Introduction
Osteoarthritis (OA) is a degenerative joint disease
that is a leading cause of physical disability and impaired quality
of life in industrialized nations. The exact etiology of OA is not
fully understood. Although age is the strongest predictor of the
development of OA, obesity, trauma and physically demanding
occupations also increase the risk of OA of the hand, knee and hip.
OA imposes a considerable functional burden on affected
individuals. NHANES III (the Third National Health and Nutrition
Examination Survey) revealed that >8% of adults in the USA have
symptomatic OA (1). A French study
found that >80% of clinical OA patients reported limitations in
their daily lives, including basic tasks, work and leisure
activities, and these limitations impacted the quality of life of
affected patients, often leading to depression and social isolation
(2). OA imposes a significant
economic burden on patients and healthcare systems (3–6).
Despite the increasing number of patients with OA,
treatments to manage the condition remain symptomatic, designed to
control pain and improve function and quality of life while
attempting to limit the adverse events associated with the
medication. Guidelines for the management of OA have been published
by the Osteoarthritis Research Society International (OARSI)
(7,8) and the European League Against
Rheumatism (EULAR) (9–11). These guidelines indicate that a
combination of pharmacological and non-pharmacological modalities
is the most effective strategy for managing the pain and
disabilities associated with OA. Acetaminophen is recommended as
the first line therapy and a switch to non steroidal
anti-inflammatory drugs (NSAIDs) is recommended if acetaminophen
does not adequately control symptoms or if there are signs of
clinical inflammation. Acetaminophen, however, is considerably less
efficient than NSAIDs in providing symptomatic relief (12,13),
and patients are, sooner or later, forced to switch to NSAIDs. The
long-term use of NSAIDs is associated with gastrointestinal, renal
and cardiovascular risks. Given the efficacy, safety and
tolerability issues associated with NSAIDs, the development of new
agents to manage OA without adverse events remains a priority.
Traditional medicines using plant-derived compounds
offer a safer alternative tool for the management of many chronic
ailments. One such formulation is evaluated in this study as a
treatment option for OA of the knee. A combination of the common
spice Curcuma longa and the active principles of
Boswellia serrata (CB formulation) was tested in OA patients
and the efficacy and safety were evaluated in direct comparison
with a selective cyclooxygenase-2 inhibitor, celecoxib. Boswellia
gum resin and turmeric have been in active use in traditional
medicine for thousands of years. Boswellia extract has shown
promise for the treatment of asthma (14), rheumatoid arthritis (15), Crohn's disease (16), OA of the knee (17–19)
and collagenous colitis (20). The
biological activity of Boswellia serrata arises from the
boswellic acids, of which the 3-O-acetyl-11-keto-boswellic acid
(AKBA) is the most active (21–24).
AKBA is an inhibitor of the lipoxygenase pathway of arachidonate
metabolism and possesses significant anti-inflammatory and
anticancer properties. The AKBA content of commercial boswellia
extracts is low at ~2% and a preparation with enhanced AKBA content
(10% AKBA) was used in this study. The oral bioavailability of
curcumin is poor (25), so the
wide range of physiological activities of curcumin have not yet
been translated into clinical benefits. A formulation of curcumin
with enhanced bioavailability was the other component of the study
drug. Curcumin, the active component of turmeric, possesses
antioxidative, anti-inflammatory and anticancer properties and may
modulate a large number of signaling pathways and thus possesses an
extremely wide range of biological activities. Glycosaminoglycan
synthesis is required for cartilage repair. Boswellia prevents a
decrease in glycosaminoglycan levels, whereas NSAIDs may disrupt
glycosaminoglycan synthesis, which, in turn, may accelerate
cartilage damage. The non-acid section of the gum has
pain-relieving qualities (26).
The curcuminoids were analyzed with an HPLC system
which used a 60/40 water THF mobile phase, C18 column and the
chromatogram was monitored at 420 nm. The assay analysis was
performed by using the USP reference standards of individual
curcuminoids (CAS numbers: 458-37-7, 24939-17-1 and 24939-16-0).
