Introduction
Gastric cancer is the second most common malignant
tumor in the world (1) and the
third leading cause of cancer-related mortality in China (2). At present, surgical resection of the
primary tumor and control of lymph node metastasis are the main
types of treatment for early gastric cancer. There is still no
effective treatment for patients with distant metastasis or
recurrence. The outcome of unresectable or metastatic gastric
cancer is extremely poor, although chemotherapy has been
demonstrated to confer a benefit in terms of survival and quality
of life (3). Therefore, a better
understanding of the molecular mechanism underlying the development
and progression of gastric cancer is necessary for developing a
more effective treatment for this disease.
The Notch family consists of four Notch proteins
(Notch1, 2, 3 and 4), which can be activated by their ligands, the
Delta-like (DLL)-1, −3, −4, Jagged-1 and −2 proteins (4). The Notch signaling pathway is
evolutionarily conserved and regulates numerous cell processes,
including proliferation, differentiation and apoptosis during
development and tumorigenesis (5).
Notch signaling can be activated by a membrane-bound Notch ligand
and alterations of the pathway may cause malignancies, including
gastric cancer (6). Once Notch
signaling is activated, Notch is cleaved to release intracellular
Notch, which is associated with transcriptional factors regulating
the expression of target genes (7). Findings of recent studies have
demonstrated that the Notch signaling pathway is involved in the
development of human malignant tumors, such as breast, lung,
pancreas, basal cell and other carcinomas, and seems to function as
an oncogene or a tumor suppressor depending on the cellular context
(8,9).
Among proteins of the Notch pathway, Notch1 and its
ligand DLL1 were found to be expressed in eight gastric cancer cell
lines (10); Notch1 is expressed
in both premalignant and cancer tissues, especially in tissues of
intestinal metaplasia and well-differentiated intestinal gastric
cancer tissues. Thus, Notch1 is considered to play an important
role in both facilitating the metaplastic transition of gastric
epithelial cells and in maintaining the sustained proliferation of
intestinalized epithelial cells (11,12).
Notch1 expression is significantly higher in gastric cancer
compared to healthy gastric tissue and correlates with tumor size,
differentiation grade, depth of invasion and vessel invasion
(13). The three-year survival
rate is significantly higher in Notch1-negative than in
Notch1-positive patients (13).
The matrix metalloproteinase (MMP) family comprises
23 enzymes which degrade almost all components of the surrounding
tissue (14), thus promoting
cancer growth and invasion (15).
Of all MMPs, MMP-2 is one of the best predictors of the invasive
ability of tumor cells. Cyclin D1 is a critical cell cycle
regulatory protein, which is required for the progression of cancer
cells from the G1 to S phase (16,17).
Cyclooxygenase-2 (COX-2) is a rate-limiting enzyme involved in the
conversion of arachidonic acid to prostaglandins and thromboxanes
(18). Overexpression of COX-2 is
directly associated with various inflammatory diseases and several
carcinogenetic processes. COX-2 promotes tumor growth through the
induction of angiogenesis, inhibition of apoptosis, by increasing
tumor invasiveness, and suppressing the immune response (19). Patients expressing Jagged-1 in
gastric cancer tissues had a poor survival rate compared to those
with no Jagged-1 expression, and the activation of the Notch1
signaling pathway promoted the progression of gastric cancer, at
least in part via the induction of COX-2 expression (20).
Downregulation of Notch1 had antineoplastic effects
in vivo and in vitro (21–24),
however, the role of the Notch1 gene in the proliferative
and invasive ability of gastric cancer cells is not clear. In this
study, we investigated the role of Notch1 in the
proliferative and invasive ability of the gastric cancer SGC-7901
cells by examining the protein expression of cyclin D1, cyclin A1,
cyclin-dependent kinase 2 (CDK2), and the mRNA expression of
MMP-2 and COX-2 after silencing of the Notch1
gene by small interfering RNA (siRNA). We found that Notch1
silencing inhibits proliferation and invasion in SGC-7901 cells by
downregulating the expression of cyclin D1, cyclin A1, MMP-2
and COX-2. Our findings may contribute in revealing the
molecular mechanism underlying the involvement of Notch1 in gastric
cancer and provide a theoretical basis for developing a new
treatment for this disease.
