Introduction
Hepatitis, cirrhosis, hepatoma and end-stage liver
disease caused by multiple factors still lack effective treatment
protocols. Treatment of chronic liver disease had become a major
problem for clinicians due to the poor efficacy of clinical
treatments and high mortality. It has been reported that ~10% of
patients with liver disease succumb to their condition while
waiting for liver sources each year (1). Liver transplantation is currently the
only therapeutic option for patients with end-stage chronic liver
disease and for severe acute liver failure. Due to limited donor
availability, surgical injury, a higher incidence of surgical
complications and the expensive cost of treatment, the development
of liver transplantation has been restricted (2).
Recent studies have focused on methods to restore
liver mass and function through cell transplantation. Stem cells
are a promising source for liver repopulation following cell
transplantation (3). Several
studies have reported that the administration of in vitro
expanded stem cells may promote liver regeneration (4,5). In
addition, hepatic stem cells have been identified in adult liver
tissues (6,7) and oval cells derived from rodent and
human livers have been demonstrated to be hepatic stem cells
(8–10). Hepatic stem cells may participate
in liver regeneration and restoration during serious liver injury
(11–14). Thus, studies on the proliferation
and differentiation of these cells are extremely useful for
elucidating the molecular mechanisms of liver development.
In the present study, partial hepatectomy (PH) was
performed to activate hepatic oval cells in rat livers, and oval
cells undergoing proliferation and differentiation were identified
by analysis of specific morphological and phenotypical
characteristics. We sought to optimize hepatic stem cell
proliferation and differentiation in in vitro culture by
comparing various conditions, including different percentage PH and
duration of collagenase perfusion.
Materials and methods
Animals
Male Wistar rats (age, 8–10 weeks old; weight,
150–180 g) were purchased from the Laboratory Animal Center of
Dalian Medical University and maintained on standard laboratory
chow and daily cycles of alternating 12 h of light and dark.
PH model
All experiments were performed in accordance with
the Principles of Laboratory Animal Care and approved by the Local
Committee for Experimental Animal Research. PH (73.1 and 83.4%) of
rat livers was performed according to the procedure described
previously (15,16). Sham-operated control animals were
treated in an identical manner with the omission of
hepatectomy.
Hepatic stem cell isolation
At 0, 7 and 14 days following PH, rat liver cells
were isolated by a two-step collagenase IV digestion method, as
described previously (17). The
liver was perfused via a cannula in the inferior vena cava with 250
ml buffer [142 mM NaCl, 6.7 mM KCl and 10 mM HEPES (pH 7.4)]
followed by 250 ml buffer containing additional 5.7 mM
CaCl2 and 0.5 mg/ml IV collagenase (Sigma-Aldrich, St.
Louis, MO, USA) for 10, 20 or 30 min.
Hepatic stem cell culture and
differentiation
Isolation and purification of oval cells were
performed according to the protocol of Pack et al(18) with specific modifications. Briefly,
the hepatocytes were dispersed and washed twice with cold
Ca2+-free perfusion buffer and resuspended in RPMI-1640
medium (Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% FBS
(Hyclone Laboratories, Inc., Logan, UT, USA), 5 ng/ml M-CSF, 0.4
ng/ml IL-3 and 140 μM β-mercaptoethanol (all PeproTech Inc., Rocky
Hill, NJ, USA) to stimulate stem cell proliferation. Following a
6-day culture period, culture medium was replaced with RPMI-1640
medium containing 10% FBS, 20 μg/l hepatocyte growth factor (HGF)
and 10 μg/l fibroblast growth factor-4 (FGF-4; both PeproTech,
Inc.) to stimulate stem cell differentiation. Cell growth was
observed under a contrast phase microscope and recorded at days 0,
7 and 14. Viability was determined by trypan blue exclusion and
only preparations with >90% viability were used. Cell number was
determined with a hemocytometer (Bio-Rad, Hercules, CA, USA).
Flow cytometric analysis
The presence of CD34+ Thy-1+
cells was determined at 0, 7 and 14 days after PH. Freshly isolated
cells (1×105) were fixed in cold acetone for 8 min at
4°C. Following centrifugation at 141 × g for 5 min at 4°C, pellets
were suspended in 0.1 ml PBS and incubated with anti-CD34 and Thy-1
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) antibodies
for 60 min at 37°C. Centrifugation was performed at 141 × g for 15
min and followed by extensive washes in PBS. Pellets were suspended
in 0.1 ml PBS in preparation for cell suspension. Binding of
primary antibody was detected by phycoerythrin (PE)-labeled IgG
(Dako, Carpinteria, CA, USA). Cells were assayed by flow cytometry
(Becton Dickinson, San Jose, CA, USA) and data were analyzed using
Cell-Quest software (Becton Dickinson). Replacement of primary
antibody with PE-labeled IgG served as a negative control.
Immunofluorescence assay of oval
cells
Immunofluorescence analysis of oval cells in culture
was performed on days 0, 7 and 14. Cells on glass slides were fixed
in cold acetone for 5 min, blocked with normal goat serum following
extensive washes in PBS (pH 7.4) and incubated with primary
antibodies including anti-cytokeratin-18 (CK-18) and
α-1-fetoprotein (AFP) at 4°C overnight. Following washing three
times in PBS, cells were cultured with PE-labeled IgG for 30 min at
37°C, washed three times again in PBS and observed microscopically
for fluorescence. Replacement of the primary antibody with
PE-labeled IgG served as a negative control.
