membranes were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit or anti-mouse secondary antibody
(1:2,000 dilution) for 2 h at room temperature with gentle
agitation. After washing, bands were visualized by an Enhanced
Chemiluminescence (ECL) Western Blotting Detection system (Amersham
Pharmacia Biotech, NJ, USA).
Statistical analysis
All data were expressed as the mean ± SE. The
statistical significance of differences in the data was determined
by Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Berberine inhibits cell viability in HCC
cell lines in a time- and dose-dependent manner
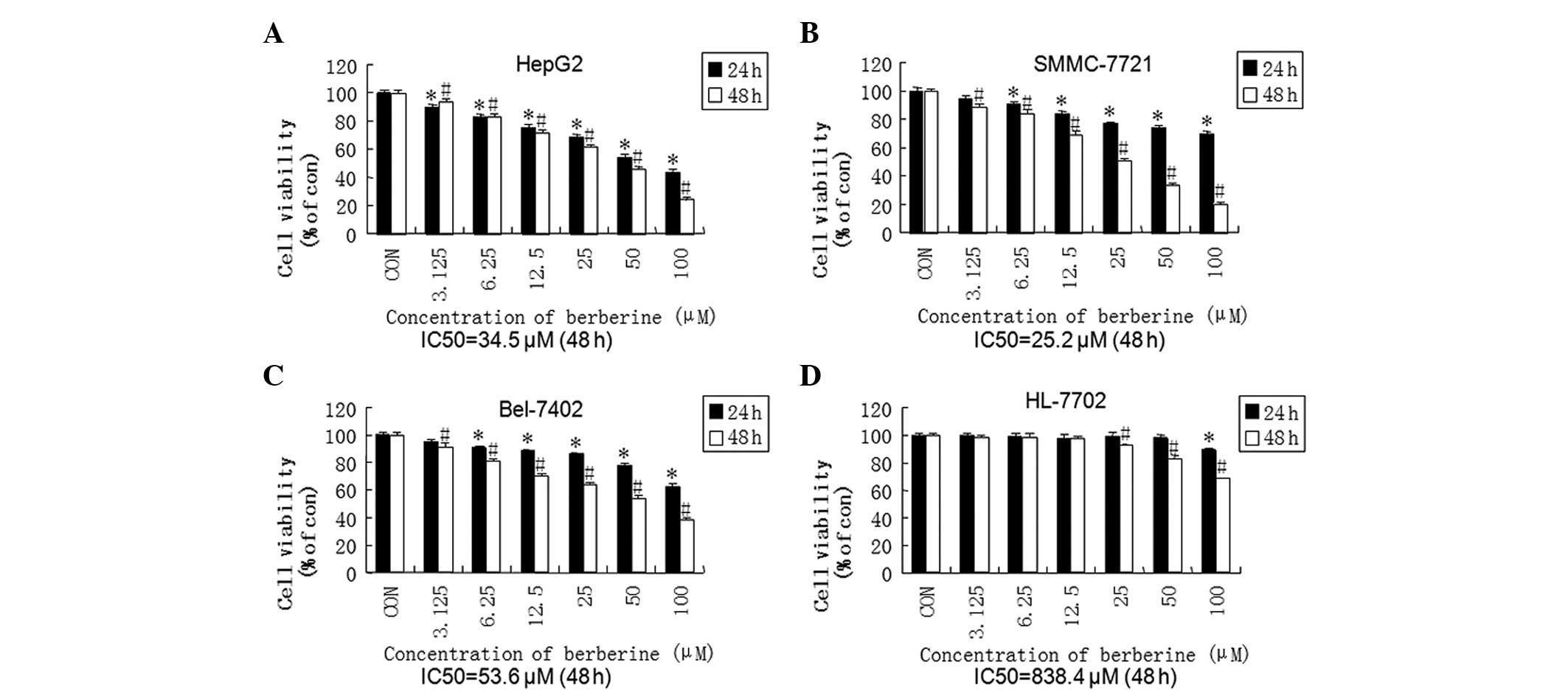
To evaluate the antitumor characteristics of
berberine, cell viability was measured by a standard SRB assay
following brief exposure to berberine (24 or 48 h). As demonstrated
in Fig. 2, berberine significantly
reduced cell viability in human hepatoma cell lines in a time- and
dose-dependent manner. Subsequently, we predicted the side effects
of berberine by exposing the normal hepatic cell line, HL-7702, to
the agent. The IC50 values in the HL-7702 cell line
(838.4 μM) at 48 h were markedly higher than those of the HCC cell
lines (34.5 μM, 25.2 μM and 53.6 μM in the HepG2, SMMC-7721 and
Bel-7402 cell lines, respectively). The differences in the
IC50 values indicated that berberine was able to
selectively decrease the cell viability of HCC cells compared with
normal hepatocytes.
