Introduction
Bladder cancer is a significant health problem,
accounting for ~7% of all human malignancies. It is the eighth most
common cause of cancer-related mortality worldwide, with an
estimated 70,980 novel cases and 14,330 deaths annually (1). Regardless of recent advancement in
multidisciplinary therapeutic strategies, bladder cancer remains
associated with relapse and deterioration (2). Therefore, defining the factors
involved in disease progression may aid in the identification of
novel strategies for the development of effective therapies against
bladder cancer.
The present study investigated exosomes, which are
small membrane vesicles, 30–100 nm in diameter, released by a
variety of mammalian cells into the extracellular space upon direct
fusion of the multi-vesicular bodies with the plasma membrane
(3). Exosomes contain lipids,
proteins and nucleic acids from their cell of origin, contents of
which are transferred to target cells and affect their function and
activity (4). Thus, exosomes may
be involved in cell-to-cell signaling. Several pioneering studies
have investigated the involvement of exosomes in reticulocyte
maturation and immune cell functions (5–6).
Recently, it has been suggested that exosomes are also involved in
a number of physiological and pathological processes, including
cancer (7). Numerous tumor cells
produce exosomes, which are emerging as a potential utility for the
early detection or control of human cancer (8–11).
Previous studies have demonstrated that exosomes derived from tumor
cells possess anti-tumor properties, such as inducing tumor cell
apoptosis (12) and enhancing
anti-tumor immunity (13).
Exosomes have also been extensively studied as a novel cell-free
source of tumor antigens to develop anti-cancer vaccines (14–15)
However, it is increasingly suggested that exosomes may also affect
tumor progression by exhibiting immunosuppressive properties,
facilitating tumor invasion and metastasis, stimulating tumor cell
proliferation or inducing drug resistance (16) A recent study showed that gastric
cancer-derived exosomes promoted tumor cell proliferation and
activated the mitogen-activated protein kinase/extracellular
signal-regulated kinase (MAPK/ERK) and lipid kinase
phoshoinositide-3-kinase (PI3K)/Akt signaling pathways (17).
To demonstrate the effects of bladder cancer
cell-derived exosomes on the regulation of tumor cell growth and
apoptosis, exosomes were isolated from bladder cancer cells and
their effects in bladder cancer cell lines were investigated in
vitro. It was demonstrated that bladder cancer cell-derived
exosomes promoted bladder cancer cell proliferation, but inhibited
apoptosis. In addition, exosomes were able to activate Akt and ERK
pathways. Thus, the results of the present study provide a novel
mechanism by which bladder cancer cell-derived exosomes participate
in tumor progression.
Materials and methods
Cell lines and culture
T24 and 5637 human bladder cancer cell lines were
provided by Professor Chun-Li Luo from the Department of Medical
Diagnostics, College of Laboratory Medicine, Chongqing Medical
University (Chongqing, China). Cells were cultured in RPMI-1640
(Gibco-BRL, Grand Island, NY, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Gibco-BRL), penicillin (100
U/ml) and streptomycin (100 g/ml) at 37° C with 5% CO2
in a humidified incubator. The cells were routinely passaged and
cells at the logarithmic growth phase were used for the
experiments.
Exosome isolation
The supernatants of T24 cells were collected and
sequentially centrifuged at 4°C at 300 × g for 10 min, 800 × g for
30 min and 10,000 × g for 30 min to remove cells and debris
(Ultracentrifuge CP100WX; Hitachi, Tokyo, Japan). The supernatants
were subjected to ultrafiltration concentration using a 100 kD MWCO
Centriplus centrifugal ultrafiltration tube (Amicon, Pineville, LA,
USA) at 1,000 × g for 30 min. The remaining supernatants were
further concentrated and subjected to ultracentrifugation on a
centrifugal ultrafiltration tube containing 30% sucrose in heavy
water (Tenglong Weibo Technology, Qingdao, China) at 100,000 × g
for 1 h at 4°C. The sucrose solution was collected and diluted with
double distilled water, followed by concentration using another 100
kD MWCO Centriplus centrifugal ultrafiltration tube at 1,000 × g
for 30 min. The remaining exosome-containing solution was
collected, filtered through a 0.22-μm filter, aliquoted and stored
at −80°C.
Transmission electron microscopy
To confirm the successful isolation of exosomes, a
transmission electron microscope (JEM-2010, Jeol Ltd., Tokyo,
Japan) was utilized to examine the morphology of the exosomes using
a standard protocol. Briefly, a 20-μl drop of exosome suspension
(~100 μg/ml) was loaded onto a copper grid to form a monolayer
(staining for 1 min). Excess solution was soaked out with clean
filter paper. Subsequently, the sample was stained with a 20-μl
drop of 2% phosphotungstic acid (12067-99-1; Damao Chemical Reagent
Factory, Tianjin, China) for 1 min and allowed to dry under an
electric incandescent lamp (Jia Yao Lighting Co., Ltd., Foshan,
China) for 10 min. The sample was reviewed under a transmission
electron microscope and images were captured (JEM-2010; Jeol,
Tokyo, Japan).
