Introduction
Simian vacuolating virus 40 (SV40) is a member of
the polyomavirus family, which also includes the JC and BK viruses.
SV40 has icosahedral capsids and contains small, circular,
double-stranded DNA genomes. The early region is alternatively
spliced to produce the small t antigen and the large T antigen, a
large phosphoprotein that has a p53-binding domain and consequently
provides essential functions to promote cellular proliferation and
viral replication, in particular the rhesus monkey. Data on the
nature of SV40 infection in humans are available (1,2,3).
When administered in high doses, oncogenic SV40 is capable of
transforming rodent and human cells in culture (3,4). The
high rates of recovery of SV40 DNA sequences from cancer tissues
have indicated that SV40 infection may be important in the
development of mesothelioma, brain tumors, bone tumors,
osteosarcoma, non-Hodgkin’s lymphoma, choroid plexus tumors and
ependymomas (5). The process of
cellular transformation induced by SV40 typically depends on the
integration of the viral DNA into the host genome and a high level
of expression of the major viral oncogenic proteins, large T
antigen and small t antigen. The large T antigen is a
multifunctional protein with a DnaJ domain, a retinoblastoma
protein (Rb)-binding domain and a p53-binding domain, that are
involved in a wide range of cellular processes, including
transcriptional activation and repression, the inhibition of
differentiation, the stimulation of the cell cycle, repression of
apoptosis and cell transformation. In particular, it can bind to
and perturb p53, Rb, p107, p130, CBP/p300 and RASSF1A, causing
unregulated cell growth and cellular immortalization (2,6,7).
As transcription of the α-crystallin gene occurs
exclusively in the lens, we established the lens tumor model of αT3
transgenic mice using the lens-specific α-crystallin A promoter and
the oncogenic SV40 T antigen gene. In the mouse lens, there
appeared, sequentially, dysplasia (2 weeks pre-birth), then
carcinoma in situ (2 months post-birth), followed by
invasion inside the eyeball (4 months) and outside the eyeball (8
months), and final metastasis into the lymph node or lung (12
months). Gene chip analysis indicated that numerous aspects of cell
function markedly changed, including the cell cycle, cell
morphology, cell development and cell-to-cell signaling due to lens
carcinogenesis. Analyses of the top canonical pathways indicated
significant differences regarding the metabolism of carbohydrates,
amino acids, nucleotides, xenobiotics and nitrogen. Cluster
analysis demonstrated a marked distinction in cell proliferation
and cell death following invasion of the carcinoma. There were
significant alterations in cell growth, the cell cycle,
cell-to-cell signaling and metabolism once carcinoma metastasis had
occurred (8).
The autogenic lens tumor model provides a tool to
screen anti-tumor reagents in vivo. Juzen-taiho-to, a
Chinese medicine that combines 10 herbs, is employed as a
nutritional agent to improve disturbances and imbalances in the
homeostasis of the body. It is used to help alleviate general
symptoms, including extreme fatigue, a pale complexion, loss of
appetite, dry or scaly skin, night sweating and dryness of the
mouth in postsurgical patients, and in patients with chronic
illnesses (9). Additionally, it
has been demonstrated to suppress tumors by enhancing phagocytosis,
cytokine induction and antibody production, and inducing the
mitogenic activity of spleen cells. In the present study, we
administered Juzen-taiho-to orally to SV40 T antigen transgenic
mice initiated by the α-crystallin promoter in order to investigate
its anti-tumor effects and the underlying mechanisms using gene
chip analysis.
Materials and methods
Animals
αT3 transgenic mice with α-crystallin/SV40 T antigen
generated on the background of wild-type FVB were produced and
maintained by our group (8). The
mice were housed in plastic cages with paper chips for bedding,
three mice per house, in pathogen-free conditions in a
temperature-controlled animal room with a 12 h light/dark
illumination cycle. All had access to standard rodent food (CE-2;
CLEA Japan, Tokyo, Japan) and water ad libitum.
Juzen-taiho-to (Tsumura, Tokyo, Japan; 1.5%) containing 10 herbs
(Astragalus membranaceus, 10.5%; ginseng, 10.5%;
cassia, 10.5%; angelica, 10.5%; rhizoma ligustici
wallichii, 10.5%; peony, 10.5%; prepared rhizome of
rehmannia, 10.5%; Atractylodes macrocephala, 10.5%;
Tuckahoe, 10.5%; liquorice, 5.5%) was mixed into the
food at the rate of 15% from 4 weeks following birth (1200–1800
mg/kg/day). Animal use procedures were in accordance with the Guide
for the Care and Use of Laboratory Animals and approved by the
Committee on Animal Experimentation of Liaoning Medical University
(Jinzhou, Liaoning, China), Kanagawa Cancer Center (Yokohama,
Kanagawa, Japan) and Kawasaki Medical School (Kurasiki, Okayama,
Japan).