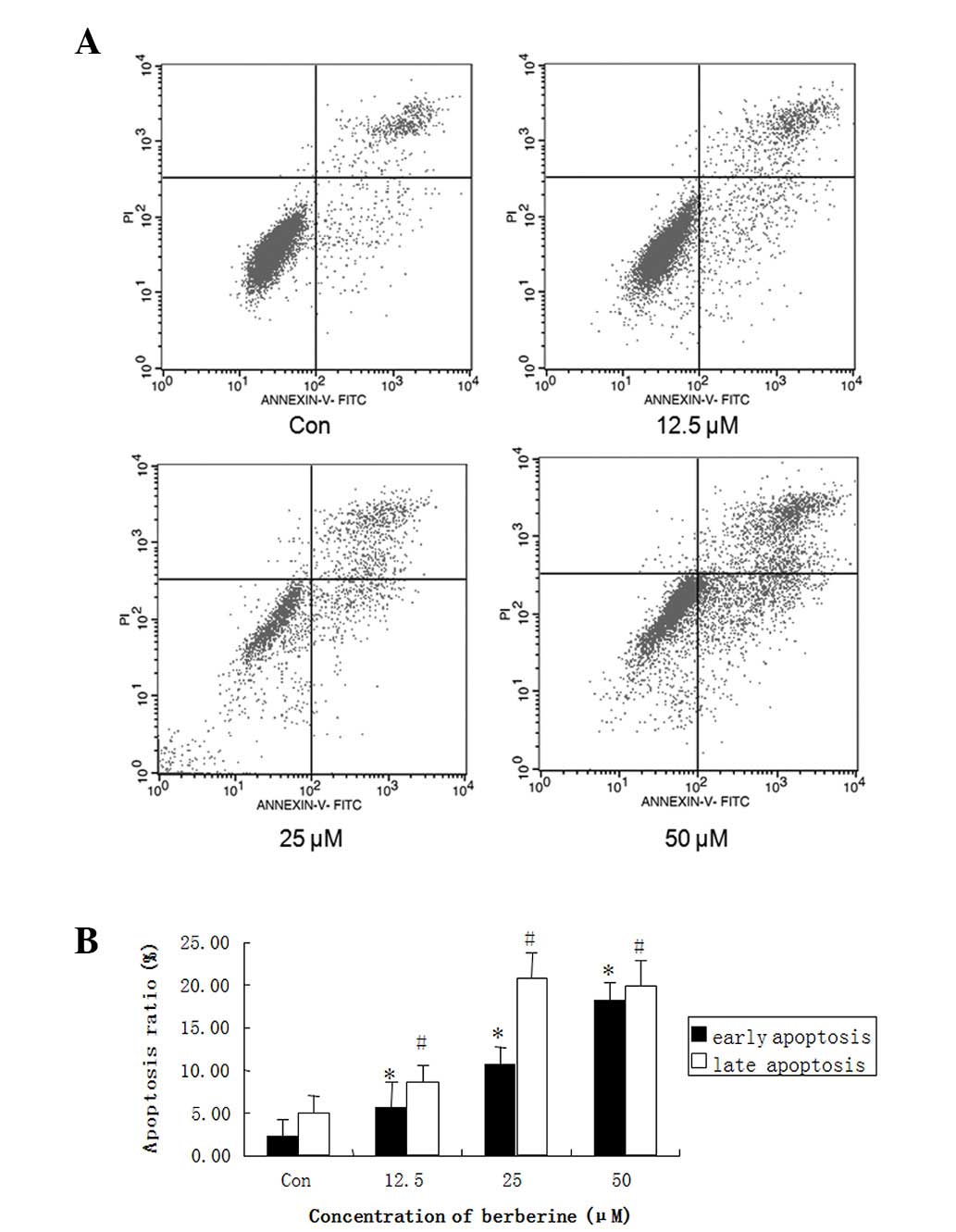
Berberine increases apoptosis in HepG2
cells
To further investigate the inhibition mechanism of
berberine in HCC, we selected HepG2 cells for further study. HepG2
cells were treated with 12.5, 25 and 50 μM berberine for 24 h, and
apoptosis was detected by flow cytometric analysis with the Annexin
V-FITC Apoptotic Detection kit (Beyotime Institute of
Biotechnology). Data revealed that the pretreatment of HepG2 cells
with berberine significantly increased the number of early
apoptotic cells (annexin V+ and PI−) and late
apoptotic cells (annexin V+ and PI+) compared
with the control group, in a dose-dependent manner (Fig. 3A). At a concentration of 50 μM,
berberine led to ≤40% apoptosis in HepG2 cells (Fig. 3B). These results suggested that
