Introduction
As the human lifespan increases, cardiovascular
diseases are starting to represent a growing health and
socioeconomic burden to society (1). Among the therapeutic strategies for
such diseases, autologous grafts, including autologous saphenous
vein and mammary artery grafts, are the common options (2). However, these approaches are limited
by the sources and donor site morbidity, and such synthetic grafts,
including expanded polytetrafluoroethylene and Dacron (polyethylene
terephthalate fibre) grafts, can often result in immunological and
thrombotic complications, particularly in the repair of
small-diameter vascular defects (3). Research has been conducted on
tissue-engineered, small-diameter vascular grafts or the
endothelialisation of the previously mentioned artificial grafts,
with endothelial cells (ECs) playing a significant role,
particularly with regard to vascular tissue engineering
applications (4). However, the
limited availability of ECs hampers the development of a suitable
vascular graft. Furthermore, the slow expansion rate and limited
proliferation capability of fully mature ECs in vitro have
presented crucial hurdles in their therapeutic use (5).
Offering the unique advantages of proliferative and
growth potential, stem cells, either embryonic or adult, and
circulating endothelial progenitors have been widely analysed as
possible sources of ECs (6,7).
Previous studies have indicated that the hair follicle (HF) is a
readily accessible mini-organ within the skin that contains stem
cells with notably broad differentiation potential, and that it may
be an alternative source of autologous ECs (8,9). HF
stem cells (HFSCs) have the broad potential to differentiate into
adipogenic, osteogenic, chondrogenic, neurogenic and myogenic
lineages under the appropriate conditions (10–13).
When compared with other stem cells, including embryonic, bone
marrow-derived or adipose stem cells, HFSCs are easier to acquire
(less invasive) and have a lower associated risk of donor site
morbidity and a higher yield at harvest (9). Thus, HFSCs could be a preferred,
novel cell source for blood vessel engineering.
Vascular endothelial growth factor (VEGF) is a
signalling protein produced by cells that stimulates vasculogenesis
and angiogenesis (14). The VEGF
family is composed of at least seven members, with VEGF-A (normally
termed VEGF) being the most significant. The targeted inactivation
of the VEGF gene in mice has been shown to cause fatal deficiencies
in vascularisation (15),
demonstrating the important role of VEGF in this process. VEGF has
been shown to affect EC differentiation in vitro(16) and is thus a component of in
vitro EC differentiation media (17–19).
Basic fibroblast growth factor (bFGF), also known as
FGF2 or FGF-β, is a member of the FGF family (20). bFGF promotes EC proliferation and
the physical organisation of ECs into tube-like structures, and is
thus critical in mediating the formation of novel blood vessels and
promoting angiogenesis (21).
The aim of the present study was to investigate the
potential of human HFSCs (hHFSCs) to differentiate into the EC
phenotype upon induction with VEGF and bFGF in a low-serum medium.
The gene and protein expression of several characteristic EC
markers was examined in the resulting hHFSCs. In addition, a
low-density lipoprotein (LDL) uptake function of the induced hHFSCs
was also demonstrated.
Materials and methods
Isolation and culture of hHFSCs
hHFSCs were isolated from human scalp tissues from
healthy adult patients undergoing cosmetic plastic surgery, as
described previously (22). All
the protocols of human tissue handling were approved by the
Research Ethical Committee of the hospital and written informed
consent was obtained from all patients. hHFSCs at the second
passage were used in the subsequent study. The characterisation of
the hHFSCs was determined by their CD marker profile (K15, K19,
integrin β1) and their ability to differentiate into osteogenic,
adipogenic and chondrogenic lineages (data not shown), as reported
previously (10,11).
Induction of EC differentiation
Cells reaching subconfluence were cultured in EC
growth medium-2 (EGM-2; Lonza, Walkersville, MD, USA) supplemented
with 50 ng/ml recombinant human VEGF (R&D Systems, Minneapolis,
MN, USA) and 10 ng/ml recombinant human bFGF (Sigma-Aldrich, St.
Louis, MO, USA), with 2% foetal bovine serum (FBS; HyClone, Logan,
UT, USA). EGM-2 supplemented with 2% FBS was defined as the basal
medium (BM). Human umbilical vein ECs (hUVECs) were used as a
positive control. The culture media were changed every 2 days. The
cell characterisation and functional evaluation were performed
subsequent to 7days of culture.
Immunofluorescence staining
hHFSCs were harvested, resuspended in
phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for
15 min and permeabilised with 0.1% Triton X-100 (both
Sigma-Aldrich) for 10 min. Subsequent to washing with PBS, the
cells were blocked with 3% bovine serum albumin (BSA) for 30 min
and then incubated with the following primary antibodies: Rabbit
polyclonal anti-von Willebrand Factor (vWF, F3520), rabbit
polyclonal anti-vascular endothelial cadherin (VE-cadherin, V1514)
and mouse monoclonal anti-CD31 (P8590) (all from Sigma-Aldrich).
Following incubation with the primary antibodies for 60 min at room
temperature, the cultures were washed with PBS three times.
Fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit
secondary antibody (Millipore, Billerica, MA, USA) was used to
detect the localisation of the anti-vWF and anti-VE-cadherin
antibodies, and FITC-conjugated goat anti-mouse secondary antibody
(Millipore) was used to detect the localisation of the anti-CD31
antibodies. The cell nuclei were stained with propidium iodide. The
control samples consisted of cells without primary antibodies and
were used to assess the background fluorescence. The images were
viewed with a fluorescence microscope (Nikon, Tokyo, Japan).