The retention times of various peaks in the sample were parallel to
those of the curcuminoid standards and the sample purity was
determined by comparing the peak areas of individual peaks present
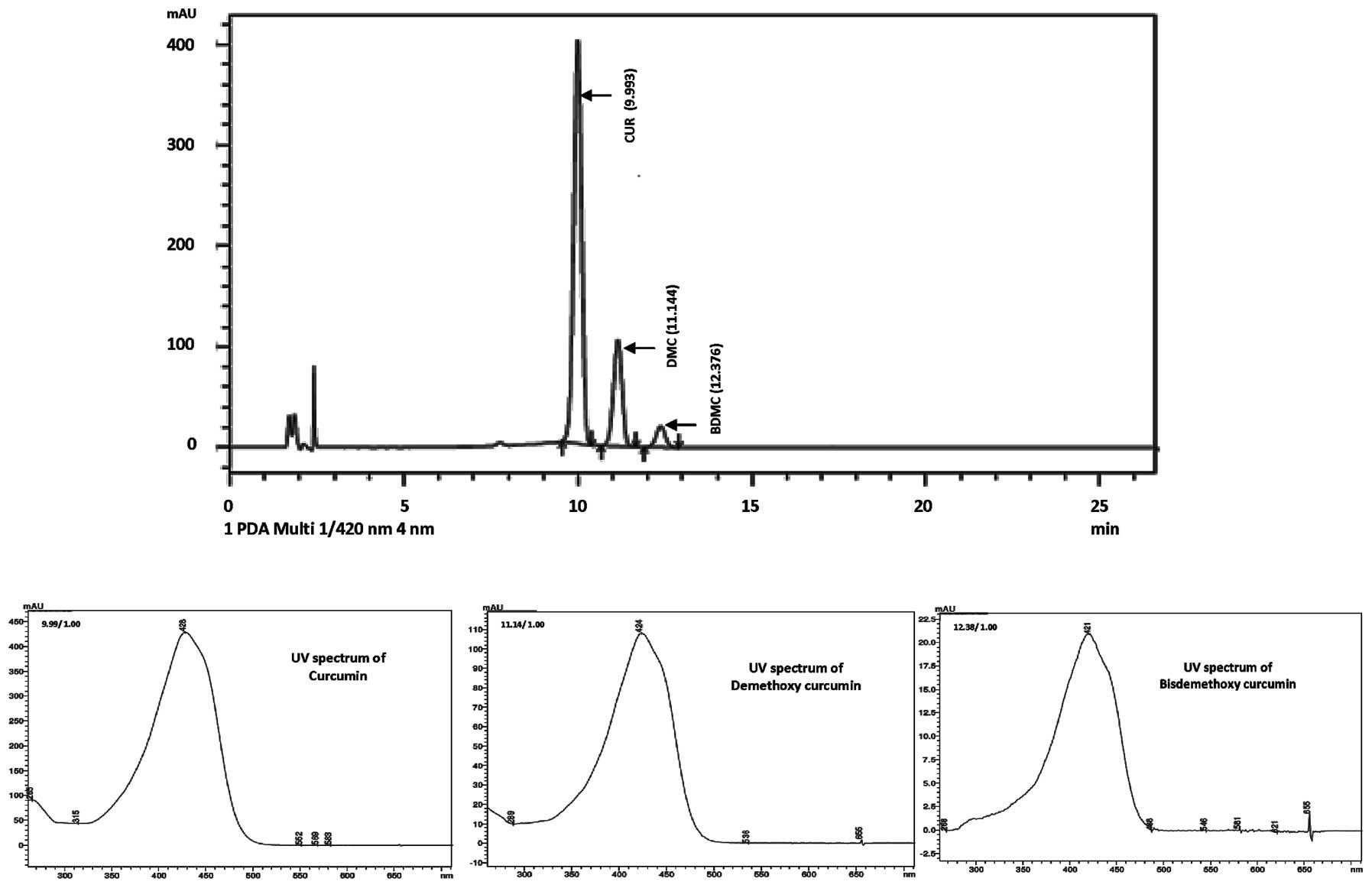
in the standard vs. the sample. The HPLC chromatogram and UV
spectra of curcuminoids in the Curcuma longa extract are
shown in Fig. 1. The retention
times for the peaks from the three curcuminoids (curcumin,
demethoxycurcumin and bis-demethoxycurcumin) in the reference
standard and sample were identical (9.993, 11.144 and 12.376 min,
respectively).
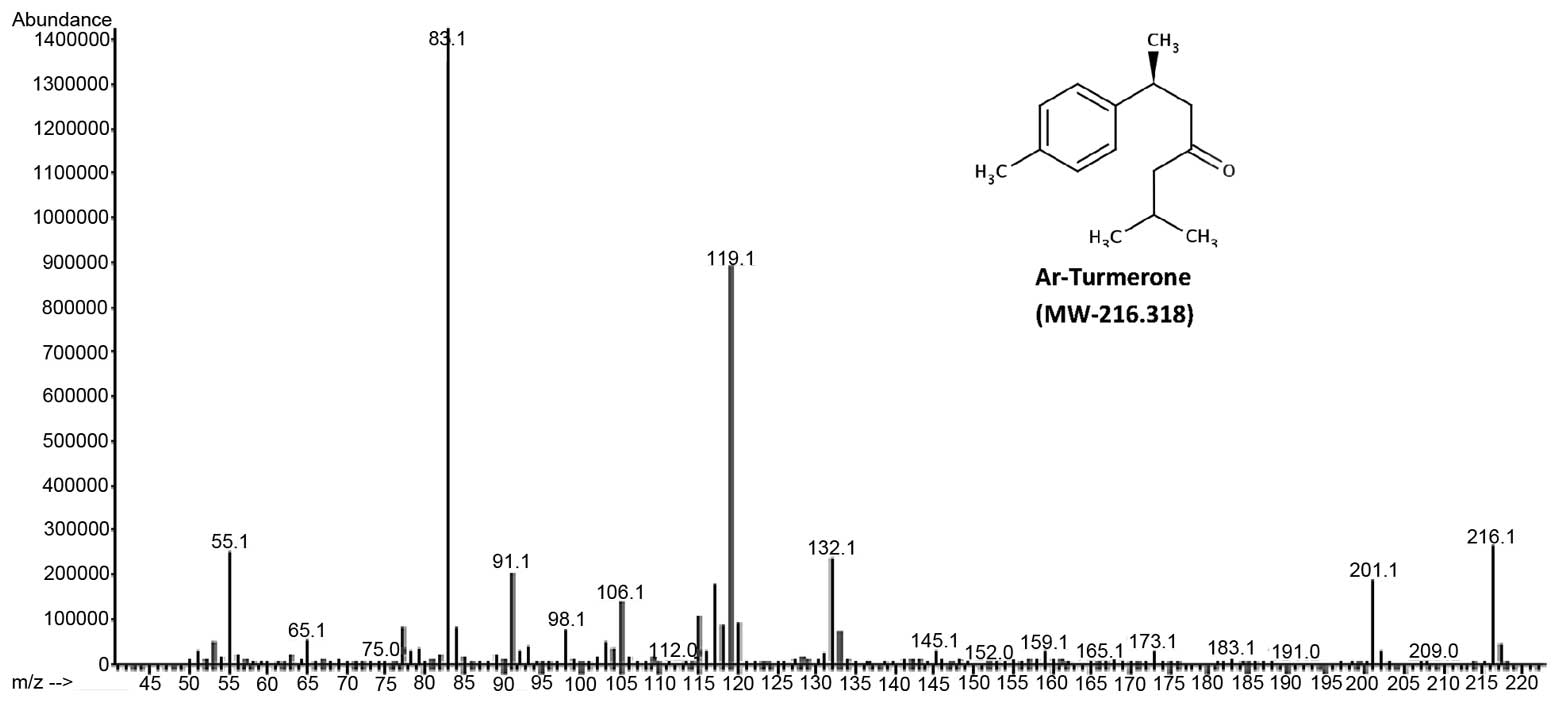
The major component of turmeric essential oils,
ar-Turmerone was characterized using GC-MS. With a molecular mass
of 216.318, ar-Turmerone is confirmed on the mass spectrum in
Fig 2.