Western blot analysis
Rat liver tissue was homogenized (TissueRuptor;
Qiagen, Hilden, Germany). Proteins were lyzed with lysis buffer
containing 25 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1%
sodium deoxycholate, 0.1% SDS, 1 mM PMSF and protease and
phosphatase inhibitor cocktails (all from Sigma-Aldrich), as
described previously (19).
Proteins were separated by SDS-PAGE and transferred to a PVDF
membrane (Bio-Rad, Hercules, CA, USA) using standard techniques.
Antibodies used for immunoblotting were as follows: CK-18, AFP and
β-catenin, and horseradish peroxidase-conjugated IgG secondary
antibodies (all Santa Cruz Biotechnology, Inc.). An ECL plus
western blotting detection kit (Amersham Biosciences, Piscataway,
NJ, USA) was used for development of the membrane.
Statistical analysis
All results are expressed as the mean ± SD.
Statistical differences were determined by Student’s t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Morphological features of freshly
isolated cells from hepatectomized rats
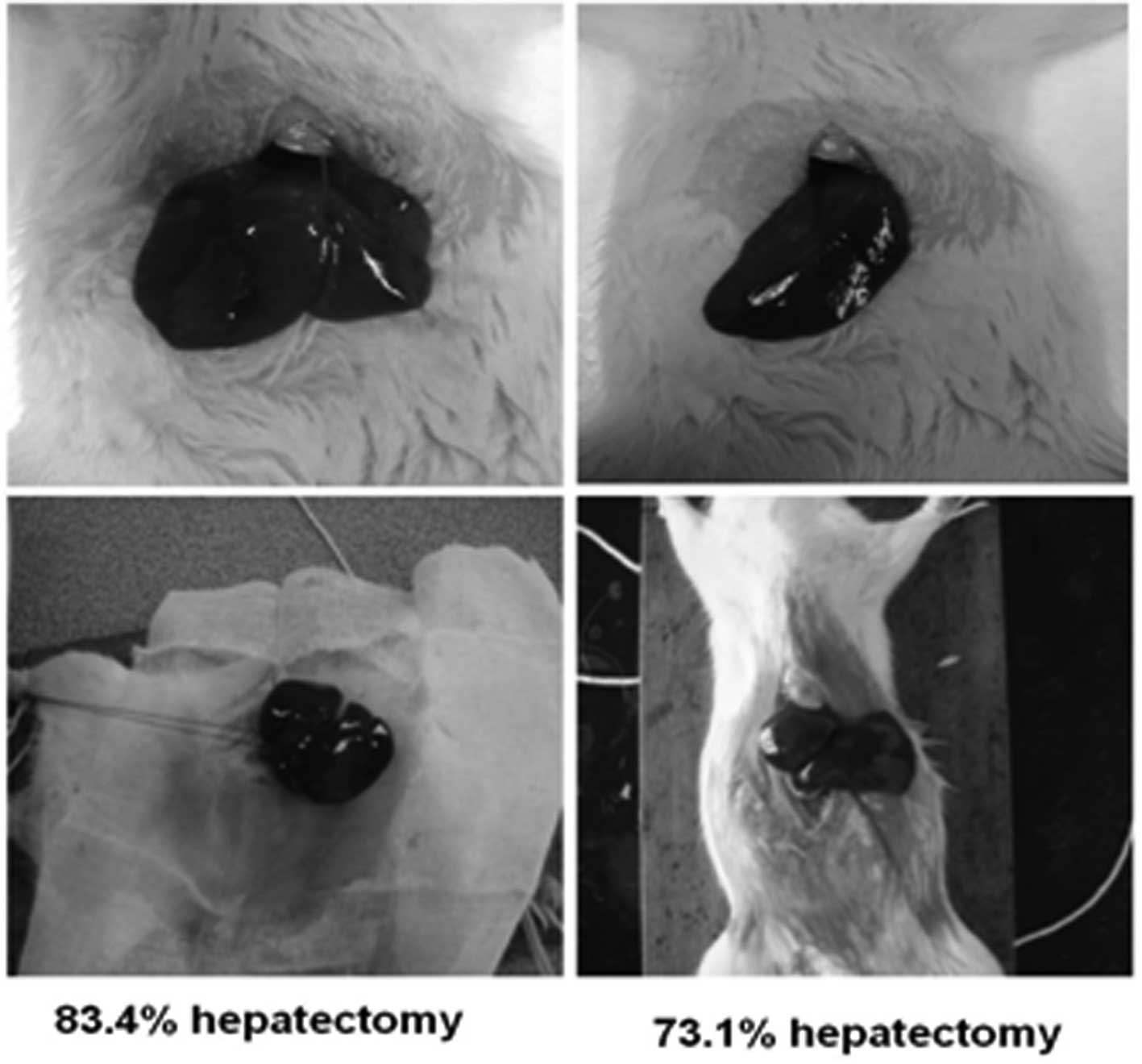