Tumor cell proliferation assay
The tumor cell proliferation assay was conducted
using a Cell Counting kit-8 (CCK-8; Qi-hai Biotech, Co., Shanghai,
China). Briefly, 5637 and T24 cells were seeded at 1,000 cells/well
in 96-well culture plates and co-cultured with increasing
concentrations of exosomes (10, 50, 100, 200 and 400 μg/ml). Cells
treated with an equal volume of phosphate-buffered saline (PBS)
served as controls and vials without cells were used as blank
controls. Following exosome treatment for 24, 48 and 72 h, 10 μl
CCK-8 solution was added to the culture medium and incubated for an
additional 2 h. Subsequent to this, the optical density of the
cells was measured with a spectrophotometer (Type 752; Shanghai
Yuanxi Instruments Co. Ltd., Shanghai, China) at an absorbance of
450 nm. The cell proliferation rate was calculated using the
following formula: proliferation rate = (experimental OD value −
blank OD value) / (control OD value − blank OD value) × 100%.
Annexin V apoptosis assay
To detect tumor cell apoptosis, an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide apoptosis
detection kit (Invitrogen Life Technologies, Carlsbad, CA, USA) was
used according to the manufacturer’s instructions. Specifically,
5637 and T24 cells were seeded into six-well plates, cultured for
24 h and treated with various concentrations of exosomes (10, 50,
100, 200 and 400 μg/ml) at different time points (24, 48 and 72 h).
Cells treated with an equal volume of PBS served as a control. The
treated and control cells were mixed with 5 μl Annexin V-FITC and
10 μl of 20 μg/ml PI reagents. The cells were then incubated at
room temperature in the dark for 20 min. After adding 400 μl PBS,
the samples were subjected to flow cytometry analysis to detect
cell apoptosis levels. Cells positive for Annexin V-FITC, but
negative for PI fluorescence, were considered to represent
apoptotic cells.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total cellular RNA was isolated from treated and
control tumor cells using TRIzol reagent (Invitrogen Life
Technologies) according to the manufacturer’s instructions.
Following quantification, the RNA samples were reverse transcribed
into cDNA using an RT-PCR kit (PrimeScript™ One Step RT-PCR Kit
Ver.2, DRR055S; Takara, Dalian, China) according to the
manufacturer’s instructions. PCR amplifications were conducted at
94°C for 5 min, 35 cycles at 94°C for 30 sec, 58°C for 40 sec and
72°C for 50 sec, with a final extension at 72°C for 5 min. The
primers used were as follows: Forward,
5′-GTAGCAGCGAGCAGCAGAGT-3′and reverse, 5′-CGGTCGTTGAGGAGGTTGG-3′
for cyclin D1; forward, 5′-CCTAGCGGATGGGTGCTATT-3′ and reverse,
5′-CGAGGTGGCAAAACAAACA-3′ for caspase 3; forward,
5′-CGGCGAATTGGAGATGAA-3′ and reverse, 5′-GTGTCCACGTCAGCAATCAT-3′
for Bax; forward, 5′-CATGCCGTCCTTAGAAAAATACA-3′ and reverse,
5′-CTGCTTTTTATTTCATGAGGTACATT-3′ for Bcl-2; and forward,
5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse, 5′-GGGCACGAAGGCTCATCATT-3′
for β-actin. The PCR products were then resolved by electrophoresis
in a 1.5% agarose gel. Subsequently, the data were quantified using
a gel imaging system (Vilber Lourmat, La Vallée, France).
Protein extraction and western blot
analysis
Bladder cancer T24 cells treated with exosomes were
lysed in a lysis buffer containing protease inhibitors for 30 min
at 4°C. Total cellular lysates were then centrifuged at 15,000 × g
for 30 min and the protein concentrations of the lysates were
determined using the Bradford method (18). To analyze the expression of exosome
proteins, 40 μl exosome suspension was sonicated, mixed with sample
buffer and boiled for 5 min. Subsequently, equal quantities of
protein samples (10 μg) were separated by sodium dodecyl sulfate
polyacrylamide gel electrophoresis, followed by transferring onto
polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA) at
250 mA. The membranes were then blocked with 5% non-fat dry milk
(w/v) in Tris-buffered saline with 0.1% Tween-20 (TBST) for 2 h at
room temperature and immunoblotted overnight at 4°C using rabbit
anti-human basic fibroblast growth factor (bFGF), rabbit anti-human
X-linked inhibitor of apoptosis protein (XIAP), rabbit anti-human
survivin (Biovision, Palo Alto, CA, USA), mouse anti-human
thymidine kinase 1 (TK1; Abnova, Taipei, Taiwan), rabbit anti-human
CD63, mouse anti-human Bcl-2, mouse anti-human Bax, rabbit
anti-human cyclin D1, rabbit anti-human caspase-3 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), rabbit anti-human Akt
and phospho-Akt1/2 (Ser 473) or rabbit anti-human ERK and
phospho-ERK1/2 (Cell Signaling Technology, Danvers, MA, USA)
antibodies. Following three rinses with TBST at 15 min intervals,
the membranes were further incubated for 1 h with horseradish
peroxidase-labeled goat anti-rabbit or anti-mouse IgG (Zhongshan
Golden Bridge, Beijing, China). The positive protein bands were
detected using an enhanced chemiluminescence (ECL) plus
chemiluminescence kit (P0018; Beyotime Institute of Biotechnology,
Haimen, China).