Flow cytometric analysis
The cells were trypsinised, centrifuged at 500 × g
for 5 min (Allegra 64R; Beckman Coulter, Brea, CA, USA),
resuspended in PBS/1% BSA and incubated with anti-vWF,
anti-VE-cadherin and anti-CD31 (all from Sigma-Aldrich) for 30 min
at room temperature on a shaking plate (Lab Rotators, Thermo
Scientific, Logan, UT, USA). The cells were then washed,
resuspended in PBS/1% BSA with FITC-conjugated secondary antibody
and incubated for 30 minutes at room temperature on a shaking
plate. The cells were then washed again and fixed, and
FITC-conjugated isotype-matching immunoglobulins were used to
determine non-specific staining. Fluorescence was determined using
a flow cytometer (Becton-Dickinson, San Jose, CA, USA), and the
data were analysed using CellQuest software (Becton-Dickinson).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
The expression levels of EC-specific markers (vWF,
VE-cadherin and CD31) were identified by isolating the total RNA
from the cells using the RNeasy total RNA isolation kit (Qiagen,
Inc., Valencia, CA, USA), and cDNA was synthesised using the
SuperScript First-strand Synthesis system (Life Technologies,
Carlsbad, CA, USA). Specific genes were amplified by PCR using the
Fast-Run Taq Master kit (Protech Technology, Taipei, Taiwan). The
primer sequences designed using the Primer Express software (Primer
Express Software Version 3.0; Applied Biosystems, Foster City, CA,
USA) are listed in Table I. The
cDNA product was amplified by PCR using standard methods, and
electrophoresed through a 2% agarose gel treated with ethidium
bromide; the bands were visualised using an ultraviolet light box.
hUVECs and human chondrocyte cells (hCs) were used as the positive
and negative controls, respectively.
 | Table IPrimers for polymerase chain
reaction. |
Table I
Primers for polymerase chain
reaction.
| RNA | Primer
sequences | Fragment size,
bp |
|---|
| vWF | F:
5′-CACCGTTTGCCCACCCTTCG-3′ | 433 |
| R:
5′-GCCCACTGGGAGCCGACACT-3′ | |
| VE-cadherin | F:
5′-ACTCACCCCTTGCAATAACG-3′ | 250 |
| R:
5′-ACAGAGCAGCCATCAGAGGT-3′ | |
| CD31 | F:
5′-TCCGATGATAACCACTGCAA-3′ | 297 |
| R:
5′-GTGGTGGAGTCTGGAGAGGA-3′ | |
LDL uptake
LDL uptake was assessed by incubating the cells for
4 h at 37°C with acetylated LDL labelled with
1,10-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI-acLDL;
Molecular Probes, Biomedical Technologies, Stoughton, MA, USA)
diluted to 10 μg/ml in a complete growth medium. The cells were
then washed three times with probe-free medium. The incorporation
of fluorochrome-labelled LDL into the cells was analysed with an
Eclipse E400 Epi-Fluorescence Microscope (Nikon, Tokyo, Japan).
Statistical analysis
Each experiment was repeated at least three times.
Since the original data were normally distributed, the results are
presented as the mean ± standard deviation. The comparisons between
groups were performed by a paired Student’s t-test and P<0.05
was used to indicate a statistically significant difference. All
the statistical analyses were performed using SPSS version 16
(SPSS, Inc., IBM Corporation, Somers, NY, USA).
Results
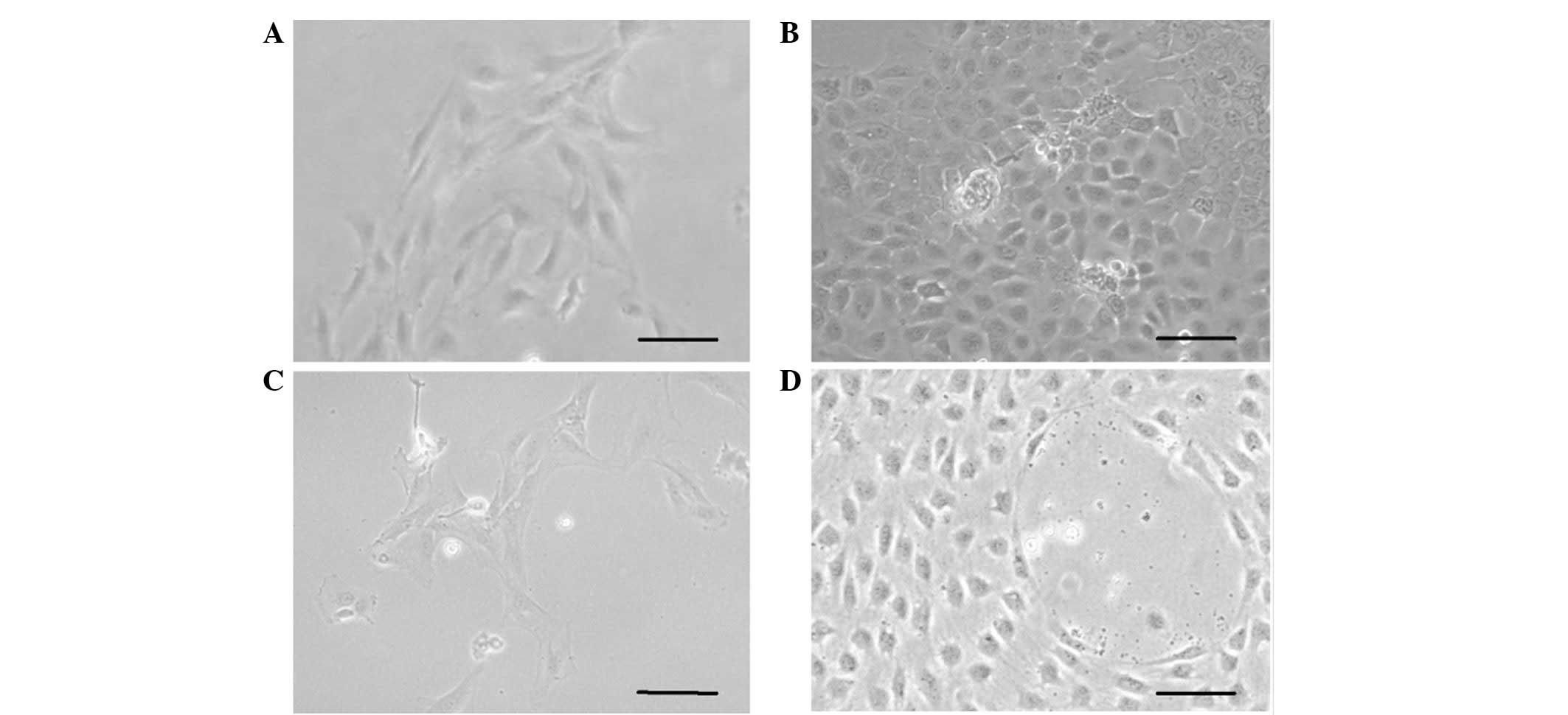
Culture of hHFSCs
The total number of cells isolated from each scalp
tissue sample ranged between 5×104 and 1×105
cells. In total, 0.5–2% of the isolated cells were found to be
adherent stem cells. The hHFSCs elongated subsequent to 1–2 days of
culture in plates (Fig. 1A); the
cells reached confluency within another 3–4 days and were
subsequently passaged onto a novel plate.