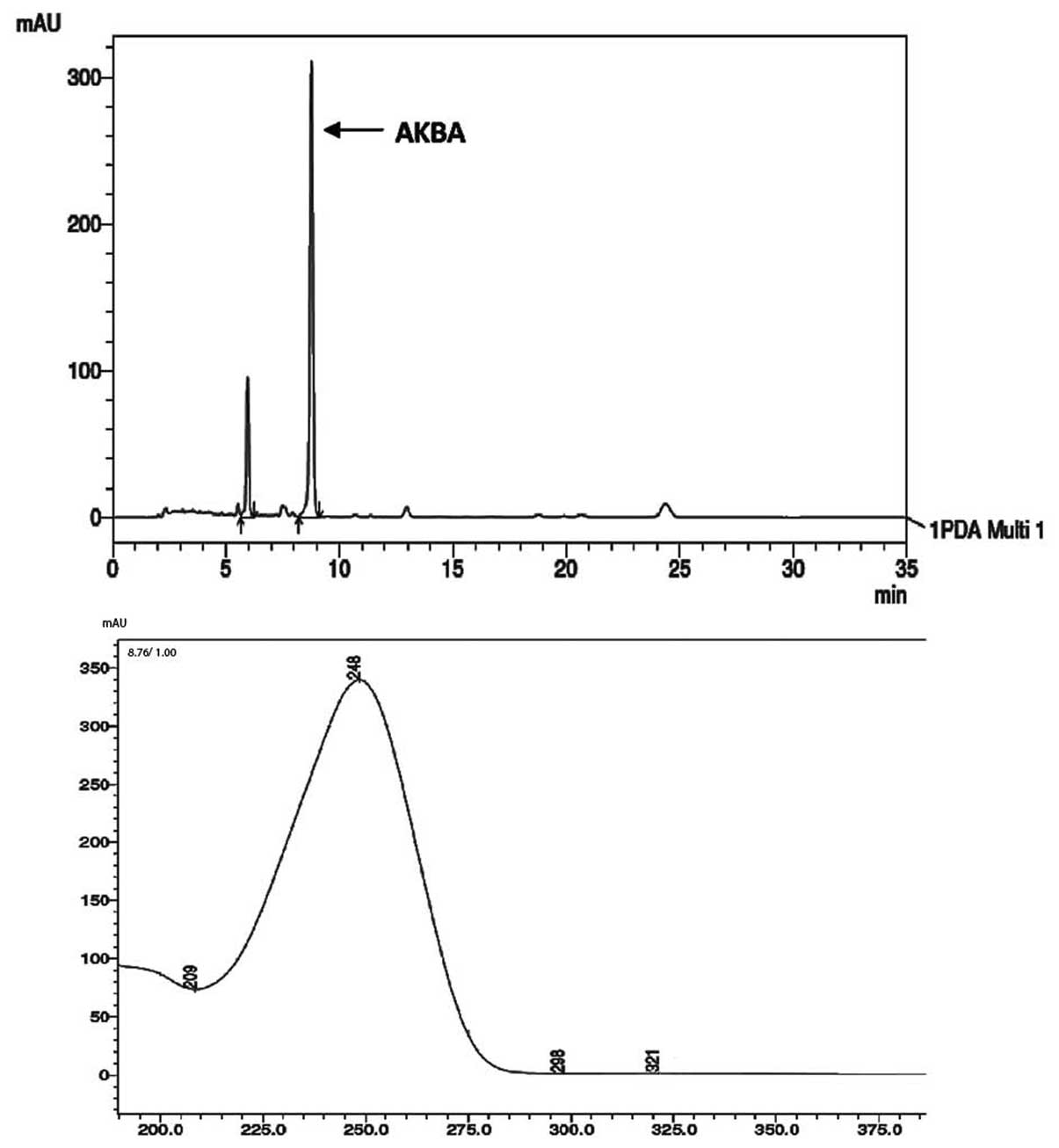
AKBA, was quantified by the HPLC system using an
acetonitrile:water (90:10) mobile phase and the chromatogram was
monitored at 254 nm. The AKBA peak was eluted at a retention time
of 8.9 min and has characteristic UV spectra (Fig. 3). The assay quantification was
performed using a reference standard (Sigma Aldrich CAS no.:
67416-61-9) and revealed 10% AKBA, a representative chromatogram is
shown in Fig. 3.