Statistical analysis
Each experiment was repeated at least three times.
All data are presented as the mean ± SD. Student’s t-test was used
to compare the differences between two groups. All statistical
analyses were performed using SPSS 17.0 software (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
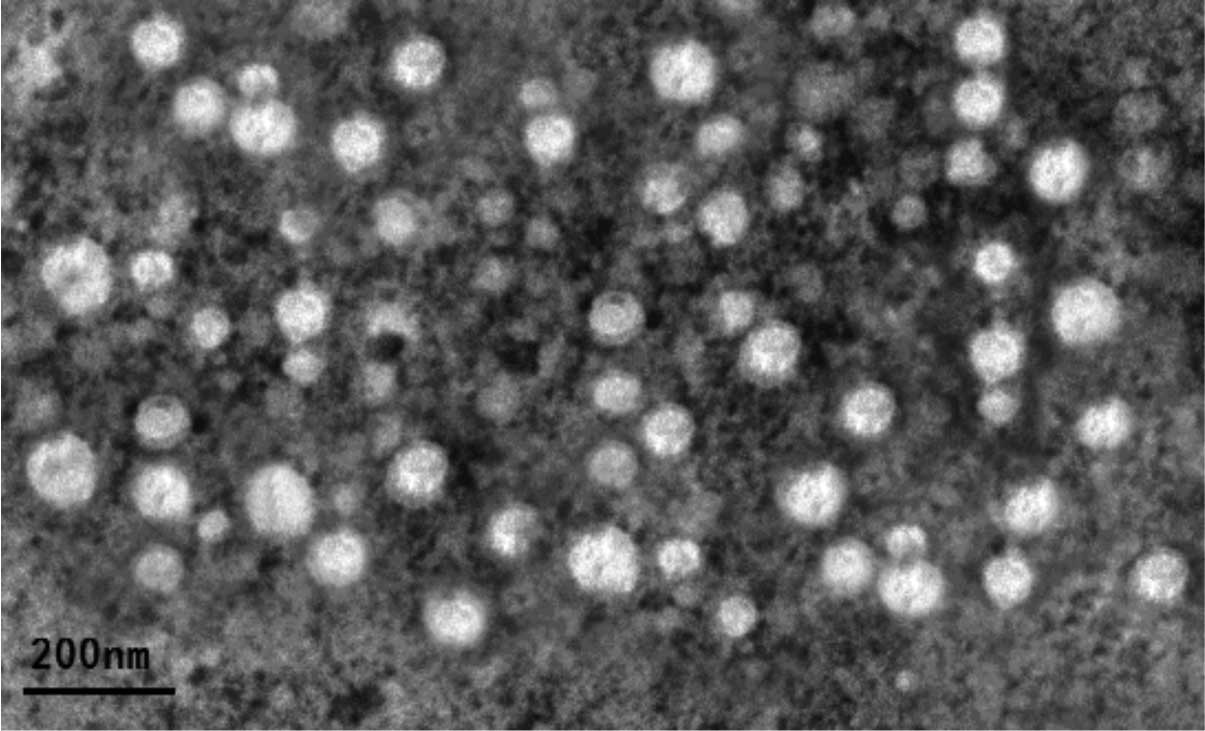
Isolation and characterization of bladder
cancer T24 cell-derived exosomes
In the present study, exosomes were purified from
T24 cells by sequential centrifugation and ultrafiltration, and
their identity was confirmed under a transmission electron
microscope. The data showed that these exosomes were round-shaped
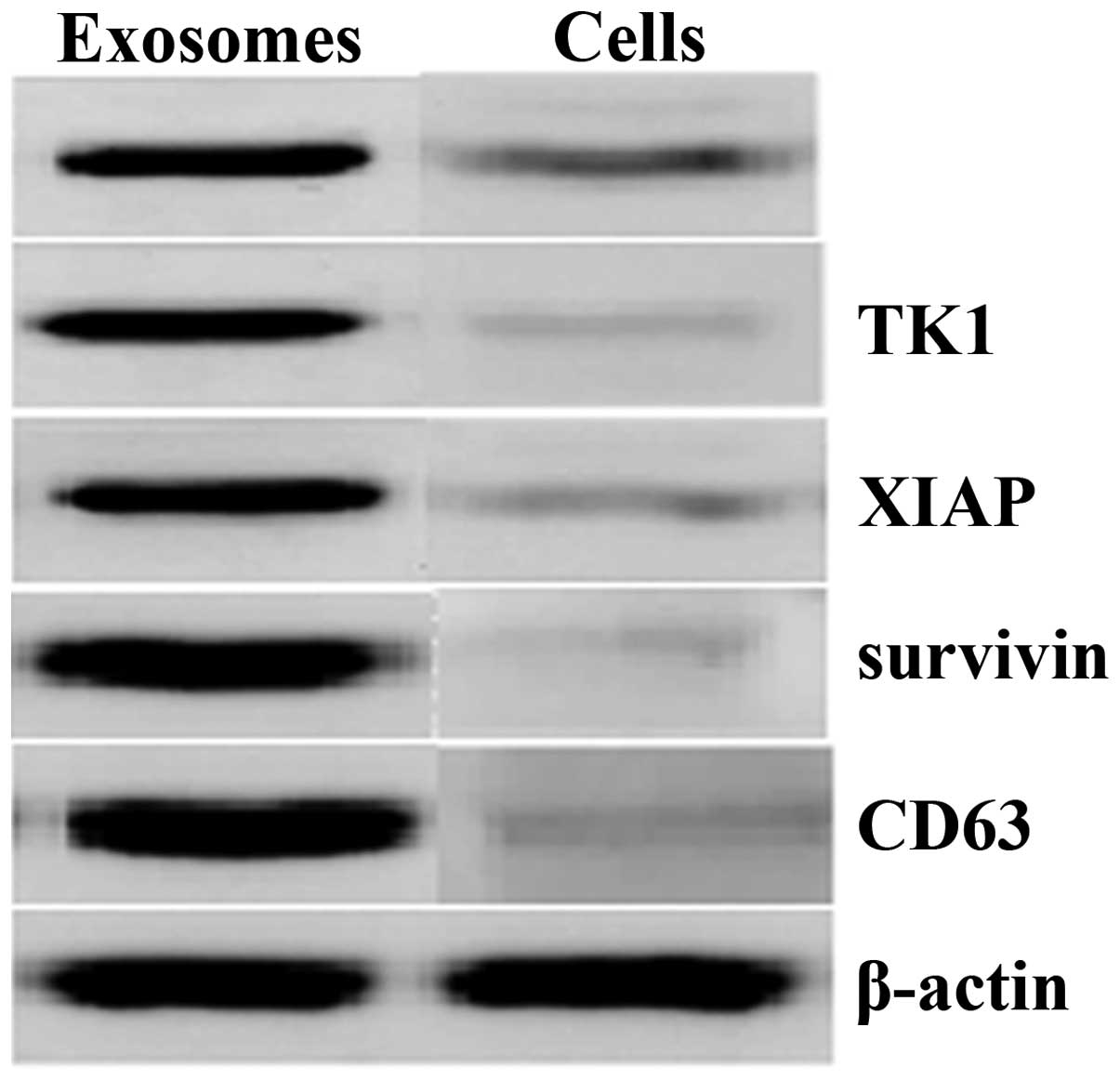
vesicular membrane structures, 30–100 nm in diameter (Fig. 1). In addition, western blot
analysis demonstrated that XIAP, survivin, bFGF, TK1 and CD63 were