VEGF and bFGF induce the differentiation
of hHFSCs to ECs
At the second passage, 50 ng/ml VEGF and 10 ng/ml
bFGF were used to induce the differentiation of hHFSCs to the EC
lineage. The hHFSCs acquired a cobblestone morphology subsequent to
treatment with VEGF and bFGF for 7 days, similar to the previous
observations in primary isolated hUVECs. No evident change was
found in the hHFSCs cultured in BM (Fig. 1B–D). At the fourth passage, the
hHFSCs appeared to comprise a relatively homogenous population that
exhibited an endothelial lineage morphology.
Expression of EC-specific markers in
hHFSCs treated with VEGF and bFGF
To determine whether VEGF and bFGF can induce the
differentiation of hHFSCs to the EC phenotype, EC-specific proteins
(vWF, VE-cadherin and CD31) were detected by immunofluorescence
staining. These three markers were also examined in hUVECs as a
positive control. As shown in Fig.
2, there was little expression of vWF, VE-cadherin or CD31 in
the undifferentiated hHFSCs cultured in BM. However, when cultured
in EGM-2 supplemented with VEGF and bFGF, the expression levels of
vWF, VE-cadherin and CD31 were enhanced, reaching a level similar
to that of the hUVECs.
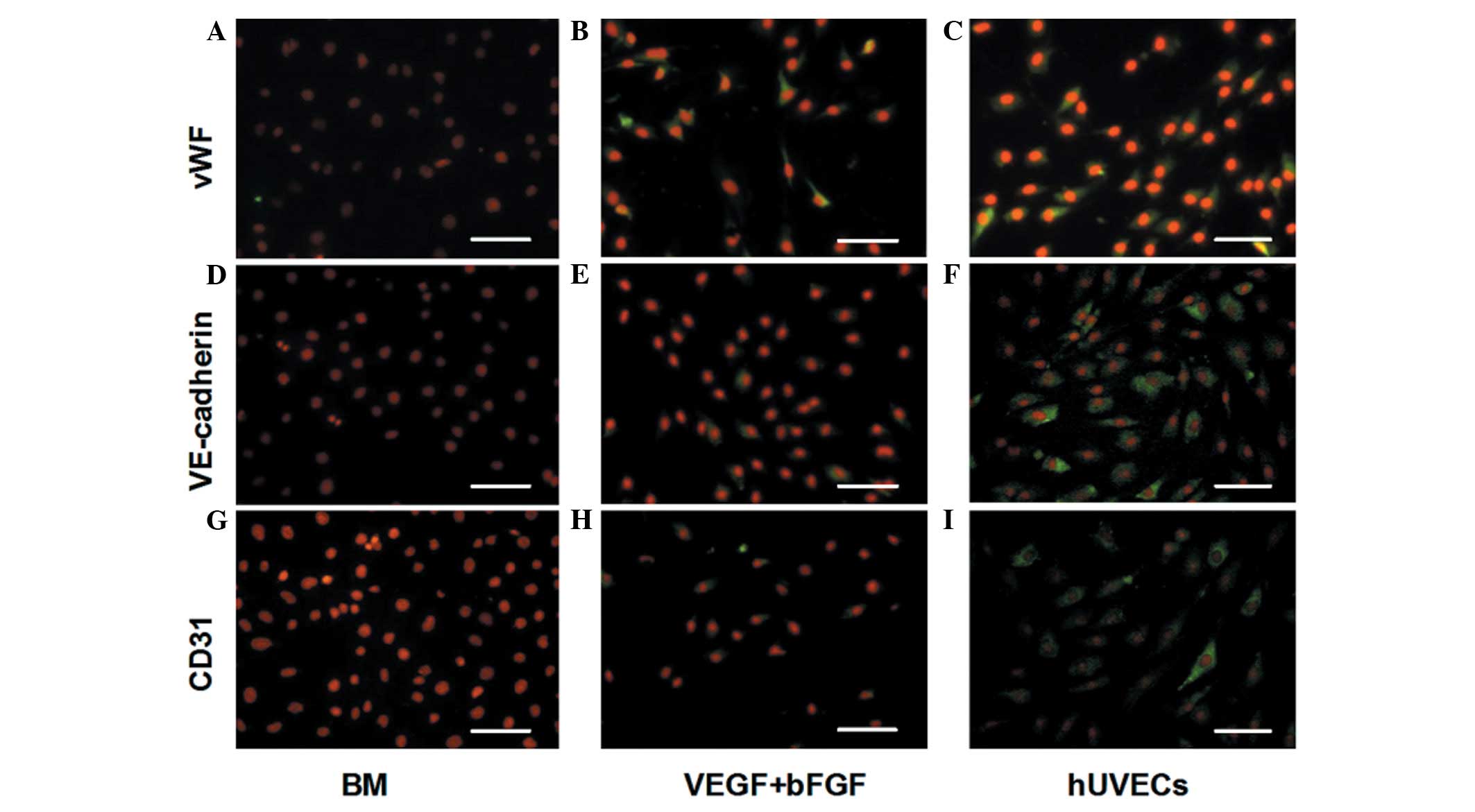 | Figure 2Expression of EC-specific proteins
(vWF, VE-cadherin and CD31) under different conditions by
immunofluorescent staining. There was little expression of (A) vWF,
(D) VE-cadherin or (G) CD31 in the undifferentiated hHFSCs cultured
in BM. The expression of (B) vWF, (E) VE-cadherin and (H) CD31 was
enhanced when cultured in BM supplemented with VEGF and bFGF,
reaching a level similar to that of (C, F, I) the hUVECs. Scale
bar, 100 μm. EC, endothelial cells; vWF, von Willebrand factor;
VE-cadherin, vascular endothelial cadherin; hHFSCs, human hair
follicle stem cells; BM, basal medium; VEGF, vascular endothelial
growth factor; bFGF, basic fibroblast growth factor; hUVECs, human
umbilical vein ECs; CD31, cluster of differentiation 31. |
To determine the percentage of EC-differentiated
cells in the hHFSC population, the expression levels of vWF,
VE-cadherin and CD31 were also analysed using flow cytometry. As
shown in Fig. 3, vWF was detected
in 3.57±0.47, 85.41±1.42 and 93.62±0.75% of undifferentiated
hHFSCs, induced hHFSCs and hUVECs, respectively. By comparison,
little expression of VE-cadherin (3.38±0.52%) or CD31 (2.63±0.56%)
was found in the undifferentiated hHFSCs, although their expression
levels reached 72.36±1.93 and 76.49±1.12%, respectively, in the
induced hHFSCs, which is much closer to the expression observed in
the hUVECs.