The purpose of this study was to evaluate the
efficacy, safety and tolerability of CB formulation compared with
celecoxib for the management of knee OA.
Materials and methods
Study design
The CB formulation consisted of 350 mg Curcuma
longa extract (BCM 95®, Arjuna Natural Extracts Ltd,
Aluva, Kerala, India) containing 70% curcumin, 17%
demethoxycurcumin, 3.5% bis-demethoxycurcumin and 7.5% turmeric
essential oils and 150 mg Boswellia serrata extract
(BosPure®, Arjuna Natural Extracts Ltd, Aluva, Kerala,
India) containing 75% boswellic acids and 10% AKBA in 500-mg hard
gelatin capsules. The study was performed at the Anugraha Medical
Centre, Kochi, India as a two arm clinical trial with a positive
control and was conducted with 30 osteoarthritic patients divided
into two groups of 15 subjects. Each patient voluntarily enrolled
in the study and received treatment for 12 weeks. The two groups
received the following treatment: Group I, oral administration of
CB formulation 500 mg capsule (twice daily). Group II, oral
administration of celecoxib 100 mg capsule (twice daily).
Subjects were enrolled on a first-come-first-served
basis and assigned a subject number sequentially. The assignment of
each subject to the treatment group was determined randomly. All
subjects visited the clinic at least eight times. At each visit,
the vital signs, symptom scoring and clinical examination results
were recorded. Liver and renal function tests were performed at the
beginning (T0), after 6 weeks (T6) and at the end of study (T12) in
order to assess the safety of the study drug. All study volunteers
provided written informed consent to participate and the study was
approved by the Independent Ethics Committee of Anugraha Medical
Centre, Kakkanad, Kochi, Kerala, India. Table I details the schedule of visits and
the procedures adopted at each visit.
 | Table ITreatment schedule. |
Table I
Treatment schedule.
| Visit no. | Tasks
accomplished |
|---|
| 1 | Informed consent
obtained, medical history and general examination
Joint examination
X-ray examination
Hemogram (TC, DC, ESR)
Liver function tests (serum bilirubin, SGOT, SGPT, SAP)
Renal function tests (blood urea, serum creatinine)
Other tests (ASO, CRP, RF, blood sugar)
Enrolled/rejected as per inclusion/exclusion criteria |
| 2 | Pre-examinations on
screened patients
Assignment to one of the treatment groups as per
randomization
T0 baseline characteristics:
Vital signs
Knee AP view and lateral view (X-ray)
Hemogram
Liver function test
Renal function test
Symptom score (joint pain, walking distance)
Patients were given a diary and trained in documentation
Treatment supplies provided for two weeks and documented
Discharge instructions regarding next visit |
| 3–7 | Follow-up for all
subjects:
Vital signs
Symptom score
Clinical examination
Treatment supplies provided for two weeks and documented
Discharge instructions regarding next visit |
| 8 | Follow-up for all
subjects:
Vital signs
Symptom score
Clinical examination
Patient diary was collected and documented
Final discharge instructions |
Patient population
Patients of both genders, aged between 18 and 65
years, who were medically stable with a moderate form of OA,
evidenced by the narrowing of the medial joint space with swelling,
were recruited for this study. Long standing OA with a gross
deformity, patients with a severe form of OA evidenced by gross
radiological findings, swelling and restricted mobility, nursing
mothers, patients with a history of rheumatoid or reactive
arthritis, or clinical findings of significant systemic disease,
drug or alcohol abuse, malnutrition or any condition which in the
opinion of the Principal Investigator would place the subject at
risk or affect the conduct of the study or interpretation of the
results, were excluded from this study.
In total, 54 subjects were screened, 30 were
enrolled and 28 subjects completed the study. Each enrolled subject
was assigned a number on a first-come-first-served basis and
assigned to a treatment group randomly.
The following criteria were used to withdraw a
subject from the study: i) any indication of an allergic reaction
to the treatment; ii) the patient developed severe symptoms that
were uncontrollable with the study drugs; iii) withdrawal of
consent; iv) administrative reasons, such as patient non-compliance
or major protocol violation (pregnancy or alcohol consumption
during the study) and v) any condition that might put the subject
at undue risk. Two patients decided to not participate further
during the course of the study: one patient from the CB formulation
group withdrew from the study due to personal reasons and one
patient from the celecoxib group withdrew from the study due to the
inadequate control of symptoms.
There were no statistically significant differences
in age, height, weight, BMI, temperature, BP-systolic,
BP-diastolic, pulse rate and respiration between the subjects in
the two groups.
Efficacy assessment
The efficacy of the CB formulation over the
treatment period was evaluated by symptom scoring and clinical
examination.
Symptom scoring was performed by an orthopedician
evaluating the subjects for joint pain (measured as ‘no’, ‘mild’,
‘moderate’ or ‘severe’) and walking distance (recorded as,
>1000, 500–1000, 100–500 or <100 m) and the responses were
documented. Clinical examination of the joints was performed for
the following parameters: i) joint tenderness, measured either as
‘no’, ‘improved’, ‘same’ or ‘worsened’; ii) crepitus, measured
either as ‘no’, ‘mild’, ‘moderate’ or ‘severe’; iii) swelling,
measured in centimeters using a measuring tape, bilaterally; iv)
range of movements, measured in degrees using a goniometer,
bilaterally; v) thigh measurements, measured using measuring tape
around the thigh at a distance 5 cm from the upper border of the
patella and recorded in centimeters; vi) warmth measured as ‘yes’
or ‘no’ and vii) gait, assessed as ‘normal’ or ‘abnormal’.