Samples
After the animals were sacrificed under sodium
pentobarbital anesthesia, eyeballs, spleens and livers were
dissected at 15, 23 and 32 weeks following the oral administration
of Juzen-taiho-to, respectively. The samples of eyeball, spleen and
liver were immediately frozen in liquid nitrogen and then stored at
−80°C until use. Total RNA was extracted from frozen tissues using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. The quality of the
RNA was assessed by running aliquots on denatured agarose gels.
Affymetrix GeneChip hybridization
For the investigation of gene expression, the
MM8-60mer-exp-X 4 array (NimbleGen, Madison, WI, USA) was applied,
which represents over 24,000 transcripts. Sample preparation was
performed according to the manufacturer’s instructions. Briefly, 10
μg of total RNA was used to synthesize double-stranded
complementary DNA with a GeneChip® Expression
3′-Amplification Reagents One-Cycle cDNA Synthesis kit (Affymetrix,
Santa Clara, CA, USA). cDNA labeled with Cy3 using a labeling kit
(NimbleGen) was hybridized to a GeneChip array at 42°C for 16 h.
The chip was washed and scanned with GenePix Personal 4100A
(NimbleGen) and the results were analyzed using the Subio Platform
(Affymetrix). Hybridization intensity data were converted into
presence/absence calls for each gene and changes in gene expression
between the experiments were detected by comparison analysis. A
difference of two-fold or more was applied to select upregulated
and downregulated genes.
Network, gene ontology and canonical
pathway analysis
Gene accession numbers were imported into DAVID
Bioinformatics Resources 6.7 (http://david.abcc.ncifcrf.gov/tools.jsp) from the
National Institute of Allergy and Infectious Diseases (NIAID;
Bethesda, MD, USA). Hierarchical clustering was used to determine
the expression consistency between the paired control and
drug-treated samples. The gene products were categorized based on
location (UP_TISSUE), cellular components (GOTERM_CC_FAT) and
reported or suggested biochemical, biological and molecular
functions (SP_PIR_ KEYWORDS). Mapping to available genetic pathways
was also performed by ranking by score the probability that a
collection of genes equal to or greater than the number in a
network could be achieved by chance alone according to the Kyoto
Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/pathway.html).
Real-time PCR
In total, 10 genes were selected for further
validation by real-time PCR. Total cDNA was synthesized using
TaqMan reverse transcription reagents (Applied Biosystems, Tokyo,
Japan). PCR reactions and analyses were conducted using an Mx3000P
QPCR System (Stratagene, La Jolla, CA, USA) with denaturation for
10 min at 95°C, followed by 50 cycles of denaturation at 95°C for
30 sec, annealing at 55°C for 1 min and extension at 72°C for 30
sec. The amount of mRNA was calculated using GAPDH as an
endogenous control. All primers were designed as follows: epidermal
growth factor receptor (Egfr; NM_007912, 2139–2338; 200 bp),
forward 5′-GCCACGCCAACTGTACCTAT-3′ and reverse
5′-GCAAGAGGGCAGAATCTGAG-3′; Rasgrf1 (NM_011245, 1910–2109;
200 bp), forward 5′-CCCTTCACTGTCATCCTGGT-3′ and reverse
5′-TTGCTGAAGCGAATGTCAAC-3′; heat shock protein 1B (Hspa1b;
NM_ 010478, 2206–2398; 193 bp), forward 5′-TGCACTTGATAGCTGCTTGG-3′
and reverse 5′-CAGTGCTGCTCCCAACATTA-3′; Rps25 (NM_024266,
190–388; 199 bp), forward 5′-TCGACAAAGCGACATACGAC-3′ and reverse
5′-CCACCC TTTGTGTTTCTGGT-3′; GAPDH (NM_ 008084, 895–998; 104
bp), forward 5′-GCGACTTCA ACAGCAACT-3′ and reverse
5′-GTATTCATTGTCATACCAGGAAATG-3′.
Statistical analysis
Statistical evaluation was performed using the
Mann-Whitney U test to analyze the means of different groups.
Kaplan-Meier survival plots were generated and comparisons made
with the log-rank statistic. P<0.05 was considered to indicate a
statistically significant difference. SPSS 10.0 software was
employed to analyze all data.