abundant in these isolated exosomes compared with the whole-cell
lysates of T24 cells (Fig. 2).
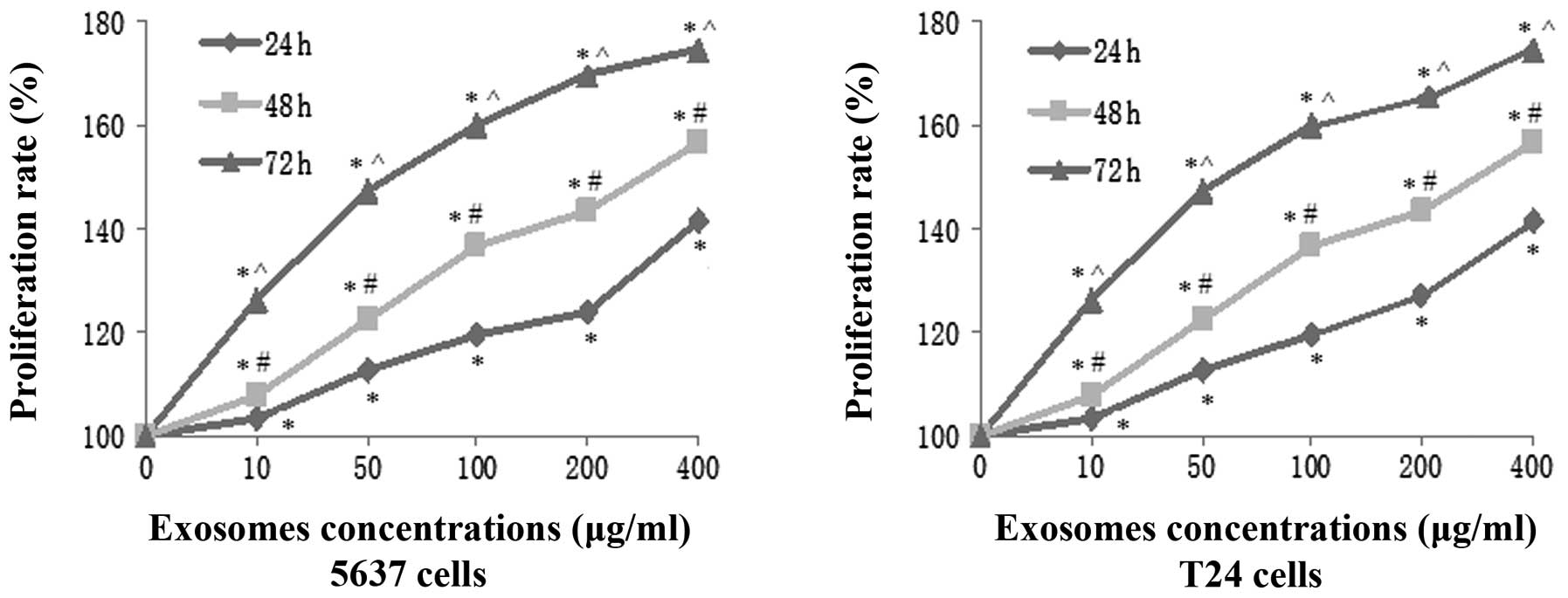
Bladder cancer cell-derived exosomes
promote tumor cell viability
To determine the effects of T24 cell-derived
exosomes on the regulation of renal clear cell carcinoma cell
proliferation, the viability of 5637 and T24 cells was analyzed
following treatment with various concentrations of exosomes (10,
50, 100, 200 and 400 μg/ml) for up to 72 h. The results of the cell
viability CCK-8 assay showed that these exosomes significantly
induced the viability of 5637 and T24 cells in a time- and
dose-dependent manner (Fig. 3). A
maximal increase in tumor cell proliferation was achieved following
treatment with 400 μg/ml exosomes for 72 h, and the viabilities of
5637 and T24 cells were increased by 76.52% and 73.26%,
respectively.