Hepatectomy in rat livers was performed at 83.4 and
73.1% (Fig. 1). Collagenase
perfusion was used to extract and isolate hepatic cells. In the
control group, the newly separated hepatic cells were observed to
exhibit a round shape, homogeneous size and strong diffraction. The
viability of freshly isolated cells was >95%, as estimated by
their ability to exclude trypan blue (data not shown). Following
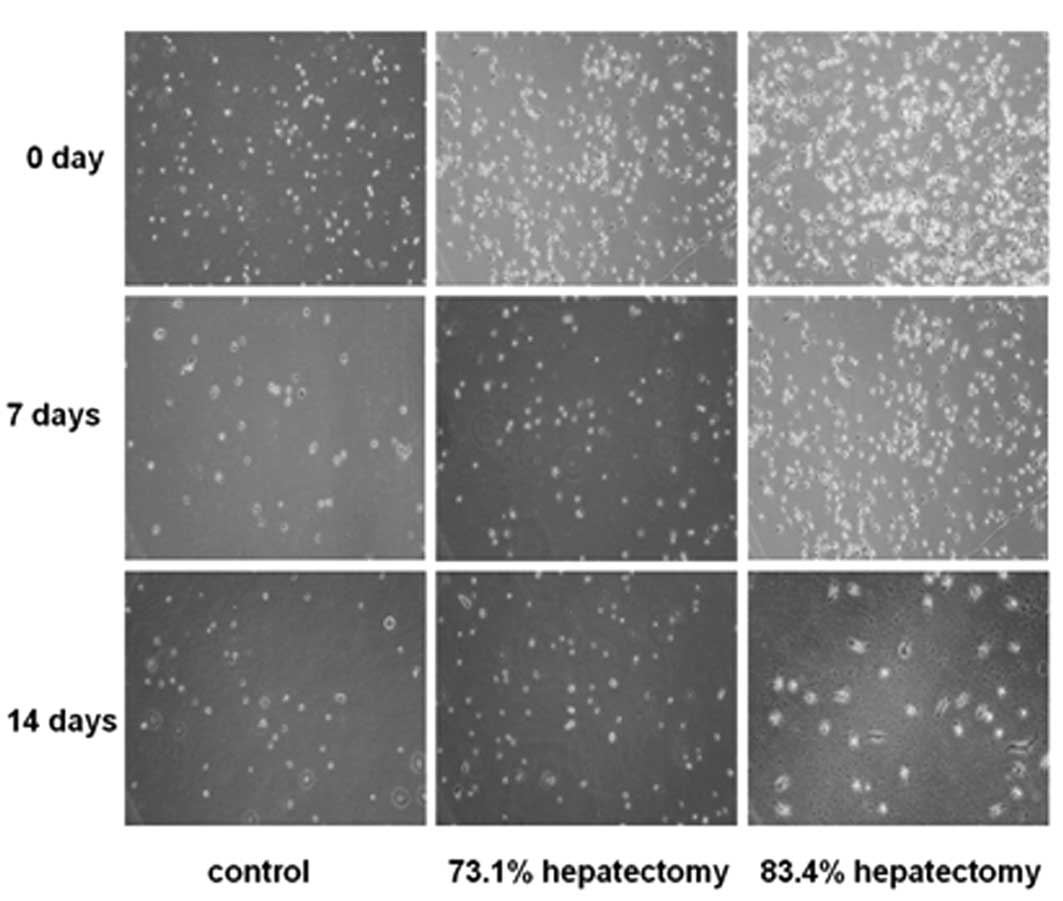
culture for 4–6 h, phase-contrast microscopy revealed that cells
attaching to the culture dishes were round in shape (Fig. 2). After culture for 7 days, cells
were observed to grow individually and no colonies were noted.
Parts of the cells gradually stretched, causing fusiform or
spindle-shaped growth. At day 14, the majority of the cells were
fibroblast-like (Fig. 2). In the
73.1% hepatectomy group, colony-like growth was observed following
culture for 6 days and a number of cells were fused, indicative of
hepatic stem cell proliferation (Fig.
2). In the 83.4% hepatectomy group, cells did not exhibit
colony-like growth (Fig. 2).