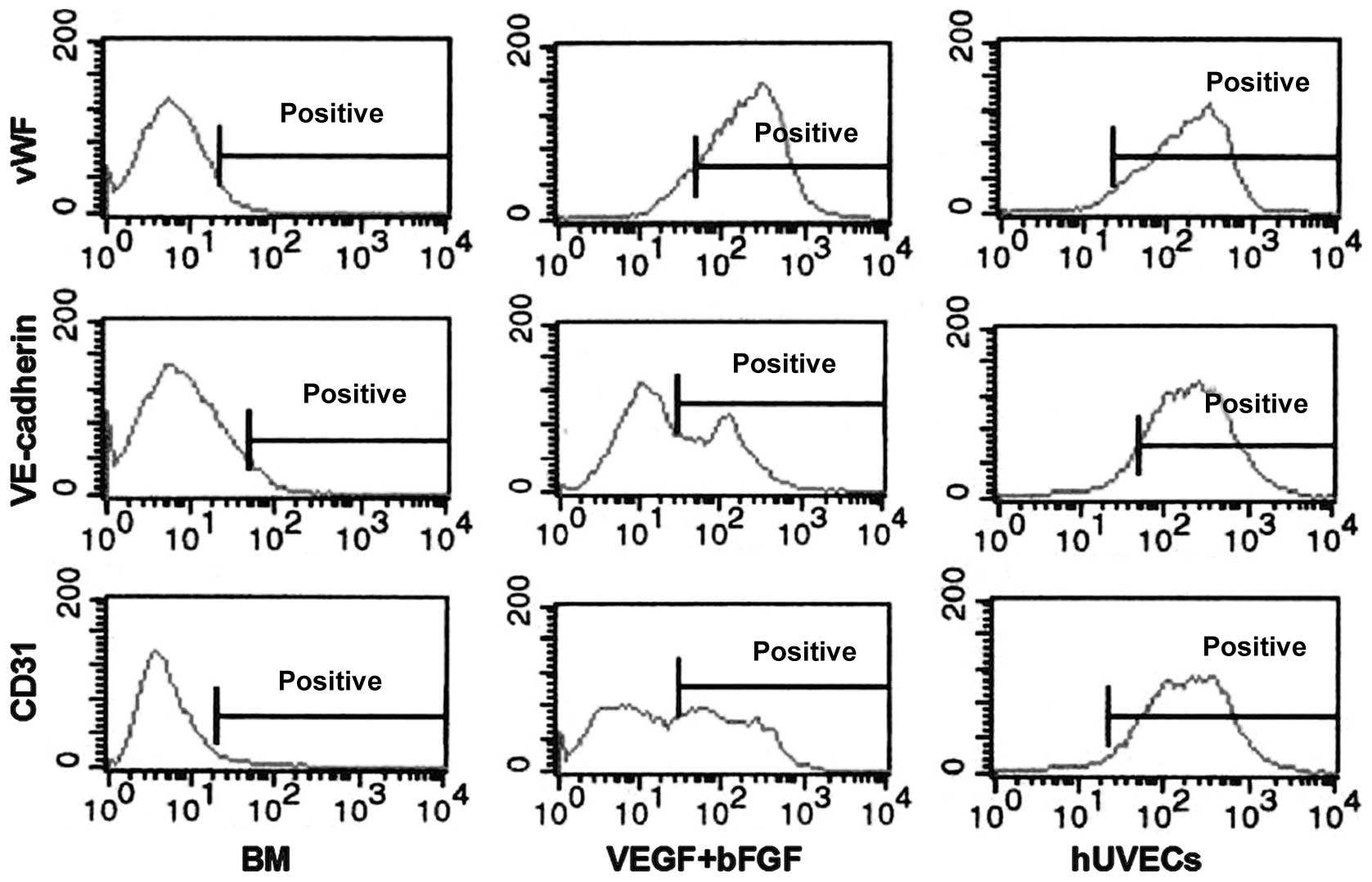 | Figure 3Flow cytometry analysis of
EC-specific proteins. Low levels of expression of vWF, VE-cadherin
and CD31 were found in the undifferentiated hHFSCs cultured in BM.
The expression was enhanced in BM supplemented with VEGF and bFGF,
similar to that in hUVECs. EC, endothelial cells; vWF, von
Willebrand factor; VE-cadherin, vascular endothelial cadherin;
hHFSCs, human hair follicle stem cells; BM, basal medium; VEGF,
vascular endothelial growth factor; bFGF, basic fibroblast growth
factor; hUVECs, human umbilical vein ECs; CD31, cluster of
differentiation 31. |
The gene expression profile analysed by RT-PCR
further confirmed the EC differentiation of the hHFSCs. As shown in
Fig. 4, the undifferentiated
hHFSCs did not express vWF, VE-cadherin or CD31, similar to the
observations in the hCs. By contrast, these factors were
upregulated in the induced hHFSCs to a similar level as that in the
hUVECs. All the data support the hypothesis that hHFSCs can
differentiate into ECs upon exposure to VEGF and bFGF.