Results
Nutrient levels and survival
No significant differences in body weight, size of
the lens tumor and liver or spleen weight were identified in αT3
transgenic mice with or without exposure to Juzen-taiho-to
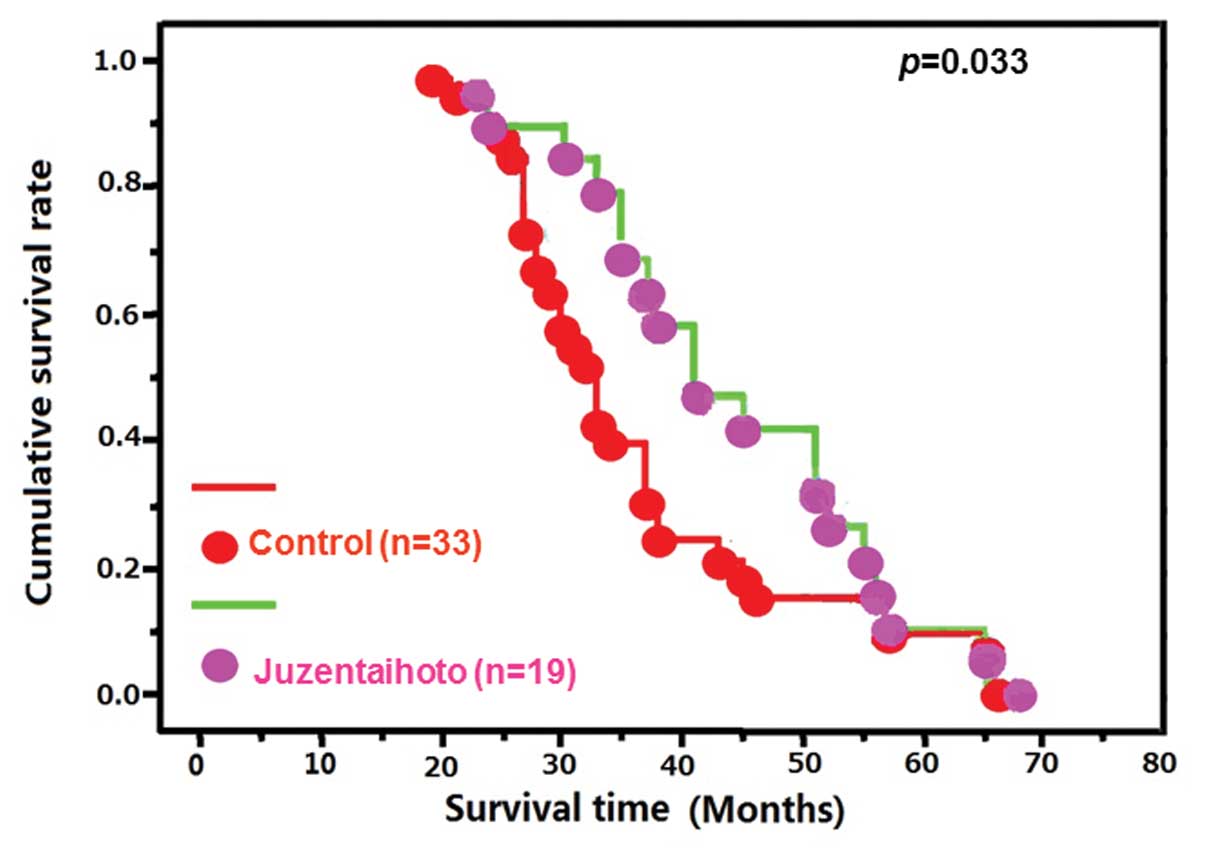
(P>0.05, data not shown). However, the αT3 mice demonstrated a
higher cumulative survival rate following exposure to
Juzen-taiho-to than the control group (P<0.05; Fig. 1).
Tissue expression, functional network,
gene ontology and pathway analysis
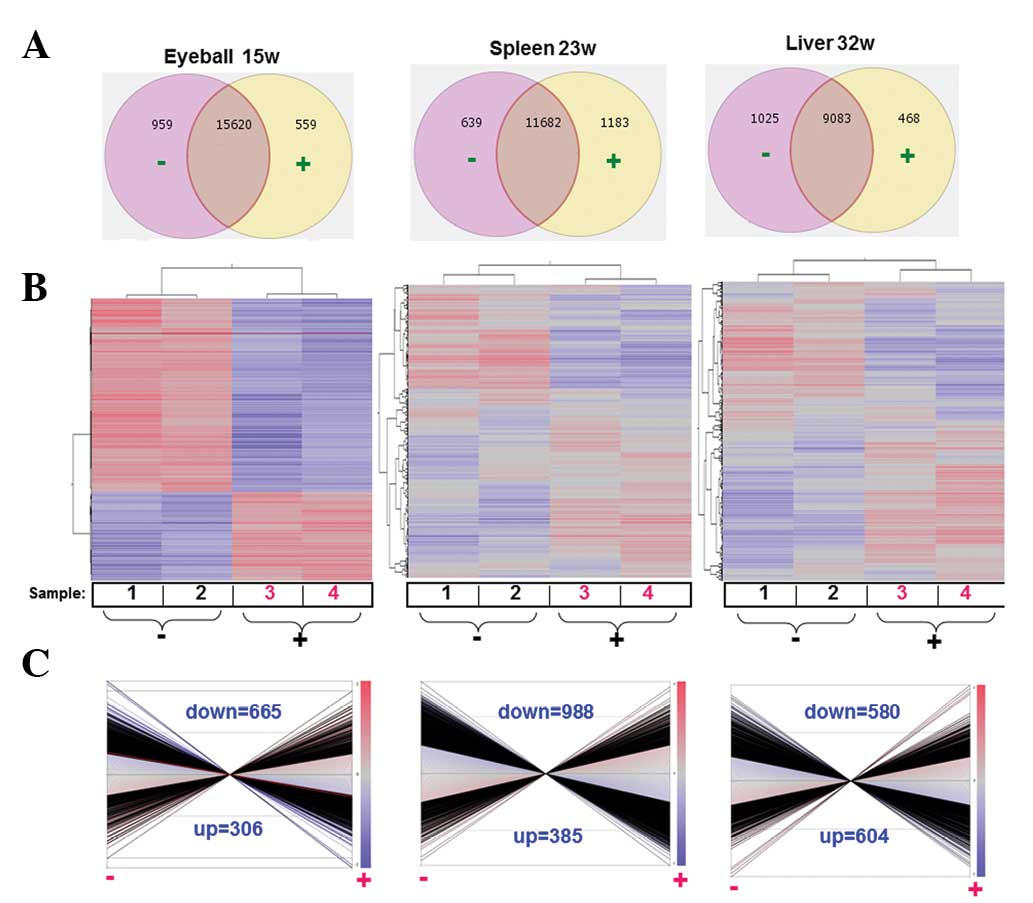
We selected the genes with raw signals above 300 for
all samples and further considered the genes with stable expression
(15,620 genes for the lens tumor; 11,682 genes for the spleen;
9,083 genes for the liver) in the control samples or the
Juzen-taiho-to-exposed samples (½<group 1:group 2 [ratio]<2;
P>0.05) for cluster analysis (Fig.
2A and B). Differentially expressed genes were identified to
analyze the tissue overexpression, function, gene ontology and
pathways between the control and the drug groups
(½>control:Juzen-taiho-to [ratio]>2; P<0.05, Fig. 2C).
As shown in Table
I, the altered genes of the lens tumor, spleen and liver were
mainly derived from the brain, erythroleukemia and liver,
respectively. As Table II
indicates, numerous aspects of the cell functions of lens tumors
were markedly altered, including membrane, glycoproteins, the cell