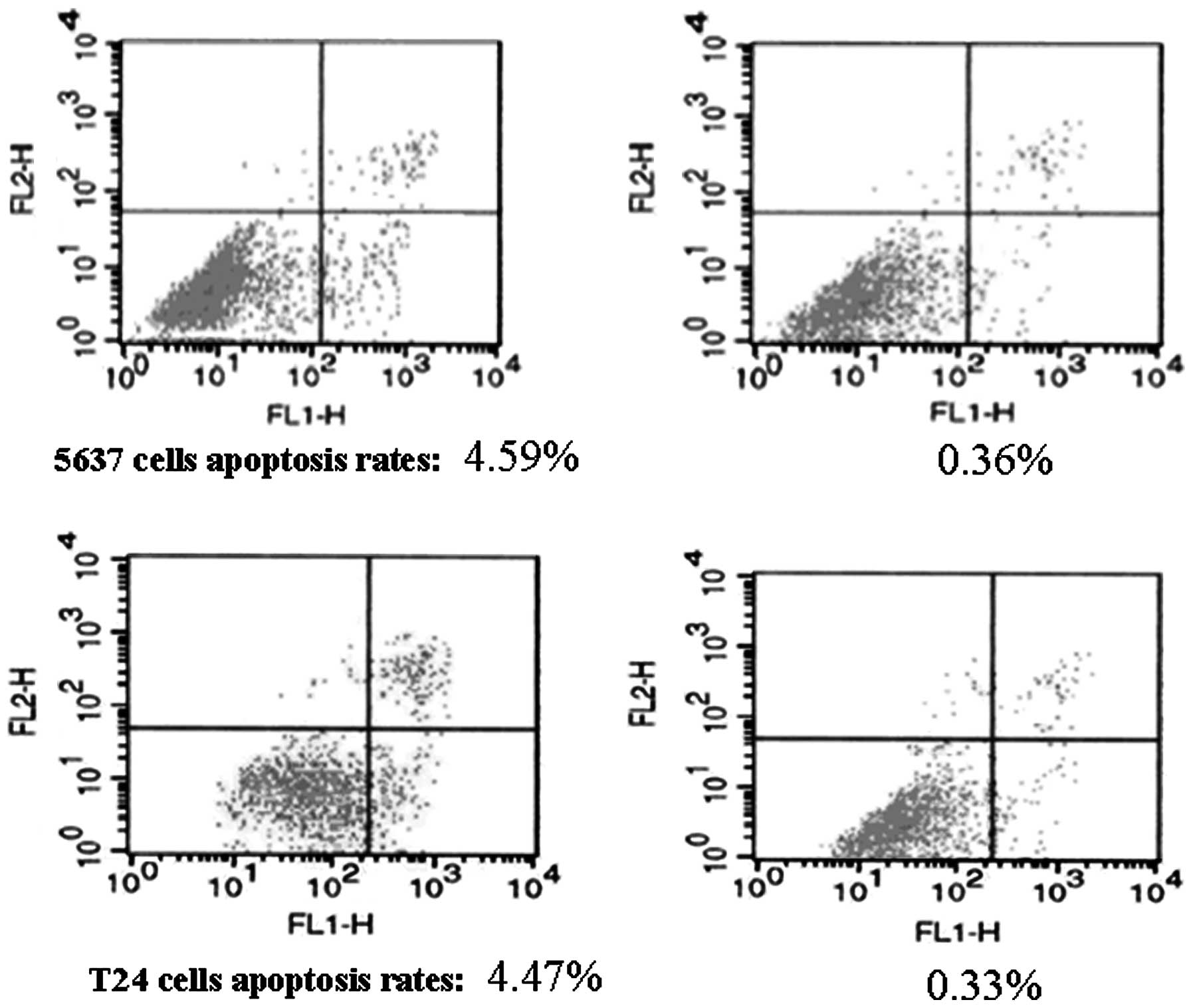
Bladder cancer cell-derived exosomes
inhibit tumor cell apoptosis
To investigate the potential cause of the induced
tumor cell viability by the exosomes, an Annexin V/flow cytometric
apoptosis assay was performed following the treatment of 5637 and
T24 cells with increasing concentrations of exosomes (10, 50, 100,
200 and 400 μg/ml) for up to 72 h. It was demonstrated that these
exosomes dose- and time-dependently inhibited the apoptosis of 5637
and T24 cells, with the maximal reduction occurring following
treatment with 400 μg/ml exosomes for 72 h (Table I, Fig.