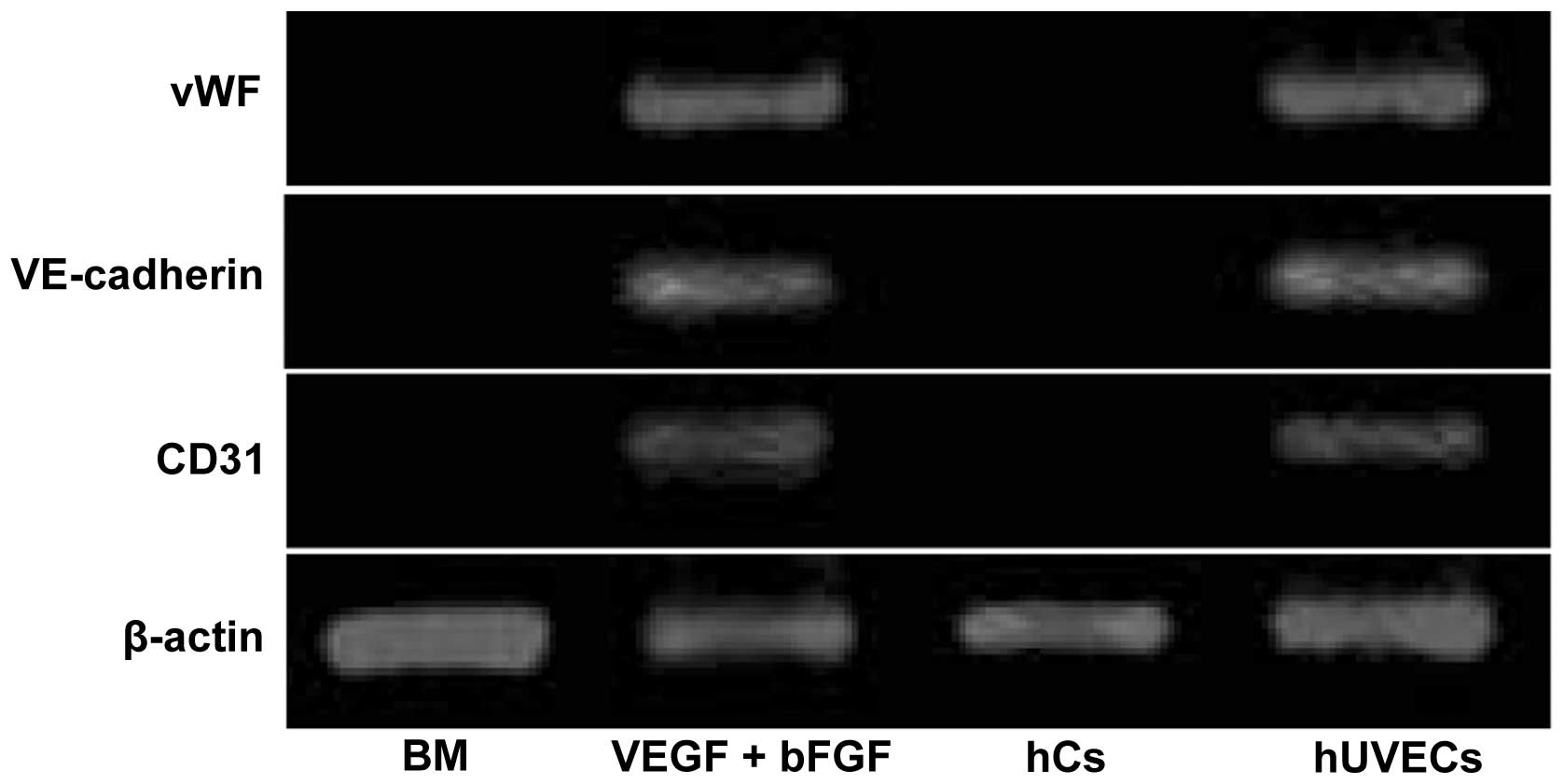 | Figure 4mRNA levels of vWF, VE-cadherin, CD31
and β-actin were determined by reverse transcription polymerase
chain reaction (RT-PCR). The expression of vWF, VE-cadherin and
CD31 was observed in hHFSCs induced by VEGF and bFGF, similar to
hUVECs. Human chondrocyte cells (hCs) were used as the negative
controls. vWF, von Willebrand factor; VE-cadherin, vascular
endothelial cadherin; hHFSCs, human hair follicle stem cells; VEGF,
vascular endothelial growth factor; bFGF, basic fibroblast growth
factor; hUVECs, human umbilical vein ECs; CD31, cluster of
differentiation 31. |
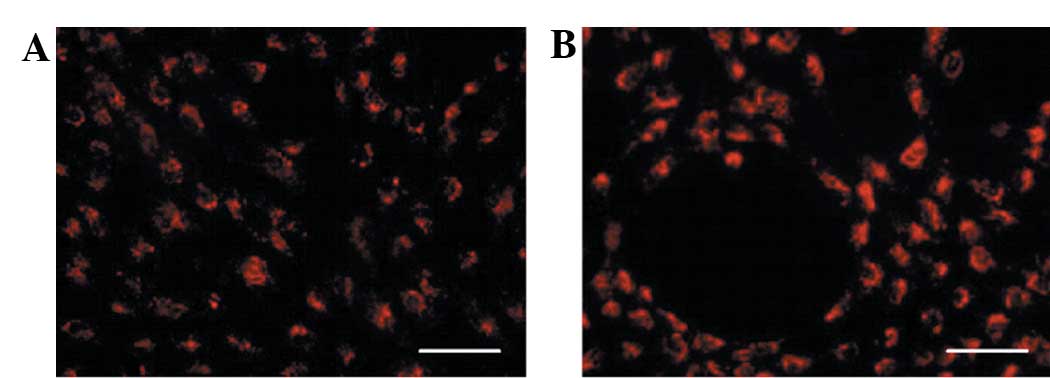
Differentiated cells can take up
DiI-Ac-LDL
The cytoplasm of the differentiated cells was
fluorescent due to the incorporation of labelled LDL (DiI-Ac-LDL;
Fig. 5A), a typical ability of
ECs, whereas the undifferentiated cells were unable to incorporate
the fluorescent DiI-Ac-LDL molecules (data not shown). Parallel
experiments were performed with the hUVECs serving as a positive
control (Fig. 5B).