membrane, signal and ionic channel. Cells of the spleen exhibited
changes in the cell cycle, DNA replication, homeobox, mitosis and
cell division. The top functional changes in the
Juzen-taiho-to-treated liver were in acetylation, mitochondria, Rb
and the ribonucleoprotein. As shown in Table III, the gene ontology of altered
genes in the lens tumor included the plasma membrane, plasma
membrane part and intrinsic to membrane. Those from the spleen
consisted of the replication fork, cytoskeleton, cytoskeletal part,
chromosomal part and chromosome. The top gene ontology of altered
genes in the liver comprised of the mitochondra, ribosome,
ribonucleoprotein complex, mitochondrial part and ribosomal
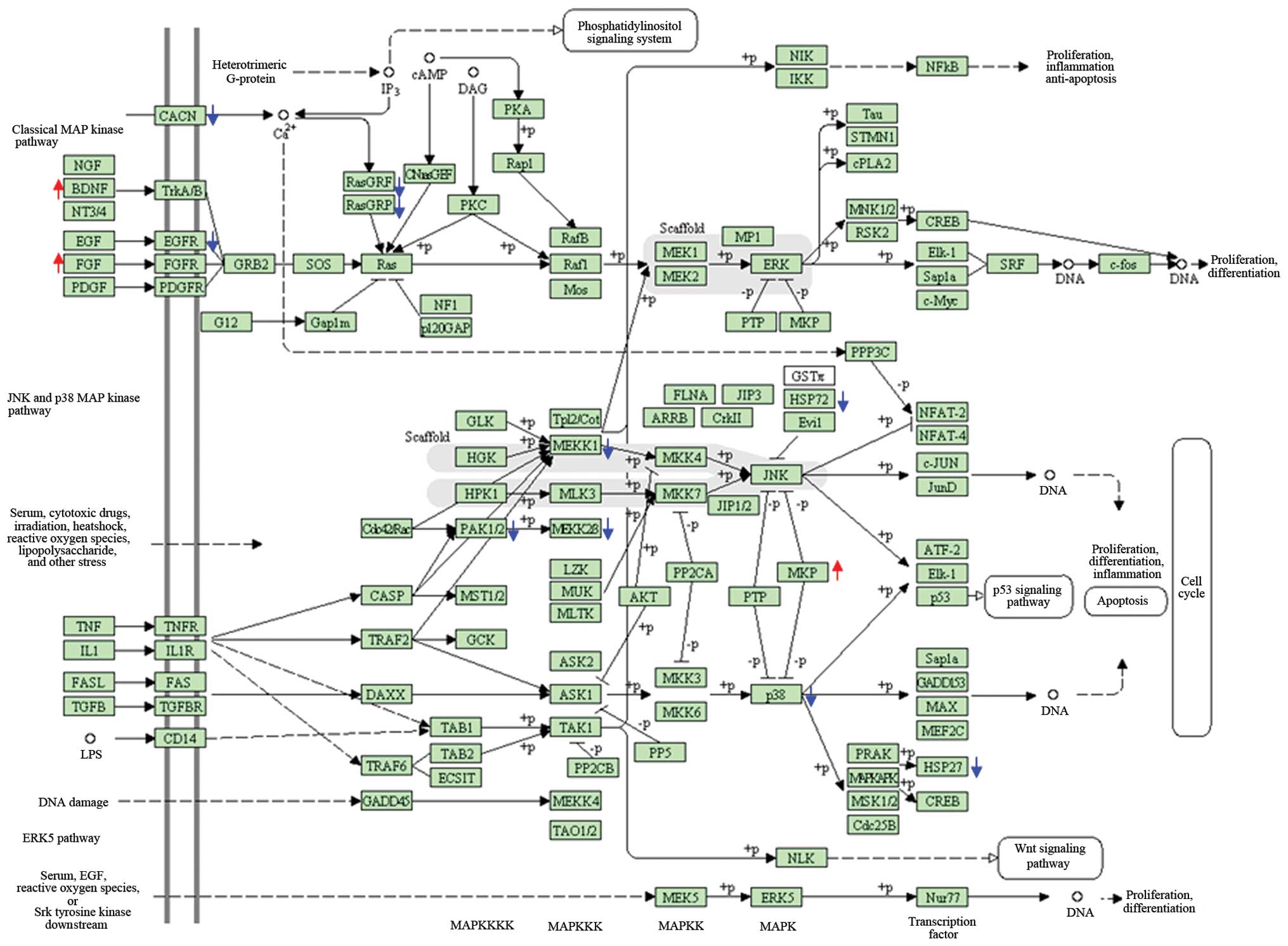
subunit. The important canonical pathways were MAPK (21 genes), the
cell cycle (18 genes) and ribosome (13 genes) for the altered genes
of the lens tumor, spleen and liver following Juzen-taiho-to
administration, respectively (Fig.
3).
 | Table ITissue expression of altered genes in
the lens tumor, spleen and liver of αT3 transgenic mice. |
Table I
Tissue expression of altered genes in
the lens tumor, spleen and liver of αT3 transgenic mice.
| Organ | Term | Count | P-value |
|---|
| Eyeball | Brain | 329 | 2.53E-04 |
| Cerebellum | 87 | 6.76E-04 |
| Medulla
oblongata | 37 | 6.88E-04 |