berberine-induced apoptosis in the liver cancer cells contributed
to its antiproliferative and cytotoxic effects.
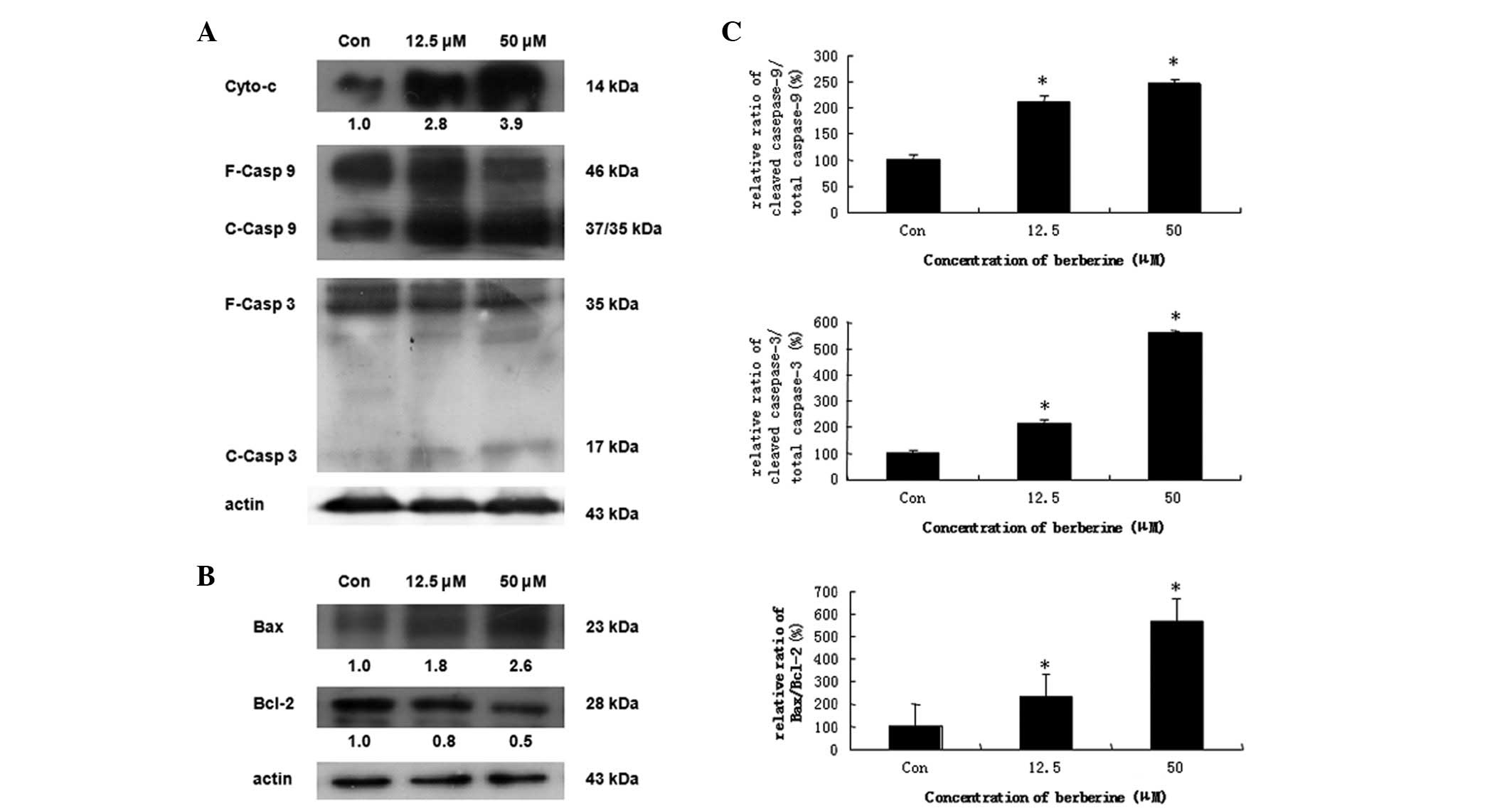
Berberine induces apoptosis via the
mitochondrial-dependent pathway
In chemically induced apoptosis, mitochondria play a
central role in the commitment of cells to apoptosis through
cytochrome c-dependent pathways. To elucidate the molecular
mechanism of berberine-induced apoptosis in HCC cells, we examined
the expression of proteins associated with apoptosis. HepG2 cells
were treated with 12.5 and 50 μM berberine for 24 h. The results
demonstrated that creatine kinase induces the release of cytochrome
c from mitochondria to the cytosol in dose-dependent manner (data
not shown). Caspase-9 and -3, well-known downstream molecules of
cytochrome c, were also cleaved in a concentration-dependent manner
(Fig. 4A). We further evaluated
the effect of berberine on the mitochondrial apoptosis signaling
pathway. We found that the expression of Bcl-2 protein, an
anti-apoptotic molecule, decreased in a dose-dependent manner,
whereas the expression of the pro-apoptotic protein, Bax, was
significantly increased (Fig. 4B).
Furthermore, the ratio of Bax/Bcl-2 was increased in a
dose-dependent manner (Fig. 4C).
These results indicated that treatment with berberine leads to a
shift from anti-apoptosis to pro-apoptosis by altering the function
of the proteins in the Bcl-2 family, which results in the release
of cytochrome c from mitochondria, thus inducing caspase-dependent
mitochondrial pathway cell apoptosis.