Phenotypic characteristics of freshly
isolated cells from hepatectomized rats
Next, the phenotypic characteristics of
differentiated cells derived from isolated cells from PH rats were
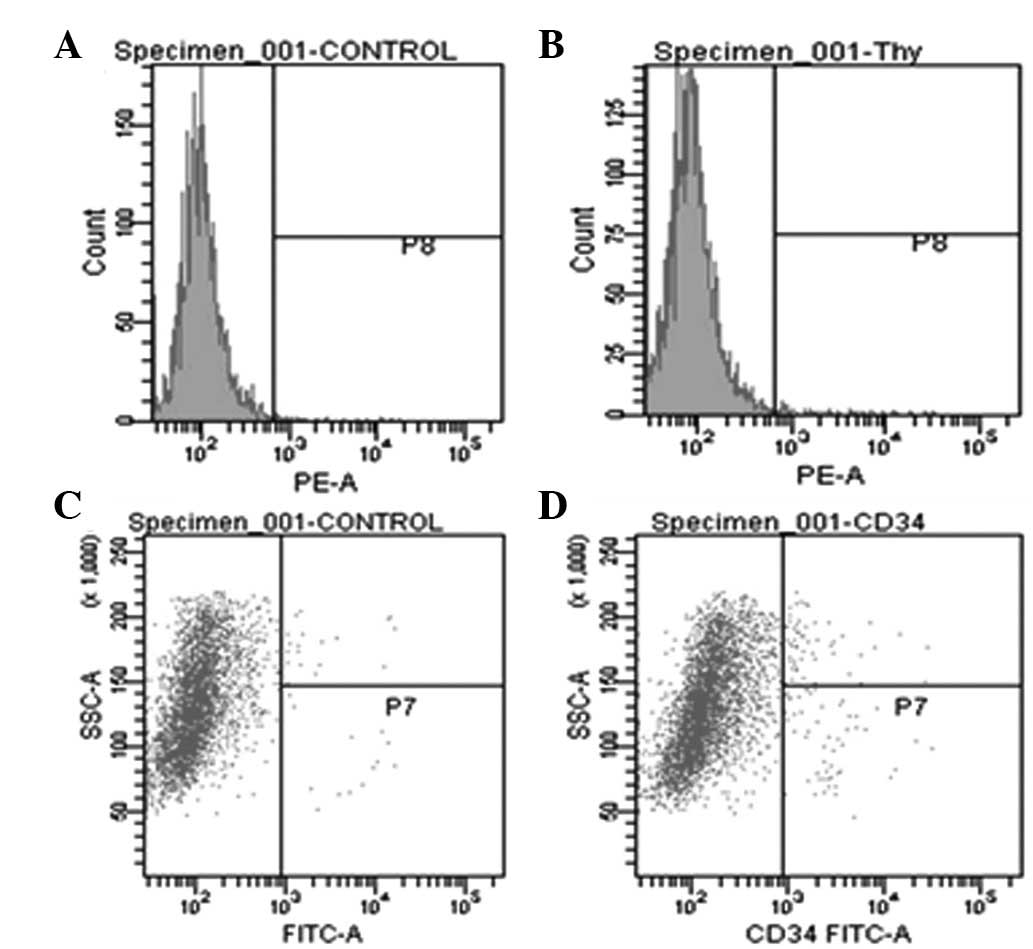
analyzed to confirm biochemical identity. Flow cytometry indicated
that all the cells isolated from PH rats were positive for hepatic
progenitor markers, CD-34 and Thy-1 (Fig. 3). In 83.4% PH rats, a significant
increase in CD-34 and Thy-1 levels were observed compared with
73.1% PH rats.
In addition, when phenotypic characteristics were
compared at 0, 7 and 14 days following 83.4% PH surgery, cells
isolated 7 days following PH were observed to express CD-34 and
Thy-1 at higher levels than cells at 0 and 14 days. In addition,
the effect of various durations of IV collagenase perfusion on
CD-34 and Thy-1 levels (Fig. 3)
was analyzed. Perfusion of IV collagenase for 20 min resulted in a
significant increase in CD-34 and Thy-1 levels, higher than that of
10 and 30 min collagenase perfusion.
Immunofluorescence analysis of
proliferative oval cells
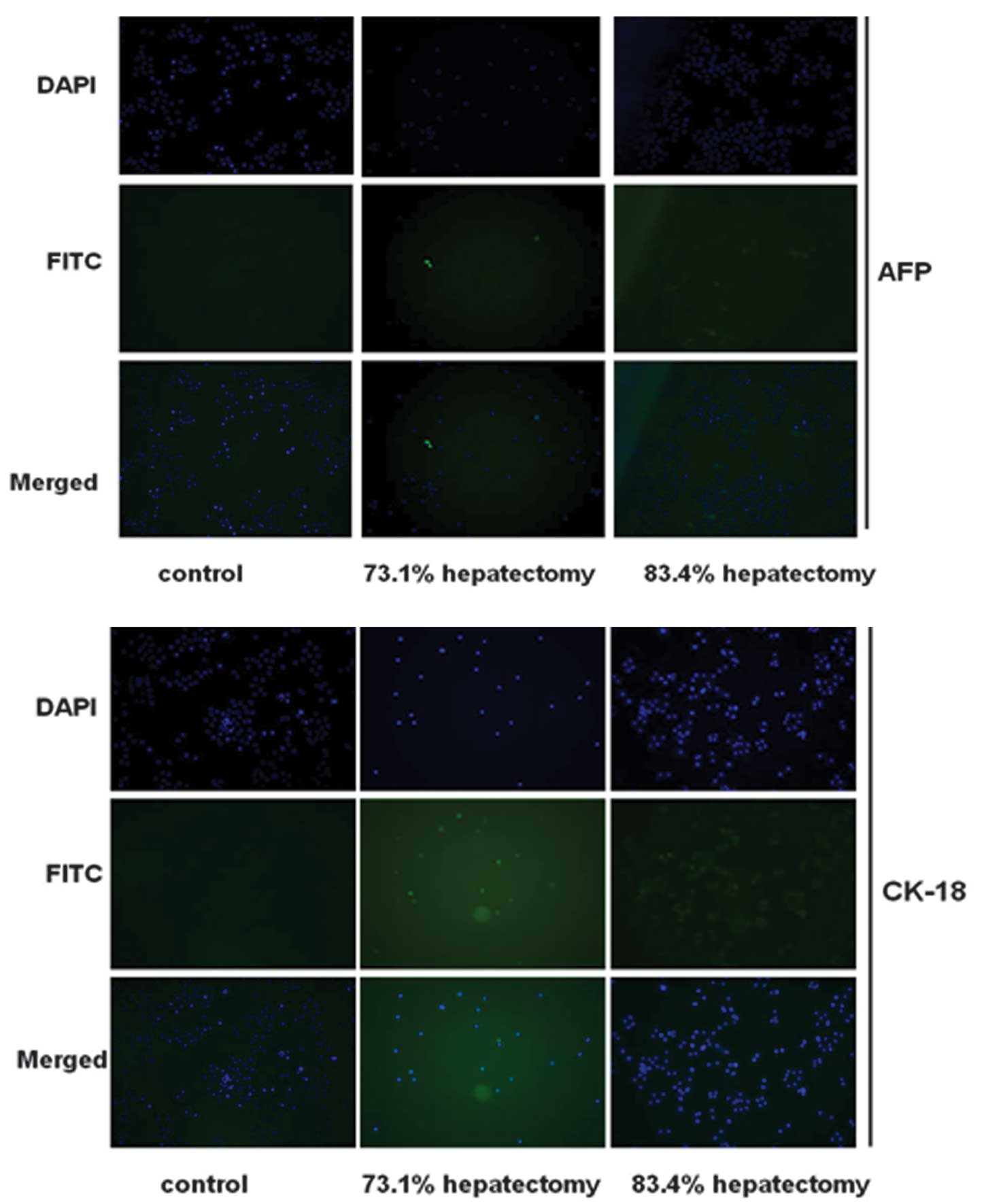
Hepatocyte differentiation was evaluated further by
immunofluorescence analysis for AFP and CK-18 (markers of
hepatocytes). Cells in the control group were negative for AFP and
CK-18 (Fig. 4). By contrast, cells
isolated from hepatectomized rats expressed AFP and CK-18,
indicating that the cells had differentiated into hepatocyte-like
cells. In the control group, expression of AFP and CK-18 was
negative.
The effect of different PH procedures and duration
of collagenase perfusion on differentiation was analyzed in
vitro. As demonstrated in Fig.
4, immunofluorescence staining revealed that isolated cells on
day 7 following 73.1 and 83.4% PH surgery expressed AFP and CK-18
at high levels. Expression was highest in cells isolated from 83.4%
PH rats. Levels of AFP and CK-18 increased in cells isolated from
PH rats perfused with collagenase for 20 min compared with 10 and