| Spinal cord | 35 | 0.002195 |
| Eye | 80 | 0.002768 |
| Spleen | Erythroleukemia | 6 | 0.00132 |
| Spleen | 57 | 0.002081 |
| Olfactory
epithelium | 24 | 0.020422 |
| Inner ear | 18 | 0.025074 |
| Submaxillary
gland | 3 | 0.025362 |
| Liver | Liver | 241 | 8.58E-19 |
| Kidney | 168 | 2.43E-16 |
| Mammary tumor | 193 | 4.25E-16 |
| Mammary gland | 151 | 9.43E-12 |
| Bone marrow | 111 | 5.15E-11 |
 | Table IIFunctional analysis of altered genes
in the lens tumor, spleen and liver of αT3 transgenic mice. |
Table II
Functional analysis of altered genes
in the lens tumor, spleen and liver of αT3 transgenic mice.
| Organ | Term | Count | P-value |
|---|
| Eyeball | Membrane | 328 | 1.14E-17 |
| Glycoprotein | 232 | 6.84E-15 |
| Cell membrane | 122 | 4.03E-10 |
| Signal | 177 | 2.43E-08 |
| Ionic channel | 35 | 5.78E-08 |
| Spleen | Cell cycle | 43 | 1.30E-07 |
| DNA replication | 14 | 2.35E-05 |
| Homeobox | 25 | 1.02E-04 |
| Mitosis | 18 | 6.39E-04 |
| Cell division | 22 | 0.001093 |
| Liver | Acetylation | 190 | 1.99E-18 |
| Mitochondrion | 85 | 3.38E-14 |
| Ribosomal
protein | 35 | 2.65E-13 |
|
Ribonucleoprotein | 43 | 4.58E-13 |
| Protein
biosynthesis | 23 | 1.67E-07 |
 | Table IIIGene ontology of altered genes in the
lens tumor, spleen and liver of αT3 transgenic mice. |
Table III
Gene ontology of altered genes in the
lens tumor, spleen and liver of αT3 transgenic mice.