Safety assessment
The safety of the CB formulation over the treatment
period was evaluated by measuring; i) vital signs; ii) hemogram
measurements including, total white blood cell count (TC),
differential white blood cell count as polymorphonuclear
neutrophils (DC-P), lymphocytes (DC-L), eosinophils (DC-E) and
erythrocyte sedimentation rate (ESR); iii) Liver function test
(LFT), including serum bilirubin, serum glutamic oxaloacetic
transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT),
serum alkaline phosphatase (SAP) and iv) renal function tests
(RFT), including blood urea and serum creatinine. Vital signs were
measured at each visit and a hemogram, LFT and RFT were measured
prior to treatment, following 6 weeks of treatment and at the end
of the treatment period (12 weeks).
Statistical analysis
Statistical analysis of the data was performed using
one-way ANOVA to analyze safety and efficacy data in order to
determine the statistically significant differences between
treatment groups or between time points within the groups. The test
was used to analyze data on joint pain measurements, walking
distance measurements, joint line tenderness, crepitus, range of
movements and swelling of the joints. P<0.05 was considered to
indicate a statistically significant difference.
Results and discussion
The baseline characteristics of the two treatment
groups are provided in Table II.
There were no statistically significant differences between the two
groups. Demographic characteristics of the groups were
comparable.
 | Table IIComparison of baseline demographic and
other characteristics between treatment groups. |
Table II
Comparison of baseline demographic and
other characteristics between treatment groups.
| Demographic
characteristic | Group I (n=14) mean ±
SD | Group II (n=14) mean
± SD |
|---|
| Age (years) | 49.70±8.20 | 47.20±9.70 |
| Males | 6 | 6 |
| Females | 8 | 8 |
| Temperature (°C) | 37.00±0.00 | 37.00±0.00 |
| Height (cm) | 154.30±9.20 | 158.70±7.50 |
| Weight (kg) | 59.60±11.30 | 65.92±13.45 |
| BMI
(kg/cm2) | 25.00±3.50 | 26.09±3.94 |
| BP-systolic
(mmHg) | 121.40±11.0 | 129.28±14.91 |
| BP-diastolic
(mmHg) | 79.29±8.29 | 80.71±8.28 |
| Pulse rate (per
min) | 78.43±5.02 | 78.14±6.29 |
| Respiration (per
min) | 22.29±1.54 | 21.21±1.25 |
The efficacy of CB formulation compared with that of
celecoxib for the management of the symptoms of OA was evaluated by
symptom scoring and clinical examination, and analyzing the data as
a function of time for the two treated groups. The CB formulation
was evaluated at a dose of 500 mg capsules twice daily and the
celecoxib capsule was administered at a dose of 100 mg twice daily.
Symptom refers to the complaints expressed by the patient and
scored depending on severity. The data were analyzed
statistically.
OA joint pain is a deep pain localized to the joint
and is measured by querying the patient and scoring it as
no/mild/moderate/severe during each visit. The results of this
analysis for the two treatment groups are presented in Table III. There was a significant
improvement in pain scores within Groups I and II over a period of
12 weeks, but there was no significant difference between the
groups. At the baseline, 85.71% of the subjects were in the
moderate/severe category in Group I and 78.57% in Group II. At the
end of the study, only 21.43% of subjects in Group I were in the
moderate/severe category whereas 50% of Group II remained in the
moderate/severe category.
 | Table IIIAnalysis of joint pain
measurements. |
Table III
Analysis of joint pain
measurements.
| | Group I | Group II |
|---|
| |
|
|
|---|
| Time points | Response | No. | % | No. | % |
|---|
| T0 | No | 0 | 0.00 | 0 | 0.00 |
| Mild | 2 | 14.29 | 3 | 21.43 |
| Moderate | 11 | 78.57 | 10 | 71.43 |
| Severe | 1 | 7.14 | 1 | 7.14 |
| T2 | No | 1 | 7.14 | 1 | 7.14 |
| Mild | 2 | 14.29 | 3 | 21.43 |
| Moderate | 10 | 71.43 | 10 | 71.43 |
| Severe | 1 | 7.14 | 0 | 0.00 |
| T4 | No | 1 | 7.14 | 1 | 7.14 |
| Mild | 4 | 28.57 | 3 | 21.43 |
| Moderate | 9 | 64.29 | 10 | 71.43 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T6 | No | 1 | 7.14 | 0 | 0.00 |
| Mild | 5 | 35.71 | 3 | 21.43 |
| Moderate | 8 | 57.14 | 11 | 78.57 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T8 | No | 1 | 7.14 | 0 | 0.00 |
| Mild | 7 | 50.00 | 4 | 28.57 |
| Moderate | 5 | 42.86 | 10 | 71.43 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T10 | No | 2 | 14.29 | 1 | 7.14 |
| Mild | 8 | 57.14 | 5 | 35.71 |
| Moderate | 4 | 28.57 | 8 | 57.14 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T12 | No | 2 | 14.29 | 1 | 7.14 |
| Mild | 9 | 64.29 | 6 | 42.86 |
| Moderate | 3 | 21.43 | 8 | 50.00 |
| Severe | 0 | 0.00 | 0 | 0.00 |
Walking distance refers to the maximum distance a
person is capable of walking without any limiting pain. The walking
distance measurements were recorded at each visit and are provided
in Table IV. Statistically
significant improvements in the proportion of individuals scoring a
walking distance of >1000 m were observed within the two groups
over a period of 12 weeks. In Group I, 92.86% of subjects could
walk >1000 m compared with 85.71% in Group II following
treatment. Between the two groups, there were no significant
changes.