4). Moreover, the expression of apoptosis-related genes was
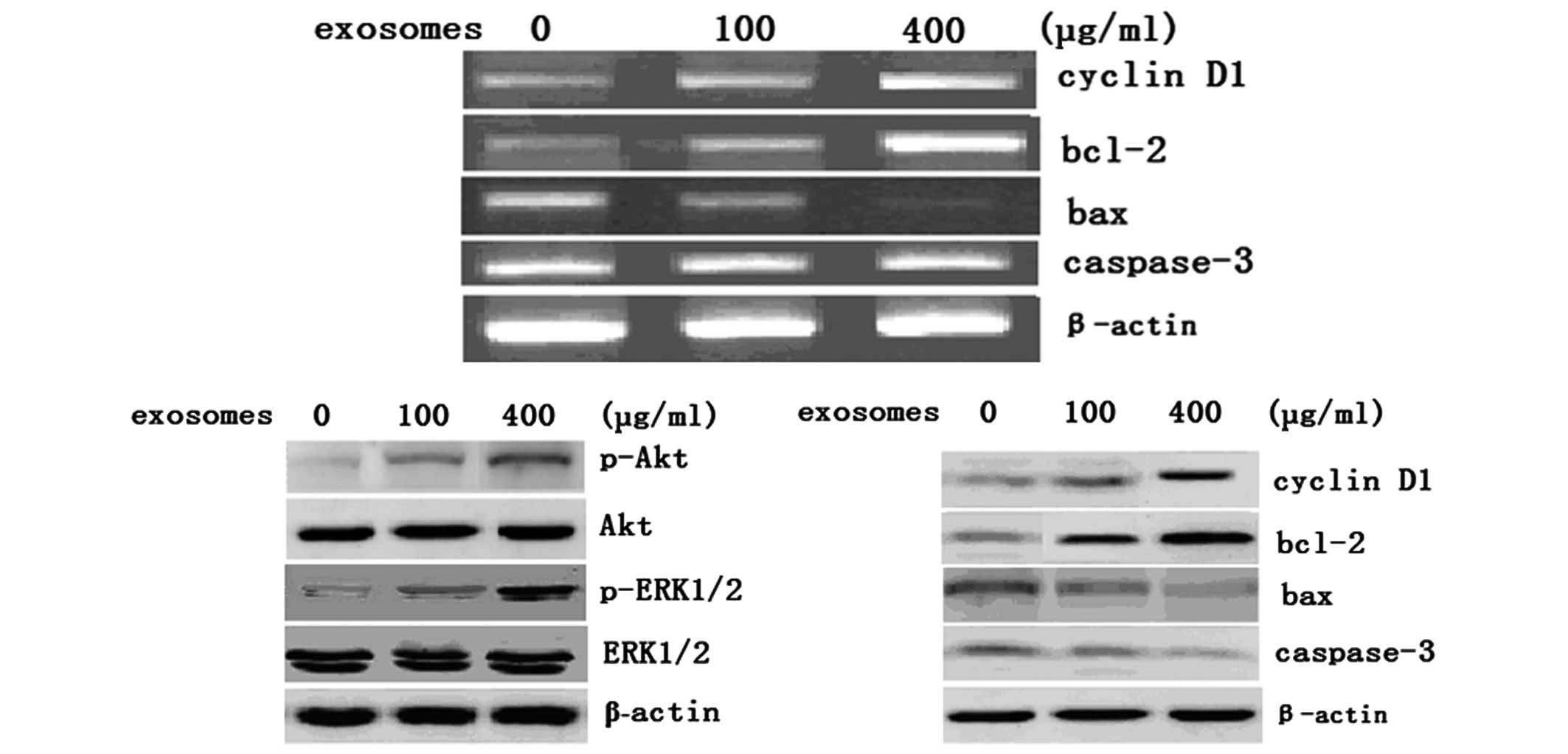
analyzed in these cells. As shown in Fig. 5, the levels of Bcl-2 mRNA and
protein were increased following 100 and 400 μg/ml exosome
treatment for 72 h, whereas the levels of Bax mRNA and protein were
markedly reduced in these cells. In addition, downregulation of
caspase-3 protein, an executioner of apoptosis, was observed.