30 min perfusion. In addition, cells isolated 7 days following PH
expressed AFP and CK-18 at higher levels than cells at 0 and 14
days (data not shown).
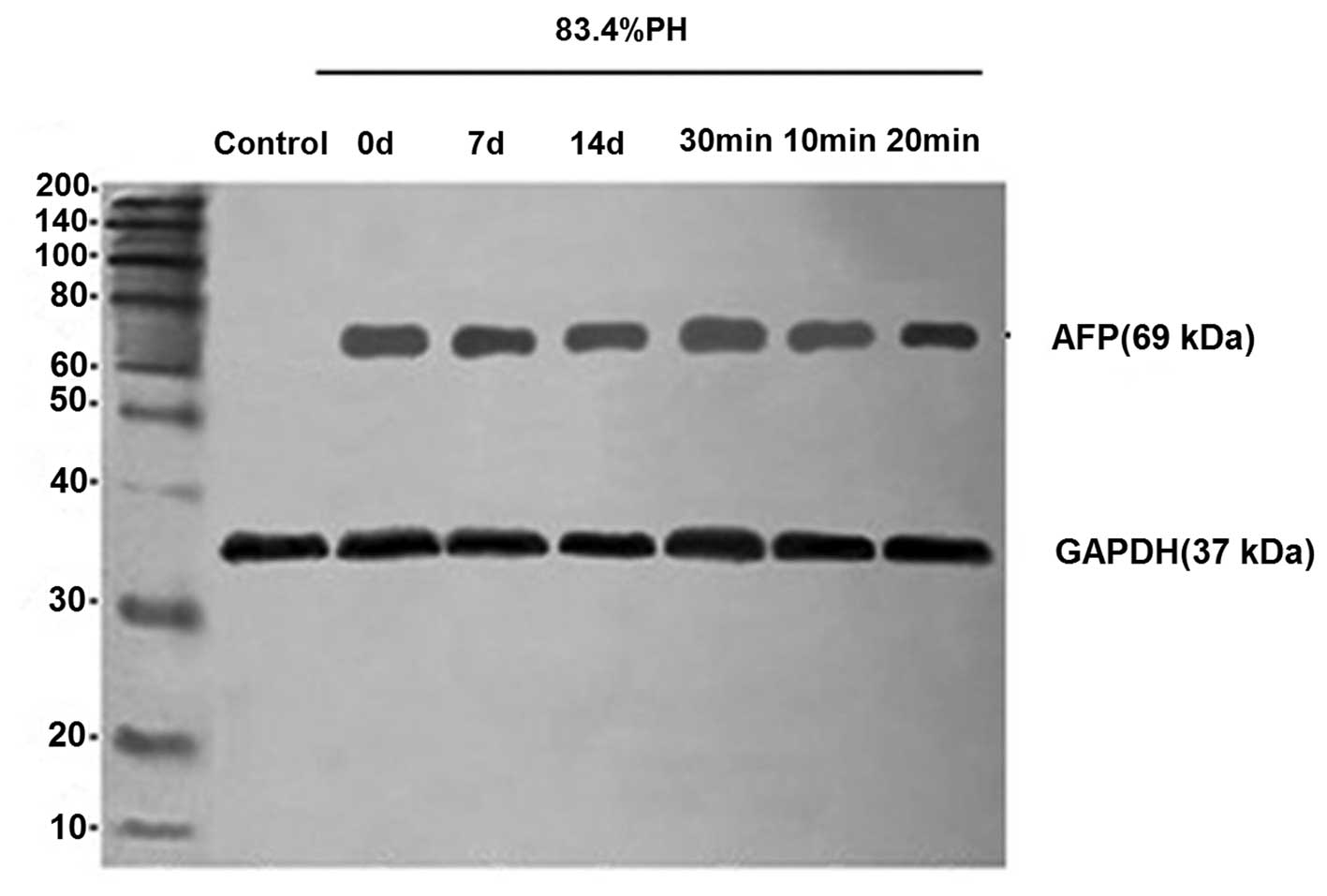
Analysis of AFP protein expression by
western blot analysis
To further evaluate the phenotypic properties of
differentiated cells, the expression of differentiation marker,
AFP, was analyzed by western blot analysis in 83.4% PH rats.
Differentiated cells were observed to express AFP protein at high
levels (Fig. 5). These results
further indicated that oval cells were specified towards a
hepatocyte lineage and that fully differentiated cells possess the
genetic characteristics of mature hepatocytes following the
isolation of cells from PH rats. In addition, consistent with
results of immunofluorescence, cells isolated from rats 7 days
following PH with collagenase perfusion for 20 min revealed
stronger ATP expression.
Discussion
In recent years, there have been a number of
advances in liver stem cell biology and studies have hypothesized
that fetal liver stem cells (hepatoblasts) and adult liver stem
cells (oval cells) may be useful for the generation of primary
hepatocytes. These hepatic progenitors have the potential to give
rise to hepatocytes or cholangiocytes (20–22).
However, it has also been demonstrated that the number of hepatic
progenitor cells in tissues are low, leading to difficulties in
isolation, purification and expansion for large-scale expansion
(23).
A large number of growth factors regulate cell
proliferation and differentiation. Oh et al(24) previously reported that HGF promotes
hepatic stem cell differentiation into hepatic cells in
vitro. Fiegel et al(25) reported that CD34+ bone
marrow hematopoietic stem cells express specific hepatic cell
markers, including AFP and CK-18, when HGF is added to the culture
medium. Additional studies demonstrated that stem cells originating
from the liver are able to be differentiated into hepatocyte-like