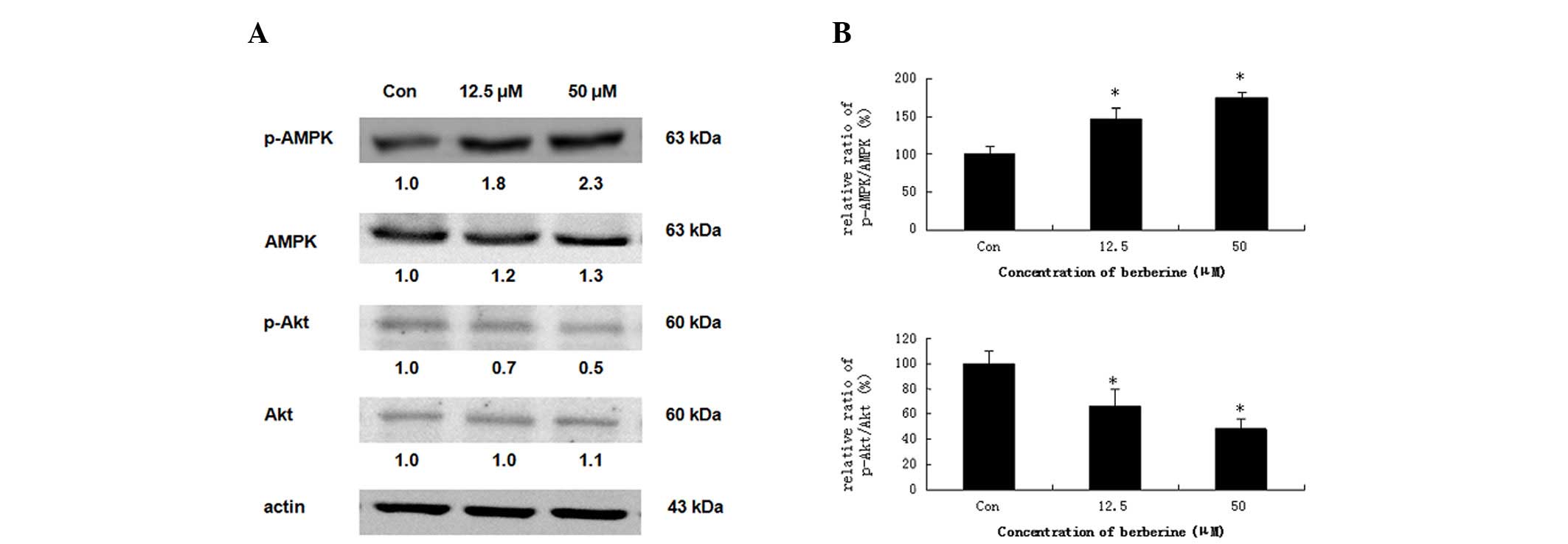
Effects of berberine on the protein
expression of AMPK and Akt
Several reports have demonstrated that activation of
AMPK leads to the induction of apoptosis in numerous human cancer
cell types (20,21). Therefore, we investigated whether
the phosphorylation of AMPK is induced by berberine. HepG2 cells
were treated with 12.5 and 50 μM berberine for 24 h. As
demonstrated in Fig. 5A, compared
with the basal level, berberine significantly stimulated the
phosphorylation of AMPK (Thr-172), which increased the ratio of
p-AMPK/total-AMPK in dose-dependent manner. The measurements of the
phosphorylation pattern of Akt revealed that the pretreatment of
HepG2 cells with berberine resulted in a marked elevation in the
levels of phosphorylated Akt (Ser-473) relative to the control
cells, without significantly altering the total protein levels of
Akt (Fig. 5B). These results
indicated that AMPK activation is correlated with the induction of
apoptosis.
Discussion
HCC has a higher prevalence and mortality rate
compared with other types of cancer. Therefore, prolonging survival
times and improving quality of life have become main objectives in
the treatment and management of patients with advanced HCC. The
antitumor property of berberine has recently attracted increasing
research attention (22). A
crucial advantage for the use of natural products as an alternative
to the chemotherapeutic approach is their low cytotoxicity. In the
present study, we examined the effect of berberine on cell
viability and apoptosis in normal and cancer cells. We observed
that berberine suppressed cell growth in a dose- and time-dependent
manner in human HepG2, SMMC-7721 and Bel-7404 HCC cell lines. This
indicates that the inhibitory effect of berberine in liver cancer
is non-species-specific, and is associated with the previously
reported types of HCC cells (23).
The normal control liver cell line, HL-7702, exhibited decreased
cell viability.
Berberine is one of the key components of Coptis
chinesis, which is frequently utilized in proprietary Chinese
herbal drugs and exerts a wide range of pharmacological effects.
Berberine exhibits high antitumor activity in various types of
tumors through different mechanisms (16,17,24).