| Organ | Term | Count | P-value |
|---|
| Eyeball | Plasma
membrane | 176 | 1.59E-07 |
| Plasma membrane
part | 105 | 1.23E-05 |
| Neuron
projection | 26 | 4.77E-05 |
| Axon | 15 | 1.74E-04 |
| Intrinsic to
membrane | 290 | 6.28E-04 |
| Spleen | Replication
fork | 7 | 2.11E-04 |
| Cytoskeleton | 63 | 0.001393 |
| Cytoskeletal
part | 47 | 0.001429 |
| Chromosomal
part | 23 | 0.004578 |
| Chromosome | 26 | 0.00466 |
| Liver | Mitochondrion | 132 | 1.80E-20 |
| Ribosome | 37 | 7.94E-14 |
| Ribonucleoprotein
complex | 52 | 8.17E-10 |
| Mitochondrial
part | 53 | 2.22E-08 |
| Ribosomal
subunit | 16 | 1.13E-07 |
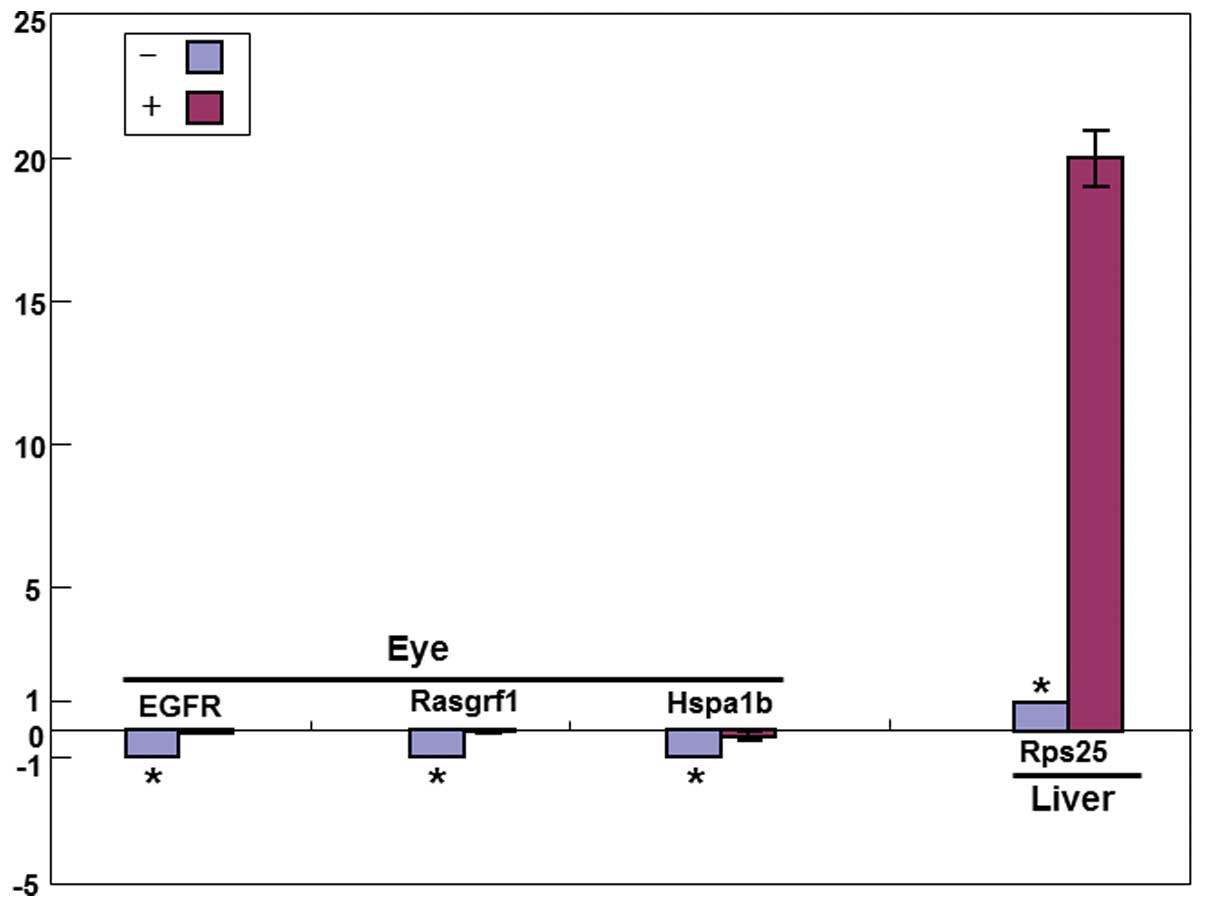
Validation of cDNA microarray gene
expression and functional changes by real-time PCR
To further validate the alterations in gene
expression by cDNA microarray, we designed the primers and probes
for real-time PCR quantification based on the nucleotide sequences
of the clones spotted on the microarray. Gene expression patterns
identified by real-time PCR were similar to those determined by
microarray analyses for these genes. For the lens tumor,
significantly less mRNA for Egfr, Rasgrf1 and
Hspa1b was found in mice treated with Juzen-taiho-to than in
the controls. By contrast, Rps25 mRNA was more abundant in
the livers of treated mice compared with the controls (Fig. 4).