Materials and methods
Materials and reagents
The human gastric cancer cell line SGC-7901 was
obtained from the Shanghai Institute for Biological Sciences
(Shanghai, China). RPMI-1640, fetal bovine serum (FBS) and trypsin
were obtained from HyClone (Logan, UT, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St.
Louis, MO, USA). Antibodies were purchased from the following
companies: anti-human cyclin D1, cyclin A1 and CDK2 from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA); anti-human β-actin,
goat-anti-mouse IgG, rabbit anti-goat IgG, goat anti-rabbit IgG
from Sigma-Aldrich. Notch1 and control siRNAs were obtained from
Santa Cruz Biotechnology, Inc. Transwell chambers were purchased
from Millipore (Billerica, MA, USA). Lipofectamine 2000 was
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
The PCR mix was obtained from Xi’an Runde Biotechnology Co., Ltd.
(Xi’an, Shaanxi, China). PCR primer sets were purchased from
DingGuo Biotechnology Co., Ltd. (Beijing, China).
Cell cultures and transfection
The human gastric cancer cell line SGC-7901 was
maintained in RPMI-1640 medium supplemented with 10% FBS, 100 μg/ml
ampicillin and 100 μg/ml streptomycin. The cells were incubated at
37°C in a humidified atmosphere containing 5% CO2. The
cells were divided into three groups for transfection:
non-transfected (normal) group, negative control group transfected
with a siRNA control (si-control group) and test group, transfected
with Notch1 siRNA (si-Notch1 group). siRNA sequences were as
follows: Notch-1, 5′-GCACGCGGAUUAAUUUGCATT-3′ and
5′-UGCAAAUUAAUCCGCGUGCTT-3′; negative control,
5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUG ACACGUUCGGAGAATT-3′.
Transfection was performed following the instructions of the
Lipofectamine 2000 kit.
Cell proliferation assays
Cells from the three experimental groups were seeded
in 96-well tissue culture plates at a density of 5,000–10,000
cells/well 24 h prior to serum starvation. After serum starvation
for 24 h, the cells were cultured in RPMI-1640 medium supplemented
with 10% FBS and incubated at 37°C. After 12, 24, 36, 48, 60 or 72
h, the medium was removed and MTT was added to each well and
incubated at 37°C for 4 h. Optical densities (OD) were measured at
492 nm on a microplate reader (BioTec Instruments Inc., Winooski,
VT, USA). The proliferation rate was defined as ODtest
plate/ODcontrol plate. Results from three separate
experiments are presented as means ± SD.
Cell invasion assays
The invasive ability of cells in each group was
assessed by a chamber-based invasion assay. The upper surface of a
filter (pore size, 8.0 μm; Millipore) was coated with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA). Prior to treatment, the
cells that had reached the log phase of growth were cultured for 24
h in 6-well plates in medium containing 1% FBS. The cells
(5×104) were suspended in RPMI-1640 medium containing 1%
FBS and then seeded in the top chamber, while the medium containing
20% FBS was added to the bottom chamber to induce the invasion of
the cancer cell line. The Matrigel invasion chamber was incubated
for 24 h in a humidified tissue culture incubator. Non-invading
cells were removed from the top of the Matrigel with a
cotton-tipped swab. Invading cells on the bottom surface of filter
were fixed in methanol and stained with crystal violet (Boster
Biological Technology Ltd., Wuhan, China). The invasive ability was
determined by counting the number of stained cells under a light
microscope. The cell invasion assay was performed in
triplicate.