Discussion
Regardless of the results obtained from using adult
blood vessel-derived ECs in vascular tissue engineering and
regenerative medicine (23–28),
their general use is hampered by their inadequate proliferation
capacity and lack of autologous sources. Considering the
procurement risk and their short lifespan, researchers have
urgently been searching for alternative sources of rapidly
proliferating and ready-to-use ECs. Although ECs can be derived
from multipotent adult stem cells (5, 29–33),
the potential of HFSCs has been less frequently investigated.
In the present study, the effect of VEGF and bFGF
was demonstrated on the differentiation of hHFSCs to the EC
lineage. A considerable variation in the expression of the EC
markers, vWF, VE-cadherin and CD31, was observed at the gene and
protein levels. Nonetheless, VEGF and bFGF induced the expression
of these three proteins in the hHFSCs. vWF is a blood glycoprotein
uniquely secreted by vascular ECs that binds to connective tissue
and recruits platelets to the sites of vascular injury. Thus, vWF
is of great importance to haemostasis (34,35).
VE-Cadherin is a strictly EC adhesive transmembrane molecule that
is specifically expressed at interendothelial junctions and is
significant in EC biology as it maintains control of the cohesion
and organisation of intercellular junctions (36). CD31, also known as platelet EC
adhesion molecule-1, is a transmembrane glycoprotein expressed on
the surface of ECs, platelets and certain haematopoietic cells
(37). CD31 is generally localised
to sites of cell-cell contact in confluent EC monolayers,
contributing to vascular permeability, coagulation and leukocyte
transmigration (38).
Additionally, according to the immunofluorescence staining in the
present study, the three markers were still expressed in the
differentiated hHFSCs subsequent to 21 days of induction and
observation (data not shown). The results of the present study
indicate that hHFSCs are fully induced to an endothelial lineage by
VEGF and bFGF.
VEGF is a major regulator of vascular development
and vascular progenitor cell differentiation in the initial phase
of blood vessel development (39),
and is a potent mitogen in embryonic and somatic angiogenesis, with
a unique specificity for vascular ECs (40). The central role of VEGF in
embryonic angiogenesis was demonstrated by the observation that
heterozygote knockout mice suffer fatal deficiencies in
vascularisation, resulting in embryonic lethality (41,42).
VEGF deficiency led to early vascular development disorders,
including vasculogenesis, angiogenesis and large vessel formation.
The role of VEGF in angiogenesis indicates that this protein could
be used to augment collateral vessel formation as an alternative to
reconstructive surgery (43), as
confirmed by the finding that treatment with DNA encoding VEGF
augmented collateral vessel formation in acute limb ischaemia
through therapeutic in situ neovascularisation and
angiogenesis (44–47). Knowledge of the function of VEGF in
endothelial differentiation and blood vessel growth stimulation may
be useful for vascular tissue engineering and regenerative
medicine, including the treatment of myocardial and lower limb
ischaemia, wound healing and skin grafting.
It has been well documented that bFGF is an
extracellular matrix component required for supporting EC growth
and promoting the formation of differentiated capillary tubes
(48,49). bFGF-mediated signalling is critical
for the proliferation of the haemangioblast and thus positively
regulates haematopoietic development (50). Several lines of evidence have
indicated that bFGF (FGF2) stimulates VEGF expression in ECs
(51–53). In the absence of bFGF signalling,
it leads to the loss of adherens and tight junctions, increased
vascular leakiness and disassembly of the existing vasculature.
The mechanism for EC differentiation induced by the
angiogenic growth factors, VEGF and bFGF, has been extensively
investigated. These factors exert their effects by specifically
binding to cell surface-expressed receptors, which are
ligand-stimulatable tyrosine kinases. The stimulation of receptor
kinase activity allows coupling to the downstream signalling
transduction pathways, which results in transcriptional changes and
biological responses, thereby regulating the proliferation,
migration and differentiation of ECs (54). Previous studies have also
demonstrated that bFGF stimulates VEGF expression in ECs. One key
factor in FGF-induced VEGF expression is the Shc protein, an
adaptor molecule recruited to FGFRs upon activation, which is
crucial in receptor tyrosine kinase-dependent VEGF gene expression
(55,56). Therefore, it was speculated that
cross-talk between VEGF and bFGF pathways may contribute to the
differentiation of hHFSCs to ECs.
To confirm a differentiated EC phenotype, the
demonstration of characteristic functional properties is required.
In the present study, the hHFSCs differentiated with VEGF and bFGF
were shown to gain the capability of transporting LDL as
endothelial differentiation progressed. These data indicate that
hHFSCs can be induced to differentiate into an EC phenotype with
conventional function when stimulated by VEGF and bFGF in
combination. Therefore, hHFSCs may be a valuable source of
functional ECs for vascular tissue engineering and therapeutic
vasculogenesis.
In conclusion, the present study demonstrated that
expanded hHFSCs acquire several endothelial-like characteristics
when cultured with VEGF and bFGF for 7 days in vitro, as
evidenced by the expression of EC-specific transcripts and
proteins, including vWF, VE-cadherin and CD31, and the ability to
incorporate fluorescent DiI-Ac-LDL molecules. It was hypothesised
that this approach has potential applications for using hHFSCs as a
candidate in cardiovascular tissue engineering, and that it
provides a tool for various clinical studies in which improved
vascularisation is desired.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81000842). The authors also
appreciate the technical support of Dr Demin Ying, Dr Lijuan Zong
and Dr Bing Zhong in the laboratory.
References
|
1
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sundaram S and Niklason LE: Smooth muscle
and other cell sources for human blood vessel engineering. Cells
Tissues Organs. 195:15–25. 2012.PubMed/NCBI
|
|
3
|
Xu ZC, Zhang WJ, Li H, Cui L, Cen L, Zhou
GD, Liu W and Cao Y: Engineering of an elastic large muscular
vessel wall with pulsatile stimulation in bioreactor. Biomaterials.