Discussion
Lens tumors in transgenic mice can be easily
observed, however, they exert little effect on mouse survival.
Consequently, this model is useful for screening reagents that aim
to reverse the precancerous lesion and treat malignancies, as well
as for clarifying the molecular mechanisms of lens carcinogenesis
and its subsequent progression. Satoh et al (10) demonstrated that Juzen-taiho-to is
able to prolong survival time with advanced lung carcinoma, which
supports our data. It also provides an explanation for the higher
survival rate observed in mice treated with Juzen-taiho-to.
αT3 transgenic mice suffered from dysplasia 2 weeks
prior to birth and invasive carcinoma 4 months after birth.
Although the eyeball sample was not examined by hematoxylin and
eosin staining, there was a lens tumor in the eyeball when it was
removed 15 weeks after the treatment with Juzen-taiho-to. In the
eyeball, Juzen-taiho-to exposure significantly weakened the MAPK
pathway. It was noted that Juzen-taiho-to may lead to downregulated
expression of the majority of the genes involved in the MAPK
pathway. Gene chip analysis and quantitative PCR indicated that
levels of Egfr, Rasgrf1 and Hspa1b mRNA were
significantly lower in the lenses of mice exposed to
Juzen-taiho-to, although there was an increased expression of the
stimulatory factors, brain-derived neurotrophic factor (BDNF) and
fibroblast growth factor (FGF). The MAPK pathway is reported to be
a chain of proteins that communicates a signal from a receptor on
the membrane surface to the nuclear DNA and it is involved in
various cellular functions, including cell proliferation,
differentiation and migration. Overall, the extracellular mitogen
binds to the membrane ligand (for example, epidermal growth factor
receptor; EGFR), which allows Ras (GTPase) to swap its GDP for a
GTP, which, in turn, activates GBR2, MAPK and the transcription
factor (11). Functionally, the
differentially expressed genes belong to membrane proteins or
signal transduction proteins, as demonstrated in the MAPK pathway
and gene ontology. As for tissue distribution, more significant
correlations were identified in the brain or the eye, which may be
due to dedifferentiation of the lens tumor or ectodermal
development. Niwa et al (12) demonstrated that Juzen-taiho-to had
an inhibitory effect on E2-related endometrial carcinogenesis in
mice, relevantly through suppression of estrogen-induced c-fos/jun
expression. Another group documented the inhibitory effect of the
oral administration of Juzen-taiho-to on experimental metastatic
liver and lung cells in vivo (13). Combined with our findings, it was
suggested that Juzen-taiho-to may inhibit the lens tumors induced
by the SV40 T antigen by suppressing the MAPK pathway.
The cell cycle is the series of events leading to
cellular division and duplication (replication) and includes the
G1, S, G2 and M phases. Two key classes of regulatory molecules,
cyclins and cyclin-dependent kinases (CDKs), determine the cell’s
progress by heterodimer formation through the cell cycle (14,15).
Juzen-taiho-to was reported to have protective effects on
hepatocarcinogenesis by impeding Kupffer cell-induced oxidative
stress (16). Additionally, immune
function may be increased via the enhancement of phagocytosis,
cytokine induction antibody production (17) and the modulation of T-cell
responses against ovalbumin towards more balanced T1/T2 responses
(18). In the present study, we
demonstrated that Juzen-taiho-to is able to upregulate the cell
cycle pathway. In the present study, the altered genes of the
spleen belonged to erythroleukemia and involved certain aspects of
cell function, including the cell cycle, DNA replication, homeobox,
mitosis and cell division following oral administration of
Juzen-taiho-to. These findings indicate that the herb
combination in Juzen-taiho-to induces protective effects against
lens tumorigenesis induced by the SV40 T antigen. It appears to
function by promoting immunity, possibly via the stimulation of
cell proliferation in the spleen.