Western blotting assays
SGC-7901 cells were lysed in situ with RIPA
buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 0.5% sodium deoxycholate,
1.0% Triton X-100, 0.1% SDS), supplemented with protease inhibitors
(Roche Diagnostics, Mannheim, Germany) and phosphatase inhibitors
(Sigma-Aldrich). Cell lysates were centrifuged at 12,000 × g for 20
min at 4°C to remove debris. Proteins (100 μg) were separated on a
10% SDS-PAGE gel and transferred to PVDF membranes (Roche
Diagnostics). the PVDF membranes were initially blocked with 5%
nonfat dry milk in Tris-buffered saline (TBS) for 2 h and incubated
with primary antibodies overnight at 4°C. After washing with TBS
and Tween-20 solution (TBST; pH 7.4) five times, each for 10 min,
the PVDF membranes were incubated with HRP-conjugated secondary
antibodies at room temperature for 2 h. The membranes were washed
again with TBST and an enhanced chemiluminescence (ECL) kit
(Gentaur, Santa Clara, CA, USA) was used to develop the
immunoblots.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer’s
protocol and RT was performed using a PrimeScript™ RT reagent kit
(Takara, Dalian, China). cDNAs (1 ml for each sample) were
amplified by PCR using the primers: Notch1, forward: 5′-GCA GTT GTG
CTC CTG AAG AA-3′ and reverse: 5′-CGG GCG GCC AGA AAC-3′; MMP-2,
forward: 5′-GTG CCC AAA GAA AGG TGC TG-3′ and reverse: 5′-AGG AGG
GGA GCC ATC CAT AG-3′; COX-2, forward: 5′-ATC CTT GCT GTT CCC ACC
CA-3′ and reverse: 5′-CTT TGA CAC CCA AGG GAG TC-3′; GAPDH,
forward: 5′-GTA AAG ACC TCT ATG CCA TCA-3′ and reverse: 5′-GGA CTC
ATC GTA CTC CTG CT3-3′. The glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) gene served as the normalization