A number of factors, including cytokines, chemokines, receptors and
downstream elements within signaling cascade pathways, are
correlated with apoptosis induced by berberine. Berberine has been
demonstrated to induce apoptosis in human HCC cells via
Fas-mediated (15) inhibition of
the mTOR-signaling pathway (16),
downregulation of MMP-9 expression (25) and the nuclear factor-κB (NF-κB) and
activator protein 1 (AP-1) pathways (26). In the current study, we
demonstrated that berberine had cytotoxic effects in HepG2 cells,
including annexin V binding, cytochrome c release and activation of
caspases, indicated by the increased cleavage of caspase-9 and -3,
via caspase-dependent mitochondrial pathway cell apoptosis.
The association between cancer and AMPK emerges as
an intriguing area of investigation. Numerous studies have
indicated that the AMPK pathway is implicated in cancer. While
traditionally regarded as a sensor of cellular energy status and a
regulator of metabolism, AMPK has been linked to tumor suppressors,
including LKB1, p53, TSC1 and TSC2, providing novel support for the
theory that AMPK may function as a suppressor of cell proliferation
(27). There has been much
speculation on the role of AMPK in cancer, as this pathway may be
either anti-apoptotic or pro-apoptotic depending on the conditions
(28). The results of the present
study demonstrated that treatment with berberine promoted AMPK
phosphorylation and inhibited Akt phosphorylation in HepG2 cells,
which led to caspase-dependent mitochondrial pathway cell
apoptosis.
In conclusion, to the best of our knowledge, our
results demonstrate for the first time that berberine exerts a
selective antitumor effect on human liver cancer cells when
compared with non-tumorigenic HL-7702 normal liver cells.
Additionally, our data provide support to the theory that AMPK may
be involved in the antitumor effect of berberine via
caspase-dependent mitochondrial pathway cell apoptosis. These
findings provide a molecular basis for the antiproliferative
activity of berberine, which may be used as a potent and
alternative chemotherapeutic agent for the treatment of HCC.
Acknowledgements
This study was supported by the China National
Nature Science Fund (no. 30671963), the China Medical Board of New
York Inc. (no. 98-681) and the Sichuan University 985 project
(Science and Technology Innovation Platform for Novel Drug
Development).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Tang ZY, Ye SL, Liu YK, et al: A decade’s
studies on metastasis of hepatocellular carcinoma. J Cancer Res
Clin Oncol. 130:187–196. 2004.
|
|
3
|
Ming L, Thorgeirsson SS, Gail MH, et al:
Dominant role of hepatitis B virus and cofactor role of aflatoxin
in hepatocarcinogenesis in Qidong, China. Hepatology. 36:1214–1220.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB: Epidemiology of
hepatocellular carcinoma in USA. Hepatol Res. 37(Suppl 2): S88–S94.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parkin DM: The global health burden of
infection-associated cancers in the year 2002. Int J Cancer.
118:3030–3044. 2006.PubMed/NCBI
|
|
6
|
Petraccia L, Onori P, Sferra R, et al: MDR
(multidrug resistance) in hepatocarcinoma clinical-therapeutic
implications. Clin Ter. 154:325–335. 2003.(In Italian).
|
|
7
|
Zhang C, Liu L, Yu Y, Chen B, Tang C and
Li X: Antitumor effects of ginsenoside Rg3 on human hepatocellular
carcinoma cells. Mol Med Rep. 5:1295–1298. 2012.PubMed/NCBI
|
|
8
|
Hardie DG, Carling D and Carlson M: The
AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of
the eukaryotic cell. Annu Rev Biochem. 67:821–855. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hardie DG: Minireview: the AMP-activated
protein kinase cascade: the key sensor of cellular energy status.
Endocrinology. 144:5179–5183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hardie DG and Sakamoto K: AMPK: a key
sensor of fuel and energy status in skeletal muscle. Physiology.