However, the levels of caspase-3 mRNA were not significantly
changed in T24 cells following treatment with 100 or 400 μg/ml
exosomes for 72 h. In addition, the expression of cyclin D1 protein
was significantly increased in T24 cells following treatment with
100 and 400 μg/ml exosomes for 72 h.
 | Table IEffect of bladder cancer cell-derived
exosomes on induction of bladder cancer cell apoptosis. |
Table I
Effect of bladder cancer cell-derived
exosomes on induction of bladder cancer cell apoptosis.
| A. Rate of 5637 cell
apoptosis following exosome treatment. |
|---|
|
|---|
| Apoptosis rate (%) at
time interval |
|---|
|
|
|---|
| Exosome concentration
(μg/ml) | 24 h | 48 h | 72 h |
|---|
| 0 | 4.46±0.27 | 4.42±0.16 | 4.59±0.18 |
| 10 | 3.82±0.10a | 3.64±0.12a | 3.54±0.23a |
| 50 | 2.86±0.05b | 2.33±0.07b | 2.19±0.08b |
| 100 | 2.04±0.07b | 1.93±0.11b | 1.84±0.05b |
| 200 | 1.36±0.05b | 1.32±0.04b | 1.23±0.04b |
| 400 | 0.95±0.05b | 0.66±0.08b | 0.36±0.03b |
|
| B. Rate of T24 cell
apoptosis following exosome treatment. |
|
| Apoptosis rate (%) at
time interval |
|
|
| Exosome concentration
(μg/ml) | 24 h | 48 h | 72 h |
|
| 0 | 4.43±0.26 | 4.35±0.31 | 4.47±0.15 |
| 10 | 3.74±0.12a | 3.63±0.12a | 3.57±0.20a |
| 50 | 2.77±0.17b | 2.34±0.10b | 2.18±0.10b |
| 100 | 2.01±0.09b | 1.95±0.11b | 1.84±0.06b |
| 200 | 1.37±0.04b | 1.32±0.08b | 1.26±0.07b |
| 400 | 0.92 ± 0.06b | 0.63 ± 0.06b | 0.33 ± 0.04b |
Bladder cancer cell-derived exosomes
activate Akt and ERK in bladder cancer cells
To demonstrate the mechanism underlying these
phenotypic changes in bladder cancer cells following exosome
treatment, the activity of Akt and ERK1/2 proteins in T24 cells was
analyzed following treatment with 100 and 400 μg/ml exosomes for 72
h. Data showed that exosome treatment dose-dependently upregulated
the expression of phosphorylated Akt and ERK1/2 compared with that
of the untreated control cells (Fig.
5B), suggesting that the activation of the Akt and ERK pathways
may participate in the exosome-stimulated proliferation of bladder
carcinoma cells.
Discussion
In the present study, bladder cancer cell-derived
exosomes were isolated and identified, and it was demonstrated that
these exosomes induced the viability of bladder cancer cells and
suppressed tumor cell apoptosis. It was also observed that exosome
treatment upregulated Bcl-2 and cyclin-D1 expression, but decreased
Bax and caspase-3 protein levels, which was correlated with the
inhibition of apoptosis in these tumor cell lines. Exosome
treatment also promoted the phosphorylation of Akt and ERK
proteins. However, the underlying mechanisms responsible for
exosome release from tumor cells and their involvement in
vivo requires further investigation. Inhibition of exosome
formation and their release from tumor cells may be a novel
strategy for effectively controlling human cancer.
Exosomes, which are secreted by various mammalian
cells, merge with and release their contents into cells that are
distant from their cell of origin, or in our case, the same origin
of the tumor cells. In addition, exosomes influence the behavior
and activity of the recipient cells, including cell growth,
differentiation and apoptosis. Exosomes are also considered to be
vital in tumor progression (19).
However, it remains unknown why and how exosomes form and are
secreted from host cells and which types of biological processes
they participate in within the target cells. The mechanism
underlying the contribution of tumor-released exosomes to
tumorigenesis remains to be fully elucidated (16).
In the present study, exosomes were successfully
isolated and purified from the supernatants of T24 human bladder
cancer cells using a commercially available exosome purification
kit. These exosomes were identified to be similar to those from
other sources (15,20–22)
and were rich in certain hallmark proteins, such as CD63. In
addition, the isolated exosomes contained concentrated levels of
different proteins, including XIAP, survivin, bFGF and TK1, which
are important in cell growth, apoptosis and angiogenesis.
Subsequent to merging with recipient cells, exosomes most likely
promote the growth of recipient cells, as well as inhibit
apoptosis.
Moreover, the current study determined the effects
of bladder cancer cell-derived exosomes on the regulation of
bladder cancer cell viability and apoptosis in vitro. It was
identified that these exosomes induced the viability of 5637 and
T24 cells in a time- and dose-dependent manner. These results are
consistent with those of previous studies showing that breast and
gastric cancer cell-released exosomes stimulated the proliferation
of their parental cells in vitro(17,23).
In addition, these results suggest that certain common molecular
events or mechanisms may be responsible for tumor-derived exosomes
in the promotion of cell proliferation and the suppression of
apoptosis. Thus, tumor-derived exosomes may exhibit crucial
growth-promoting effects that are involved in cancer progression.
Furthermore, the upregulation of Bcl-2 and downregulation of Bax
and caspase-3 in exosome-treated bladder cancer cell lines was also
observed. A previous study showed that a variety of cancer cells
were able to take up survivin, an anti-apoptotic protein, from
extracellular media, thereby inhibiting apoptosis and increasing
metastatic capability (24). These
data may provide molecular insight into the involvement of exosomes
in cell-to-cell signaling. Further studies are required to
investigate molecular signaling in the formation and release of
exosomes from the host cells and their affect on the recipient
cells.