cells under HGF and epidermal growth factor (EGF) induction
(25,26). To date, HGF, FGF, EGF and
transforming growth factor have been extensively applied in stem
cell studies worldwide (28,29).
Jang et al(30) found that damaged hepatic cells
excrete specific factors when co-cultured with purified
hematopoietic stem cells, inducing stem cells to differentiate into
hepatocyte-like cells expressing hepatoid cells markers, including
CK-18 and hepatocyte nuclear factor-3β (27). The ability of a number of factors
to induce multipotent adult progenitor cell (MAPC) differentiation
into functional hepatocyte-like cells was analyzed and optimal
differentiation was observed when cells were cultured with FGF-4
and HGF. Human, mouse and rat MAPCs cultured with FGF-4 and HGF
differentiated into epithelioid cells expressing hepatic cell
markers, acquired functional characteristics of hepatocytes and
maintained the accordant metabolic activity of humans (27). Ruhnke et al(31) reported that peripheral blood
monocytes cultured with M-CSF and IL-3 expressed the hematopoietic
stem cell marker, CD90, and monocyte marker, CD14, and then
differentiated into hepatocyte-like (NeoHap) cells following
induction by FGF-4 and HGF. The NeoHep cells resembled primary
human hepatocytes with regard to morphology, expression of
hepatocyte markers, various secretory and metabolic functions and
drug detoxification activities. Following transplantation of NeoHep
cells into the liver of severe combined immunodeficiency
disease/non-obese diabetic mice, neohepatocytes integrated well
into the liver tissue and revealed a morphology and albumin
expression level similar to that of primary human hepatocytes
transplanted under identical conditions (32).
In this context, to optimize hepatocyte formation,
we first induced hepatic stem cell proliferation by PH, and the
liver was removed 0, 7 and 14 days following PH. To obtain viable
cells from the liver, two-step collagenase perfusion was performed.