29:1464–1472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poh M, Boyer M, Solan A, Dahl SL, Pedrotty
D, Banik SS, McKee JA, Klinger RY, Counter CM and Niklason LE:
Blood vessels engineered from human cells. Lancet. 365:2122–2124.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McKee JA, Banik SS, Boyer MJ, Hamad NM,
Lawson JH, Niklason LE and Counter CM: Human arteries engineered in
vitro. EMBO Rep. 4:633–638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bajpai VK and Andreadis ST: Stem cell
sources for vascular tissue engineering and regeneration. Tissue
Eng Part B Rev. 18:405–425. 2012.PubMed/NCBI
|
|
7
|
Matsumura G, Miyagawa-Tomita S, Shin’oka
T, Ikada Y and Kurosawa H: First evidence that bone marrow cells
contribute to the construction of tissue-engineered vascular
autografts in vivo. Circulation. 108:1729–1734. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin’oka T, Matsumura G, Hibino N, Naito
Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M and Kurosawa H:
Midterm clinical result of tissue-engineered vascular autografts
seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg.
129:1330–1338. 2005.
|
|
9
|
Gong ZD and Niklason LE: Small-diameter
human vessel wall engineered from bone marrow-derived mesenchymal
stem cells (hMSCs). FASEB J. 22:1635–1648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heydarkhan-Hagvall S, Schenke-Layland K,
Yang JQ, Heydarkhan S, Xu Y, Zuk PA, MacLellan WR and Beygui RE:
Human adipose stem cells: a potential cell source for
cardiovascular tissue engineering. Cells Tissues Organs.
187:263–274. 2008.PubMed/NCBI
|
|
11
|
Wang C, Cen L, Yin S, Liu Q, Liu W, Cao Y
and Cui L: A small diameter elastic blood vessel wall prepared
under pulsatile conditions from polyglycolic acid mesh and smooth
muscle cells differentiated from adipose-derived stem cells.
Biomaterials. 31:621–630. 2010. View Article : Google Scholar
|
|
12
|
Harris LJ, Abdollahi H, Zhang P, McIlhenny
S, Tulenko TN and DiMuzio PJ: Differentiation of adult stem cells
into smooth muscle for vascular tissue engineering. J Surg Res.
168:306–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsu YC, Pasolli HA and Fuchs E: Dynamics
between stem cells, niche, and progeny in the hair follicle. Cell.
144:92–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mistriotis P and Andreadis ST: Hair
Follicle: a novel source of multipotent stem cells for tissue
engineering and regenerative medicine. Tissue Eng Part B Rev.
19:265–278. 2013.PubMed/NCBI
|
|
15
|
Jahoda CA, Whitehouse J, Reynolds AJ and
Hole N: Hair follicle dermal cells differentiate into adipogenic
and osteogenic lineages. Exp Dermatol. 12:849–859. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu H, Fang D, Kumar SM, Li L, Nguyen TK,
Acs G, Herlyn M and Xu X: Isolation of a novel population of
multipotent adult stem cells from human hair follicles. Am J
Pathol. 168:1879–1888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drewa T, Joachimiak R, Kaznica A, Sarafian
V and Pokrywczynska M: Hair stem cells for bladder regeneration in
rats: preliminary results. Transplant Proc. 41:4345–4351. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin H, Liu F, Zhang C, Zhang Z, Kong Z,
Zhang X and Hoffman RM: Characterization of nerve conduits seeded
with neurons and Schwann cells derived from hair follicle neural
crest stem cells. Tissue Eng Part A. 17:1691–1698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dickson MC, Martin JS, Cousins FM,
Kulkarni AB, Karlsson S and Akhurst RJ: Defective haematopoiesis
and vasculogenesis in transforming growth factor-beta 1 knock out
mice. Development. 121:1845–1854. 1995.PubMed/NCBI
|
|
20
|
Shah NM, Groves AK and Anderson DJ:
Alternative neural crest cell fates are instructively promoted by
TGFbeta superfamily members. Cell. 85:331–343. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grainger DJ, Metcalfe JC, Grace AA and
Mosedale DE: Transforming growth factor-beta dynamically regulates
vascular smooth muscle differentiation in vivo. J Cell Sci.
111:2977–2988. 1998.PubMed/NCBI
|
|
22
|
Hirschi KK, Rohovsky SA and D’Amore PA:
PDGF, TGFbeta, and heterotypic cell-cell interactions mediate
endothelial cell-induced recruitment of 10T1/2 cells and their
differentiation to a smooth muscle fate. J Cell Biol. 141:805–814.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S and Lechleider RJ: Transforming
growth factor-beta-induced differentiation of smooth muscle from a
neural crest stem cell line. Circ Res. 94:1195–1202. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirschi KK, Burt JM, Hirschi KD and Dai C:
Gap junction communication mediates transforming growth factor-beta
activation and endothelial-induced mural cell differentiation. Circ
Res. 93:429–437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross JJ, Hong Z, Willenbring B, Zeng L,
Isenberg B, Lee EH, Reyes M, Keirstead SA, Weir EK, Tranquillo RT
and Verfaillie CM: Cytokine-induced differentiation of multipotent
adult progenitor cells into functional smooth muscle cells. J Clin
Invest. 116:3139–3149. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lindahl P, Johansson BR, Levéen P and
Betsholtz C: Pericyte loss and microaneurysm formation in
PDGF-B-deficient mice. Science. 277:242–245. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hellström M, Kalén M, Lindahl P, Abramsson
A and Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of
vascular smooth muscle cells and pericytes during embryonic blood
vessel formation in the mouse. Development. 126:3047–3055.
1999.PubMed/NCBI
|
|
28
|
Kim YM, Jeon ES, Kim MR, Jho SK, Ryu SW
and Kim JH: Angiotensin II-induced differentiation of adipose
tissue-derived mesenchymal stem cells to smooth muscle-like cells.
Int J Biochem Cell Biol. 40:2482–2491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Long JL and Tranquillo RT: Elastic fiber
production in cardiovascular tissue-equivalents. Matrix Biol.
22:339–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong Z and Niklason LE: Blood vessels
engineered from human cells. Trends Cardiovasc Med. 16:153–156.
2006. View Article : Google Scholar
|
|
31
|
Isenberg BC, Williams C and Tranquillo RT:
Small-diameter artificial arteries engineered in vitro. Circ Res.