The PI3K/Akt and MAPK/ERK pathways are prototypic
survival signaling pathways and are critical in mediating survival
signals in a wide range of cell types. A previous study
demonstrated that gastric cancer-derived exosomes promoted tumor
cell proliferation through the activation of MAPK/ERK and PI3K/Akt
signaling pathways (17). Thus,
the present study was shown to be concurrent with this data by
demonstrating that bladder cancer cell-derived exosomes
dose-dependently increased the expression of phosphorylated Akt and
ERK1/2. However, in these cell lines, it remains to be determined
how signaling transduction of these genes results in the
upregulation of Bcl-2 and cyclin D1 and downregulation of Bax and
caspase-3.
Tumor cell-derived exosomes may exert diverse
biological functions leading to tumor progression and metastasis.
Future studies are required to investigate the potential of
exosomes to effectively deliver therapeutic drugs for cancer
therapy.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Hurst R: Does the biomarker search
paradigm need re-booting? BMC Urol. 9:12009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vlassov AV, Magdaleno S, Setterquist R and
Conrad R: Exosomes: Current knowledge of their composition,
biological functions, and diagnostic and therapeutic potentials.
Biochim Biophys Acta. 1820:940–948. 2012. View Article : Google Scholar
|
|
4
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar
|
|
5
|
Johnstone RM, Adam M, Hammond JR, et al:
Vesicle formation during reticulocyte maturation. Association of
plasma membrane activities with released vesicles (exosomes). J
Biol Chem. 262:9412–9420. 1987.
|
|
6
|
Potolicchio I, Carven GJ, Xu X, et al:
Analysis of microglia-derived exosomes: metabolic role of the
aminopeptidase CD13 in neuropeptide catabolism. J Immunol.
175:2237–2243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Henderson MC and Azorsa DO: The genomic
and proteomic content of cancer cell-derived exosomes. Front Oncol.
2:382012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosell R, Wei J and Taron M: Circulating
microRNA signatures of tumor-derived exosomes for early diagnosis
of non-small-cell lung cancer. Clin Lung Cancer. 10:8–9. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rabinowits G, Gerçel-Taylor C, Day JM,
Taylor DD and Kloecker GH: Exosomal microRNA: a diagnostic marker
for lung cancer. Clin Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simpson RJ, Lim JW, Moritz RL and
Mathivanan S: Exosomes: proteomic insights and diagnostic
potential. Expert Rev Proteomics. 6:267–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ristorcelli E, Beraud E, Verrando P,
Villard C, Lafitte D, Sbarra V, et al: Human tumor nanoparticles
induce apoptosis of pancreatic cancer cells. FASEB J. 22:3358–3369.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Luo CL, He BC, Zhang JM, Cheng G
and Wu XH: Exosomes derived from IL-12-anchored renal cancer cells
increase induction of specific antitumor response in vitro: a novel
vaccine for renal cell carcinoma. Int J Oncol. 36:133–140.
2010.
|
|
14
|
Wolfers J, Lozier A, Raposo G, Regnault A,
Théry C, Masurier C, et al: Tumor-derived exosomes are a source of
shared tumor rejection antigens for CTL cross-priming. Nat Med.
7:297–303. 2001.PubMed/NCBI
|
|
15
|
Andre F, Schartz NE, Movassagh M, Flament
C, Pautier P, Morice P, et al: Malignant effusions and immunogenic
tumour-derived exosomes. Lancet. 360:295–305. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang C and Robbins PD: The roles of
tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol.
2011:8428492011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y,
Hou KZ, et al: Gastric cancer exosomes promote tumour cell
proliferation through PI3K/Akt and MAPK/ERK activation. Dig Liver
Dis. 41:875–880. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Camussi G, Deregibus MC, Bruno S, Grange
C, Fonsato V and Tetta C: Exosome/microvesicle-mediated epigenetic
reprogramming of cells. Am J Cancer Res. 1:98–110. 2011.PubMed/NCBI
|
|
20
|
Raposo G, Nijman HW, Stoorvogel W,
Liejendekker R, Harding CV, Melief CJ and Geuze HJ: B lymphocytes
secrete antigen-presenting vesicles. J Exp Med. 183:1161–1172.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Théry C, Regnault A, Garin J, Wolfers J,
Zitvogel L, Ricciardi-Castagnoli P, et al: Molecular
characterization of dendritic cell-derived exosomes. Selective
accumulation of the heat shock protein hsc73. J Cell Biol.
147:599–610. 1999.PubMed/NCBI
|
|
22
|
Lamparski HG, Metha-Damani A, Yao JY,
Patel S, Hsu DH, Ruegg C and Le Pecq JB: Production and
characterization of clinical grade exosomes derived from dendritic
cells. J Immunol Methods. 270:211–226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koga K, Matsumoto K, Akiyoshi T, Kubo M,
Yamanaka N, Tasaki A, et al: Purification, characterization and
biological significance of tumor-derived exosomes. Anticancer Res.
25:3703–3707. 2005.PubMed/NCBI
|
|
24
|
Khan S, Aspe JR, Asumen MG, Almaguel F,
Odumosu O, Acevedo-Martinez S, et al: Extracellular, cell-permeable
survivin inhibits apoptosis while promoting proliferative and
metastatic potential. Br J Cancer. 100:1073–1086. 2009. View Article : Google Scholar : PubMed/NCBI
|