The first step consists of an isotonic buffer to flush blood cells
and platelets from the vasculature. The second perfusate contained
collagenase, which digests the extracellular matrix, allowing for
the collection of a single cell suspension suitable for the
isolation of discrete cell populations. IV collagenase was perfused
for 10, 20 or 30 min.
Next, 5 μg/l M-CSF, 0.4 μg/l IL-3 and 140 μmol/l
β-mercaptoethanol was added to RPMI-1640 medium for 6 days,
followed by the addition of 20 μg/l HGF and 10 μg/l FGF-4. Cell
morphology was analyzed and proteins levels were determined by
immunochemistry and western blot analysis. The results revealed
that putative hepatic stem cells (oval cells) differentiate in
vitro into cells that are morphologically, phenotypically and
functionally representative of hepatocytes via a 2-step induction
protocol. HGF and FGF-4 are likely to initiate a stable hepatic
phenotype and are key to oval cell specification toward
hepatocytes, as indicated by the observation that these substances
induced oval cells to change into small hepatocytes with hepatic
characteristics. These results further confirmed that HGF and FGF-4
are important for hepatocyte differentiation.
Hematopoietic markers, including CD34 and Thy-1,
although restricted to hematopoietic stem cells, have been used in
a number of previous studies to identify and isolate hepatic
progenitors (33,34). The results of our study
demonstrated that rat-derived stem cells differentiate in
vitro into an endodermal cell type with hepatocyte phenotype.
Hepatectomy is necessary to trigger CD34+ stem cells.
The CD34 and Thy-1 contents of 83.4% PH cells were higher than that
of 73.1% PH cells. However, in the control group, cells were
negative for CD34 and Thy-1, indicating that the difference of
percentage in PH had an effect on the stem cell quantity. By
contrast, differences in the isolation times following surgery were
found to be associated with stem cell generation. At day 0, no
expression of CD34 and Thy-1 was observed, as the sorted cells were
immature. Over time, the expression of CD34 and Thy-1 increased as
the cells underwent maturation, with higher expression of CD34 and
Thy-1 observed at day 7. However, on day 14, CD34 and Thy-1
expression began to decrease.
The expression of CD34 and Thy-1 was also analyzed
under various durations of collagenase perfusion. Following 10 min
perfusion, cells exhibited a polygonal or circle appearance and
were negative for the hepatic stem cell markers, CD34 and Thy-1. By
contrast, after 20 min perfusion, cells exhibited a stretched
fibroblast-like appearance and expressed hepatic markers. However,
the expression of CD34 and Thy-1 decreased following perfusion for
30 min. These results indicated that the optimal conditions for
generation of hepatic cells following PH include 83.4% PH in rats
followed by cell isolation at 7 days using perfusion for 20 min.
Long-term collagenase perfusion may inhibit stem cell
formation.
Following cell culture in medium containing HGF and
FGF-4, cells were characterized by the presence of hepatic markers,
including AFP and CK-18. The expression of AFP and CK-18 was
highest in cells isolated under perfusion for 20 min at day 7
following PH, indicating that the optimal differentiation of
hepatic stem cells to CK-18- and AFP-positive cells is observed in
stem cells isolated from 83.4% PH rats (7 days following surgery)
perfused with IV collagenase for 20 min.
In conclusion, results of the current study indicate
that cells from PH were able to proliferate and differentiate into
cells of the hepatic lineage. Differences in the isolation times
following surgery and duration of perfusion were observed to affect
stem cell generation and differentiation. The establishment of an
in vitro protocol for the optimization of hepatic stem cell
culture as described in this study is likely to be useful for the
development of in vitro techniques for liver tissue or organ
culture.
References
|
1
|
Kim WR, Therneau TM, Benson JT, et al:
Deaths on the liver transplant waiting list: an analysis of
competing risks. Hepatology. 43:345–351. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duan BW, Lu SC, Wang ML, et al: Liver
transplantation in acute-on-chronic liver failure patients with
high model for end-stage liver disease (MELD) scores: a single
center experience of 100 consecutive cases. J Surg Res.
183:936–943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verfaillie CM, Pera MF and Lansdorp PM:
Stem cells: hype and reality. Hematology Am Soc Hematol Educ
Program. 2002:369–391. 2002. View Article : Google Scholar
|
|
4
|
Fiegel HC, Lange C, Kneser U, et al: Fetal
and adult liver stem cells for liver regeneration and tissue
engineering. J Cell Mol Med. 10:577–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alison MR, Choong C and Lim S: Application
of liver stem cells for cell therapy. Semin Cell Dev Biol.
18:819–826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilson JW and Leduc EH: Role of
cholangioles in restoration of the liver of the mouse after dietary
injury. J Pathol Bacteriol. 76:441–449. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arber N, Zajicek G and Ariel I: The
streaming liver. II. Hepatocyte life history. Liver. 8:80–87. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haruna Y, Saito K, Spaulding S, Nalesnik
MA and Gerber MA: Identification of bipotential progenitor cells in
human liver development. Hepatology. 23:476–481. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petersen BE, Bowen WC, Patrene KD, et al:
Bone marrow as a potential source of hepatic oval cells. Science.