98:25–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cho SW, Lim SH, Kim IK, Hong YS, Kim SS,
Yoo KJ, Park HY, Jang Y, Chang BC, Choi CY, et al: Small-diameter
blood vessels engineered with bone marrow-derived cells. Ann Surg.
241:506–515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rodriguez LV, Alfonso Z, Zhang R, Leung J,
Wu B and Ignarro LJ: Clonogenic multipotent stem cells in human
adipose tissue differentiate into functional smooth muscle cells.
Proc Natl Acad Sci USA. 103:12167–12172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beltrami AP, Cesselli D, Bergamin N,
Marcon P, Rigo S, Puppato E, D’Aurizio F, Verardo R, Piazza S,
Pignatelli A, et al: Multipotent cells can be generated in vitro
from several adult human organs (heart, liver, and bone marrow).
Blood. 110:3438–3446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miano JM: Mammalian smooth muscle
differentiation: origins, markers and transcriptional control.
Results Probl Cell Differ. 38:39–59. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grainger DJ: Transforming growth factor
beta and atherosclerosis: so far, so good for the protective
cytokine hypothesis. Arterioscler Thromb Vasc Biol. 24:399–404.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Björkerud S: Effects of transforming
growth factor-beta 1 on human arterial smooth muscle cells in
vitro. Arterioscler Thromb. 11:892–902. 1991.PubMed/NCBI
|
|
38
|
Deaton RA, Su C, Valencia TG and Grant SR:
Transforming growth factor-beta1-induced expression of smooth
muscle marker genes involves activation of PKN and p38 MAPK. J Biol
Chem. 280:31172–31181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hautmann MB, Madsen CS and Owens GK: A
transforming growth factor beta (TGFbeta) control element drives
TGFbeta-induced stimulation of smooth muscle alpha-actin gene
expression in concert with two CArG elements. J Biol Chem.
272:10948–10956. 1997. View Article : Google Scholar
|
|
40
|
Kawai-Kowase K, Sato H, Oyama Y, Kanai H,
Sato M, Doi H and Kurabayashi M: Basic fibroblast growth factor
antagonizes transforming growth factor-beta1-induced smooth muscle
gene expression through extracellular signal-regulated kinase 1/2
signaling pathway activation. Arterioscler Thromb Vasc Biol.
24:1384–1390. 2004. View Article : Google Scholar
|
|
41
|
Papetti M, Shujath J, Riley KN and Herman
IM: FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte
alpha-smooth muscle actin expression: a role for myf-5 and
Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci.
44:4994–5005. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kucich U, Rosenbloom JC, Abrams WR, Bashir
MM and Rosenbloom J: Stabilization of elastin mRNA by TGF-beta:
initial characterization of signaling pathway. Am J Respir Cell Mol
Biol. 17:10–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kucich U, Rosenbloom JC, Abrams WR and
Rosenbloom J: Transforming growth factor-beta stabilizes elastin
mRNA by a pathway requiring active Smads, protein kinase C-delta,
and p38. Am J Respir Cell Mol Biol. 26:183–188. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hong HH, Uzel MI, Duan C, Sheff MC and
Trackman PC: Regulation of lysyl oxidase, collagen, and connective
tissue growth factor by TGF-beta1 and detection in human gingiva.
Lab Invest. 79:1655–1667. 1999.PubMed/NCBI
|
|
45
|
Ross JJ and Tranquillo RT: ECM gene
expression correlates with in vitro tissue growth and development
in fibrin gel remodeled by neonatal smooth muscle cells. Matrix
Biol. 22:477–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yao L, Swartz DD, Gugino SF, Russell JA
and Andreadis ST: Fibrin-based tissue-engineered blood vessels:
differential effects of biomaterial and culture parameters on
mechanical strength and vascular reactivity. Tissue Eng.
11:991–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sinha S, Hoofnagle MH, Kingston PA,
McCanna ME and Owens GK: Transforming growth factor-beta1 signaling
contributes to development of smooth muscle cells from embryonic
stem cells. Am J Physiol Cell Physiol. 287:C1560–C1568. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kinner B, Zaleskas JM and Spector M:
Regulation of smooth muscle actin expression and contraction in
adult human mesenchymal stem cells. Exp Cell Res. 278:72–83. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang D, Park JS, Chu JS, Krakowski A, Luo
K, Chen DJ and Li S: Proteomic profiling of bone marrow mesenchymal
stem cells upon transforming growth factor beta1 stimulation. J
Biol Chem. 279:43725–43734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Collins T, Pober JS, Gimbrone MA Jr,
Hammacher A, Betsholtz C, Westermark B and Heldin CH: Cultured
human endothelial cells express platelet-derived growth factor A
chain. Am J Pathol. 126:7–12. 1987.PubMed/NCBI
|
|
51
|
Westermark B, Siegbahn A, Heldin CH and
Claesson-Welsh L: B-type receptor for platelet-derived growth
factor mediates a chemotactic response by means of ligand-induced
activation of the receptor protein-tyrosine kinase. Proc Natl Acad
Sci USA. 87:128–132. 1990. View Article : Google Scholar
|
|
52
|
Holmgren L, Glaser A, Pfeifer-Ohlsson S
and Ohlsson R: Angiogenesis during human extraembryonic development
involves the spatiotemporal control of PDGF ligand and receptor
gene expression. Development. 113:749–754. 1991.
|
|
53
|
Hyytiäinen M, Penttinen C and Keski-Oja J:
Latent TGF-beta binding proteins: extracellular matrix association
and roles in TGF-beta activation. Crit Rev Clin Lab Sci.
41:233–264. 2004.PubMed/NCBI
|
|
54
|
Derynck R and Akhurst RJ: Differentiation
plasticity regulated by TGF-beta family proteins in development and
disease. Nat Cell Biol. 9:1000–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liang MS and Andreadis ST: Engineering
fibrin-binding TGF-β1 for sustained signaling and contractile
function of MSC based vascular constructs. Biomaterials.
32:8684–8693. 2011.PubMed/NCBI
|
|
56
|
Gallagher JT: Heparan sulfate: growth
control with a restricted menu. J Clin Invest. 108:357–361. 2001.
View Article : Google Scholar : PubMed/NCBI
|