21:48–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garcia-Gil M, Pesi R, Perna S, et al:
5′-Aminoimidazole-4-carboxamide riboside induces apoptosis in human
neuroblastoma cells. Neuroscience. 117:811–820. 2003.
|
|
12
|
Akhter MH, Sabir M and Bhide NK:
Anti-inflammatory effect of berberine in rats injected locally with
cholera toxin. Indian J Med Res. 65:133–141. 1977.PubMed/NCBI
|
|
13
|
Hwang JM, Wang CJ, Chou FP, Tseng TH,
Hsieh YS, Lin WL and Chu CY: Inhibitory effect of berberine on
tert-butyl hydroperoxide-induced oxidative damage in rat liver.
Arch Toxicol. 76:664–670. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai CS and Ochillo RF: Pharmacological
effects of berberine on the longitudinal muscle of the guinea-pig
isolated ileum. Arch Int Pharmacodyn Ther. 310:116–131.
1991.PubMed/NCBI
|
|
15
|
Wang GY, Lv QH, Dong Q, Xu RZ and Dong QH:
Berbamine induces Fas-mediated apoptosis in human hepatocellular
carcinoma HepG2 cells and inhibits its tumor growth in nude mice. J
Asian Nat Prod Res. 11:219–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang N, Feng Y, Zhu M, et al: Berberine
induces autophagic cell death and mitochondrial apoptosis in liver
cancer cells: the cellular mechanism. J Cell Biochem.
111:1426–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Patil JB, Kim J and Jayaprakasha GK:
Berberine induces apoptosis in breast cancer cells (MCF-7) through
mitochondrial-dependent pathway. Eur J Pharmacol. 645:70–78. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ho YT, Lu CC, Yang JS, et al: Berberine
induced apoptosis via promoting the expression of caspase-8, -9 and
-3, apoptosis-inducing factor and endonuclease G in SCC-4 human
tongue squamous carcinoma cancer cells. Anticancer Res.
29:4063–4070. 2009.
|
|
19
|
Vichai V and Kirtikara K: Sulforhodamine B
colorimetric assay for cytotoxicity screening. Nat Protoc.
1:1112–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim YM, Hwang JT, Kwak DW, et al:
Involvement of AMPK signaling cascade in capsaicin-induced
apoptosis of HT-29 colon cancer cells. Ann N Y Acad Sci.
1095:496–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji CB, Yang B, Yang YL, et al: Exogenous
cell-permeable C6 ceramide sensitizes multiple cancer cell lines to
Doxorubicin-induced apoptosis by promoting AMPK activation and
mTORC1 inhibition. Oncogene. 29:6557–6568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dorai T and Aggarwal BB: Role of
chemopreventive agents in cancer therapy. Cancer Lett. 5:129–140.
2004. View Article : Google Scholar
|
|
23
|
Hwang JM, Kuo HC, Tseng TH, Liu JY and Chu
CY: Berberine induces apoptosis through a mitochondria/caspases
pathway in human hepatoma cells. Arch Toxicol. 80:62–73. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hur JM, Hyun MS, Lim SY, Lee WY and Kim D:
The combination of berberine and irradiation enhances anti-cancer
effects via activation of p38 MAPK pathway and ROS generation in
human hepatoma cells. J Cell Biochem. 107:955–964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu B, Wang G, Yang J, Pan X, Yang Z and
Zang L: Berberine inhibits human hepatoma cell invasion without
cytotoxicity in healthy hepatocytes. PLoS One. 6:e214162011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chao DC, Lin LJ, Kao ST, et al: Inhibitory
effects of Zuo-Jin-Wan and its alkaloidal ingredients on activator
protein 1, nuclear factor-κB, and cellular transformation in HepG2
cells. Fitoterapia. 82:696–703. 2011.PubMed/NCBI
|
|
27
|
Motoshima H, Goldstein BJ, Igata M and
Araki E: AMPK and cell proliferation - AMPK as a therapeutic target
for atherosclerosis and cancer. J Physiol. 574:63–71. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo Z, Saha AK, Xiang X and Ruderman NB:
AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci.
26:69–76. 2005. View Article : Google Scholar : PubMed/NCBI
|