 | Table IVAnalysis of walking distance
measurements. |
Table IV
Analysis of walking distance
measurements.
| | Group I | Group II |
|---|
| |
|
|
|---|
| Time points | Distance (m) | No. | % | No. | % |
|---|
| T0 | <100 | 1 | 7.14 | 1 | 7.14 |
| 100–500 | 5 | 35.71 | 3 | 21.43 |
| 500–1000 | 5 | 35.71 | 6 | 42.86 |
| >1000 | 3 | 21.43 | 4 | 28.57 |
| T2 | <100 | 0 | 0.00 | 0 | 0.00 |
| 100–500 | 2 | 14.29 | 1 | 7.14 |
| 500–1000 | 8 | 57.14 | 9 | 64.29 |
| >1000 | 4 | 28.57 | 4 | 28.57 |
| T4 | <100 | 0 | 0.00 | 0 | 0.00 |
| 100–500 | 1 | 7.14 | 1 | 7.14 |
| 500–1000 | 3 | 21.43 | 2 | 14.29 |
| >1000 | 10 | 71.43 | 11 | 78.57 |
| T6 | <100 | 0 | 0.00 | 0 | 0.00 |
| 100–500 | 1 | 7.14 | 1 | 7.14 |
| 500–1000 | 2 | 14.29 | 3 | 21.43 |
| >1000 | 11 | 78.57 | 10 | 71.43 |
| T8 | <100 | 0 | 0.00 | 0 | 0.00 |
| 100–500 | 1 | 7.14 | 0 | 0.00 |
| 500–1000 | 1 | 7.14 | 3 | 21.43 |
| >1000 | 12 | 85.71 | 11 | 78.57 |
| T10 | <100 | 0 | 0.00 | 0 | 0.00 |
| 100–500 | 0 | 0.00 | 0 | 0.00 |
| 500–1000 | 2 | 14.29 | 2 | 14.29 |
| >1000 | 12 | 85.71 | 12 | 85.71 |
| T12 | <100 | 0 | 0.00 | 0 | 0.00 |
| 100–500 | 0 | 0.00 | 0 | 0.00 |
| 500–1000 | 1 | 7.14 | 2 | 14.29 |
| >1000 | 13 | 92.86 | 12 | 85.71 |
Joint line tenderness was elicited by palpating
along the joint line and was measured by querying the patient and
recording the response as no/mild/moderate/severe and was recorded
at each visit, the results of which are presented in Table V. Significant improvements were
observed in the two groups. The percentage of patients in the
moderate/severe category decreased from 85.71 to 7.14% in Group I
over the 12 week period, whereas in Group II a decrease from 78.57
to 21.43% was observed. This showed that 92.85% of the patients in
Group I demonstrated an improvement or had no joint line tenderness
compared with 78.57% in Group II.
 | Table VAnalysis of joint line
tenderness. |
Table V
Analysis of joint line
tenderness.
| | Group I | Group II |
|---|
| |
|
|
|---|
| Time points | Response | No. | % | No. | % |
|---|
| T0 | No | 2 | 14.29 | 3 | 21.43 |
| Mild | 0 | 0.00 | 0 | 0.00 |
| Moderate | 12 | 85.71 | 11 | 78.57 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T2 | No | 4 | 28.57 | 3 | 21.43 |
| Mild | 1 | 7.14 | 2 | 14.29 |
| Moderate | 9 | 64.29 | 9 | 64.29 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T4 | No | 5 | 35.71 | 4 | 28.57 |
| Mild | 2 | 14.29 | 2 | 14.29 |
| Moderate | 7 | 50.00 | 8 | 57.14 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T6 | No | 6 | 42.86 | 4 | 28.57 |
| Mild | 3 | 21.43 | 4 | 28.57 |
| Moderate | 4 | 35.71 | 6 | 42.86 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T8 | No | 6 | 42.86 | 5 | 35.71 |
| Mild | 4 | 28.57 | 4 | 28.57 |
| Moderate | 3 | 28.57 | 5 | 35.71 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T10 | No | 7 | 50.00 | 6 | 42.86 |
| Mild | 3 | 28.57 | 4 | 28.57 |
| Moderate | 3 | 21.43 | 4 | 28.57 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T12 | No | 8 | 57.14 | 6 | 42.86 |
| Mild | 3 | 35.71 | 5 | 35.71 |
| Moderate | 1 | 7.14 | 3 | 21.43 |
| Severe | 0 | 0.00 | 0 | 0.00 |
Crepitus (a crackling or grating feeling or sound in
the joints) is elicited by palpating the joint on movement and
scoring it as no/mild/moderate/severe. These were recorded at each
visit and are presented in Table
VI. The range of movement of the knee is measured for
flexion/extension movement and the normal range is from 0–135° (0
being a neutral position and the increasing flexion of the joint is
normally up to 135°). It is measured using a goniometer and is
measured by asking the patient to flex the joint to the greatest
possible extent and the maximum value was recorded. Results for the
left and right knee are recorded in Table VII. A significant improvement in
crepitus and the range of movement were seen within both groups.