284:1168–1170. 1999.PubMed/NCBI
|
|
10
|
Wang X, Foster M, Al-Dhalimy M, et al: The
origin and liver repopulating capacity of murine oval cells. Proc
Natl Acad Sci USA. 100(Suppl 1): 11881–11888. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Li M, Niu B and Gong J: Therapeutic
potential of stem cell in liver regeneration. Front Med. 5:26–32.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takami T, Terai S and Sakaida I: Stem cell
therapy in chronic liver disease. Curr Opin Gastroenterol.
28:203–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Russo FP and Parola M: Stem and progenitor
cells in liver regeneration and repair. Cytotherapy. 13:135–144.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He ZP, Tan WQ, Tang YF, Zhang HJ and Feng
MF: Activation, isolation, identification and in vitro
proliferation of oval cells from adult rat livers. Cell Prolif.
37:177–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colletti LM, Green M, Burdick MD, Kunkel
SL and Strieter RM: Proliferative effects of CXC chemokines in rat
hepatocytes in vitro and in vivo. Shock. 10:248–257. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higgins GM and Anderson RM: Restoration of
the liver of the white rat following partial surgical removal. Arch
Pathol. 12:186–202. 1931.
|
|
17
|
Shupe TD, Piscaglia AC, Oh SH, Gasbarrini
A and Petersen BE: Isolation and characterization of hepatic stem
cells, or ‘oval cells’, from rat livers. Methods Mol Biol.
482:387–405. 2009.
|
|
18
|
Pack R, Heck R, Dienes HP, Oesch F and
Steinberg P: Isolation, biochemical characterization, long-term
culture and phenotype modulation of oval cells from carcinogen-fed
rats. Exp Cell Res. 204:198–209. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bussolati B, Deambrosis I, Russo S, et al:
Altered angiogenesis and survival in humantumor-derived endothelial
cells. FASEB J. 17:1159–1161. 2003.PubMed/NCBI
|
|
20
|
Herrera MB, Bruno S, Buttiglieri S, et al:
Isolation and characterization of a stem cell population from adult
human liver. Stem Cells. 24:2840–2850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lazaro CA, Rhim JA, Yamada Y and Fausto N:
Generation of hepatocytes from oval cell precursors in culture.
Cancer Res. 58:5514–5522. 1998.PubMed/NCBI
|
|
22
|
Rogler LE: Selective bipotential
differentiation of mouse embryonic hepatoblasts in vitro. Am J
Pathol. 150:591–602. 1997.PubMed/NCBI
|
|
23
|
Czyz J, Wiese C, Rolletschek A, et al:
Potential of embryonic and adult stem cells in vitro. Biol Chem.
384:1391–1409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oh SH, Miyazaki M, Kouchi H, et al:
Hepatocyte growth factor induces differentiation of adult rat bone
marrow cells into a hepatocyte lineage in vitro. Biochem Biophys
Res Commun. 279:500–504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fiegel HC, Lioznov MV, Cortes-Dericks, et
al: Liver-specific gene expression in cultured human hematopoietic
stem cells. Stem Cells. 21:98–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyazaki M, Akiyama I, Sakaguchi M, et al:
Improved conditions to induce hepatocytes from rat bone marrow
cells in culture. Biochem Biophys Res Commun. 298:24–30. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schwartz RE, Reyes M, Koodie L, et al:
Multipotent adult progenitor cells from bone marrow differentiate
into functional hepatocyte like cells. J Clin Invest.
109:1291–1302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Block GD, Locker J, Bowen WC, et al:
Population expansion, clonal growth, and specific differentiation
patterns in primary cultures of hepatocytes induced by HGF/SF, EGF
and TGF alpha in a chemically defined (HGM) medium. J Cell Biol.
132:1133–1149. 1996. View Article : Google Scholar
|
|
29
|
Michalopoulos GK, Bowen WC, Mule K, et al:
HGF-, EGF- and dexamethas-one-induced gene expression patterns
during formation of tissue in hepatic organoid cultures. Gene Expr.
11:55–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jang YY, Collector MI, Baylin SB, et al:
Hematopoietic stem cells convert into liver cells within days
without fusion. Nat Cell Biol. 6:532–539. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ruhnke M, Nussler AK, Ungefroren H, et al:
Human monocyte-derived neohepatocytes: a promising alternative to
primary human hepatocytes for autologous cell therapy.
Transplantation. 79:1097–1103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ruhnke M, Ungefroren H, Nussler AK, et al:
Differentiation of in vitro-modified human peripheral blood
monocytes into hepatocyte-like and pancreatic islet-like cells.
Gastroenterology. 128:1774–1786. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Terrace JD, Currie IS, Hay DC, et al:
Progenitor cell characterization and location in the developing
human liver. Stem Cells Dev. 16:771–778. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petersen BE, Goff JP, Greenberger JS and
Michalopoulos GK: Hepatic oval cells express the hematopoietic stem
cell marker Thy-1 in the rat. Hepatology. 27:433–445. 1998.
View Article : Google Scholar : PubMed/NCBI
|