The ribosome is an organelle (an internal component
of a biological cell) that functions to assemble the 20 specific
amino acid molecules to form the particular protein molecule
determined by the nucleotide sequence of an RNA molecule (19). In the liver, the altered genes
responding to Juzen-taiho-to were mainly in the liver and the
kidney, which have high levels of protein synthesis. Therefore, the
most significant pathway is ribosomal, as also indicated by
functional analysis and gene ontology. The phenomenon could be
explained by the upregulated liver intoxication under
Juzen-taiho-to stimulation. The higher level of protein synthesis
may also have protected the liver from more injury. We conclude
that there was no hepatic toxicity from the Juzen-taiho-to used to
treat the transgenic lens tumors of mice. Juzen-taiho-to may
prolong the survival time of transgenic mice with lens tumors
induced by the SV40 T antigen by improving the nutritional
condition of the mice, inhibiting the MAPK pathway (20) and strengthening immunity without
causing hepatic toxicity. However, the molecular targets of
Juzen-taiho-to require clarification in this model in future
investigations.
Acknowledgements
This study was supported by the Shenyang Outstanding
Talent Foundation of China, Shenyang Science and Technology Grant
(F11-264-1-10), the Natural Scientific Foundation of China (no.
81172371) and Grant-in aid for Scientific Research from the
Ministry of Education, Culture, Sports and Technology of Japan (no.
23659958 and no. 21790624).
References
|
1
|
Reddy VB, Thimmappaya B, Dhar R, et al:
The genome of simian virus 40. Science. 200:494–502. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ahuja D, Sáenz-Robles MT and Pipas JM:
SV40 large T antigen targets multiple cellular pathways to elicit
cellular transformation. Oncogene. 24:7729–7745. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poulin DL and DeCaprio JA: Is there a role
for SV40 in human cancer? J Clin Oncol. 24:4356–4365. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kroczynska B, Cutrone R, Bocchetta M, et
al: Crocidolite asbestos and SV40 are cocarcinogens in human
mesothelial cells and in causing mesothelioma in hamsters. Proc
Natl Acad Sci USA. 103:14128–14133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shah KV: SV40 and human cancer: a review
of recent data. Int J Cancer. 120:215–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mutti L, Carbone M, Giordano GG and
Giordano A: Simian virus 40 and human cancer. Monaldi Arch Chest
Dis. 53:198–201. 1998.PubMed/NCBI
|
|
7
|
Matker CM, Rizzo P, Pass HI, et al: The
biological activities of simian virus 40 large-T antigen and its
possible oncogenic effects in humans. Monaldi Arch Chest Dis.
53:193–197. 1998.PubMed/NCBI
|
|
8
|
Zheng HC, Nakamura T, Zheng Y, et al: SV40
T antigen disrupted the cell metabolism and the balance between
proliferation and apoptosis in lens tumors of transgenic mice. J
Cancer Res Clin Oncol. 135:1521–1532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saiki I: A Kampo medicine
“Juzen-taiho-to”--prevention of malignant progression and
metastasis of tumor cells and the mechanism of action. Biol Pharm
Bull. 23:677–688. 2000.
|
|
10
|
Satoh H, Ishikawa H, Ohtsuka M and
Sekizawa K: Japanese herbal medicine in patients with advanced lung
cancer: prolongation of survival. J Altern Complement Med.
8:107–108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niwa K, Hashimoto M, Morishita S, et al:
Preventive effects of Juzen-taiho-to on N-methyl-N-nitrosourea and
estradiol-17B-induced endometrial carcinogenesis in mice.
Carcinogenesis. 22:587–591. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Onishi Y, Yamaura T, Tauchi K, et al:
Expression of the anti-metastatic effect induced by Juzen-taiho-to
is based on the content of Shimotsu-to constituents. Biol Pharm
Bull. 21:761–765. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Satyanarayana A and Kaldis P: Mammalian
cell-cycle regulation: several Cdks, numerous cyclins and diverse
compensatory mechanisms. Oncogene. 28:2925–2939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Csikász-Nagy A, Palmisano A and Zámborszky
J: Molecular network dynamics of cell cycle control: transitions to
start and finish. Methods Mol Biol. 761:277–291. 2011.PubMed/NCBI
|
|
16
|
Tsuchiya M, Kono H, Matsuda M, Fujii H and
Rusyn I: Protective effect of Juzen-taiho-to on
hepatocarcinogenesis is mediated through the inhibition of Kupffer
cell-induced oxidative stress. Int J Cancer. 123:2503–2511. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamiyama H, Takano S, Ishikawa E, et al:
Anti-angiogenic and immunomodulatory effect of the herbal medicine
‘Juzen-taiho-to’ on malignant glioma. Biol Pharm Bull. 28:2111–116.
2005.PubMed/NCBI
|
|
18
|
Iijima K, Sun S, Cyong JC and Jyonouchi H:
Juzen-taiho-to, a Japanese herbal medicine, modulates type 1 and
type 2 T cell responses in old BALB/c mice. Am J Chin Med.
27:191–203. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rodnina MV and Wintermeyer W: The ribosome
as a molecular machine: the mechanism of tRNA-mRNA movement in
translocation. Biochem Soc Trans. 39:658–662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signaling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|