The other parameters studied, namely, joint swelling, warmth of
joint, gait and thigh measurements were unaffected by any of the
drugs or combination tested.
 | Table VIAnalysis of crepitus at various time
points. |
Table VI
Analysis of crepitus at various time
points.
| | Group I | Group II |
|---|
| |
|
|
|---|
| Time points | Response | No. | % | No. | % |
|---|
| T0 | No | 1 | 7.14 | 2 | 14.29 |
| Mild | 6 | 42.86 | 5 | 35.71 |
| Moderate | 7 | 50.00 | 6 | 42.86 |
| Severe | 0 | 0.00 | 1 | 7.14 |
| T2 | No | 2 | 14.29 | 2 | 14.29 |
| Mild | 6 | 42.86 | 6 | 42.86 |
| Moderate | 6 | 42.86 | 5 | 35.71 |
| Severe | 0 | 0.00 | 1 | 7.14 |
| T4 | No | 2 | 14.29 | 2 | 14.29 |
| Mild | 7 | 50.00 | 7 | 50.00 |
| Moderate | 5 | 35.71 | 4 | 28.57 |
| Severe | 0 | 0.00 | 1 | 7.14 |
| T6 | No | 2 | 14.29 | 2 | 14.29 |
| Mild | 7 | 50.00 | 9 | 64.29 |
| Moderate | 5 | 35.71 | 3 | 21.43 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T8 | No | 4 | 28.57 | 3 | 21.43 |
| Mild | 5 | 35.71 | 9 | 64.29 |
| Moderate | 5 | 35.71 | 2 | 14.29 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T10 | No | 4 | 28.57 | 3 | 21.43 |
| Mild | 7 | 50.00 | 10 | 71.43 |
| Moderate | 3 | 21.43 | 1 | 7.14 |
| Severe | 0 | 0.00 | 0 | 0.00 |
| T12 | No | 5 | 35.71 | 4 | 28.57 |
| Mild | 9 | 64.29 | 10 | 71.43 |
| Moderate | 0 | 0.00 | 0 | 0.00 |
| Severe | 0 | 0.00 | 0 | 0.00 |
 | Table VIIAnalysis of range of movement of
knees. |
Table VII
Analysis of range of movement of
knees.
| Group I | Group II |
|---|
|
|
|
|---|
| Time points | Left knee | Right knee | Left knee | Right knee |
|---|
| T0 | 128.2 | 126.4 | 125.4 | 125.4 |
| T2 | 130.4 | 131.4 | 126.1 | 126.1 |
| T4 | 131.1 | 131.0 | 131.1 | 130.7 |
| T6 | 131.9 | 132.1 | 129.6 | 129.6 |
| T8 | 132.4 | 132.4 | 132.5 | 132.5 |
| T10 | 133.1 | 133.2 | 132.1 | 131.8 |
| T12 | 133.9 | 133.2 | 133.2 | 133.2 |
The safety of the CB formulation was evaluated by
measuring vital signs (systolic and diastolic BP, pulse rate,
respiratory rate), hemogram measurement (TC, DC-P, DC-L, DC-E,
ESR), LFTs (SGOT, SGPT, SAP, bilirubin), RFTs (blood urea, serum
creatinine). None of these parameters were adversely modified by CB
formulation. There were also no adverse events reported in the
study.
In conclusion, the CB formulation 500 mg,
administered twice daily demonstrated a greater improvement in the
treatment of OA than celecoxib 100 mg twice daily in the scores for
pain, walking distance and joint line tenderness. The CB
formulation was equally as effective as celecoxib in alleviating
crepitus, and increasing the range of joint movements. The CB
formulation was well tolerated and no dose-related toxicity was
found. The efficacy and tolerability of CB formulation used in the
current study was shown to be superior to those of celecoxib
(NSAID) for treating active OA.
Acknowledgements
The author would like to acknowledge Anugraha
Medical Center, Kakkanad, Kochi for financial support and Arjuna
Natural Extracts Ltd., Aluva, Kerala for providing the
extracts.
References
|
1
|
Dillion CF, Hirsch R, Rasch EK and Gu Q:
Symptomatic hand osteoarthritis in the United States: prevalence
and functional impairment estimates from the third U.S. National
Health and Nutrition Examination Survey 1991–1994. Am J Phys Med
Rehabil. 86:12–21. 2007.PubMed/NCBI
|
|
2
|
Fautrel B, Hilliquin P, Rozenberg S, et
al: Impact of osteoarthritis: results of nationwide survey of
10,000 patients consulting for OA. Joint Bone Spine. 72:235–240.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rabenda V, Manette C, Lemmens R, et al:
Direct and indirect costs attributable to osteoarthritis in active
subjects. J Rheumatol. 33:1152–1158. 2006.PubMed/NCBI
|
|
4
|
Gabriel SE, Crowson CS, Campion ME and
O'Fallon WM: Direct medical costs unique to people with arthritis.
J Rheumatol. 24:719–725. 1997.PubMed/NCBI
|
|
5
|
Gupta S, Hawker GA, Laporte A, Croxford R
and Coyte PC: The economic burden of disabling hip and knee
osteoarthritis (OA) from the perspective of individuals living with
this condition. Rheumatology (Oxford). 44:1531–1537. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Le Pen C, Reygrobellet C and Gérentes I:
Financial costs of osteoarthritis in France. The ‘COART’ France
study Joint Bone. Spine. 72:567–570. 2005.
|
|
7
|
Zhang W, Moskowitz RW, Nuki G, et al:
OARSI recommendations for the management of hip and knee
osteoarthritis, Part I: critical appraisal of existing treatment
guidelines and systematic review of current research evidence.
Osteoarthritis Cartilage. 15:981–1000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Moskowitz RW, Nuki G, et al:
OARSI recommendations for the management of hip and knee
osteoarthritis, Part II: OARSI evidence-based, expert consensus
guidelines. Osteoarthritis Cartilage. 16:137–162. 2008. View Article : Google Scholar
|
|
9
|
Zhang W, Doherty M, Arden N, et al: EULAR
evidence-based recommendations for the management of hip
osteoarthritis: report of a task force of the EULAR Standing
Committee for International Clinical Studies Including Therapeutics
(ESCISIT). Ann Rheum Dis. 64:669–681. 2005. View Article : Google Scholar
|
|
10
|
Zhang W, Doherty M, Leeb BF, et al: EULAR
evidence-based recommendations for the management of hand
osteoarthritis: report of a task force of the EULAR Standing
Committee for International Clinical Studies Including Therapeutics
(ESCISIT). Ann Rheum Dis. 66:377–388. 2007. View Article : Google Scholar
|
|
11
|
Jordan KM, Arden NK, Doherty M, et al:
EULAR Recommendations 2003: an evidence-based approach to the
management of knee osteoarthritis: report of a task force of the
Standing Committee for International Clinical Studies Including
Therapeutics (ESCISIT). Ann Rheum Dis. 62:1145–1155. 2003.
View Article : Google Scholar
|
|
12
|
Pincus T, Koch GG, Sokka T, et al: A
randomized, double-blind, crossover clinical trial of diclofenac
plus misoprostol versus acetaminophen in patients with
osteoarthritis of the hip or knee. Arthritis Rheum. 44:1587–1598.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Case JP, Baliunas AJ and Block JA: Lack of
efficacy of acetaminophen in treating symptomatic knee
osteoarthritis: a randomized, double-blind, placebo-controlled
comparison trial with diclofenac sodium. Arch Intern Med.
163:169–178. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta I, Gupta V, Parihar A, et al:
Effects of Boswellia serrata gum resin in patients with bronchial
asthma: results of a double-blind, placebo-controlled, 6-week
clinical study. Eur J Med Res. 3:511–514. 1998.PubMed/NCBI
|
|
15
|
Sander O, Herborn G and Rau R: Is H15
(resin extract of Boswellia serrata, ‘incense’) a useful supplement
to establish drug therapy of chronic polyarthritis? Results of a
double-blind pilot study. Z Rheumatol. 57:11–16. 1998.(In
German).
|
|
16
|
Gerhardt H, Seifert F, Buvari P, et al:
Therapy of active Crohn disease with Boswellia serrata extract H15.
Z Gastroenterol. 39:11–17. 2001.(In German).
|
|
17
|
Kimmatkar N, Thawani V, Hingorani L and
Khiyani R: Efficacy and tolerability of Boswellia serrata extract
in treatment of osteoarthritis of knee - a randomized,
double-blind, placebo-controlled trial. Phytomedicine. 10:3–7.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sontakke S, Thawani V, Pimpalkhute P, et
al: Open, randomized, controlled clinical trial of Boswellia
serrata extract as compared to valdecoxib in osteoarthritis of the
knee. Indian J Pharmacol. 39:27–29. 2007. View Article : Google Scholar
|
|
19
|
Sengupta K, Alluri KV, Satish AR, et al: A
double-blind, randomized, placebo-controlled study of the efficacy
and safety of 5-Loxin for treatment of osteoarthritis of the knee.
Arthritis Res Ther. 10:R852008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Madisch A, Miehlke S, Eichele O, et al:
Boswellia serrata extract for the treatment of collagenous colitis.
A double-blind, randomized, placebo-controlled, multicenter trial.
Int J Colorectal Dis. 22:1445–1451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Safayhi H, Mack T, Sabieraj J, Anazodo MI,
Subramanian LR and Ammon HP: Boswellic acids: novel, specific,
nonredox inhibitors of 5-lipoxygenase. J Pharmacol Exp Ther.
261:1143–1146. 1992.PubMed/NCBI
|
|
22
|
Sailer ER, Subramanian LR, Rall B,
Hoernlein RF, Ammon HP and Safayhi H: Acetyl-11-keto-beta-boswellic
acid (AKBA): structure requirements for binding and 5-lipoxygenase
inhibitory activity. Br J Pharmacol. 117:615–618. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sailer ER, Schweizer S, Boden SE, Ammon HP
and Safayhi H: Characterization of an acetyl-11-keto-beta-boswellic
acid and arachidonate-binding regulatory site of 5-lipoxygenase
using photoaffinity labeling. Eur J Biochem. 256:364–368. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu JJ, Nilsson A, Oredsson S, Badmaev V,
Zhao WZ and Duan RD: Boswellic acids trigger apoptosis via a
pathway dependent on caspase-8 activation but independent on
Fas/Fas ligand interaction in colon cancer HT-29 cells.
Carcinogenesis. 23:2087–2093. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shoba G, Joy D, Joseph T, Majeed M,
Rajendran R and Srinivas PS: Influence of piperine in
pharmacokinetics of curcumin in animals and human volunteers.
Planta Med. 64:353–356. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schweizer S, von Brocke AF, Boden SE,
Bayer E, Ammon HP and Safayhi H: Workup-dependent formation of
5-lipoxygenase-inhibitory boswellic acid analogues. J Nat Prod.
63:1058–1061. 2000. View Article : Google Scholar : PubMed/NCBI
|