control. RT-PCR products were resolved by 1.5% agarose gel
electrophoresis. The results were analyzed and photographed using a
UV transilluminator. Each measurement was carried out in
triplicate.
Statistical analysis
Results are shown as means ± standard error.
Differences were evaluated with unpaired two-tailed Student’s
t-tests with unequal variance for multiple comparisons using the
SPSS software, version 16.0 (SPSS, Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Experiments were repeated independently at least three times.
Results
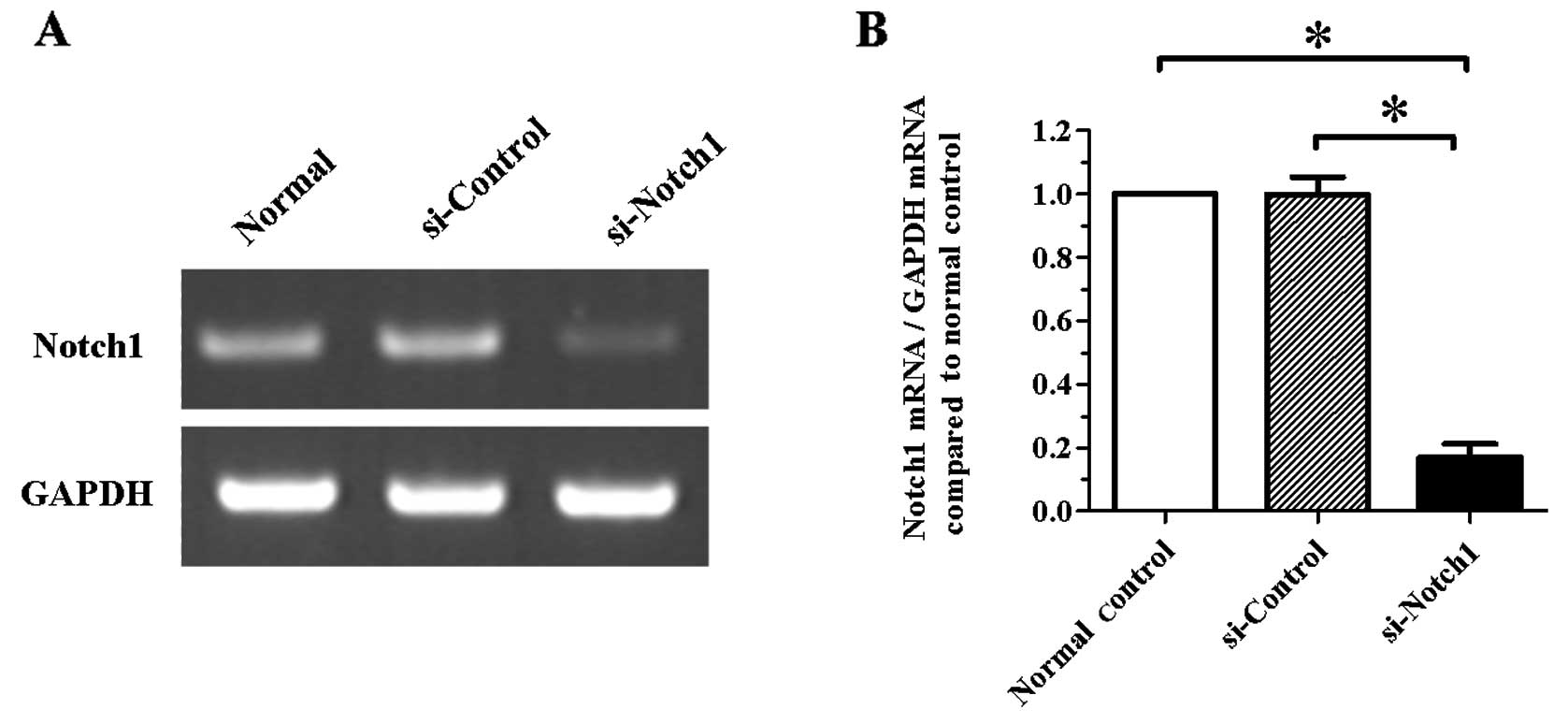
Expression of Notch1 is significantly
inhibited by a Notch1-specific siRNA in SGC-7901 cells
In a first set of experiments, we examined the
silencing efficiency of a specific siRNA targeting the
Notch1 gene in SGC-7901 cells. Following cell transfection
for 24 h, Notch1 silencing was confirmed by RT-PCR. As shown
in Fig. 1, the mRNA level of
Notch1 was significantly reduced in the si-Notch1 group
compared to the si-control group (P<0.05).
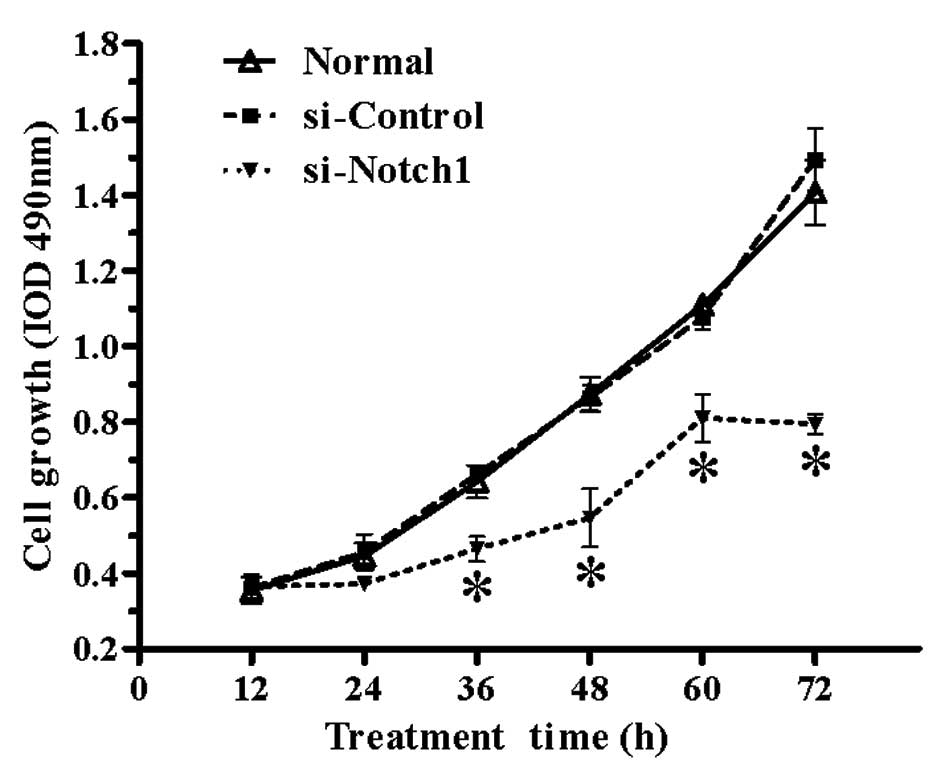
Proliferation rate of SGC-7901 cells is
significantly impaired by Notch1 silencing
To investigate the effect of Notch1 silencing
on gastric cancer cell proliferation, non-transfected and
si-RNA-transfected SGC-7901 cells were seeded in 96-well plates,
incubated for 12, 24, 36, 48, 60 or 72 h, and their proliferation
rate was investigated by the MTT assay. As shown in Fig. 2, the proliferation rate of SGC-7901
cells was markedly reduced by Notch1 silencing compared to
the normal and si-control groups from 36 h onwards (P<0.05).
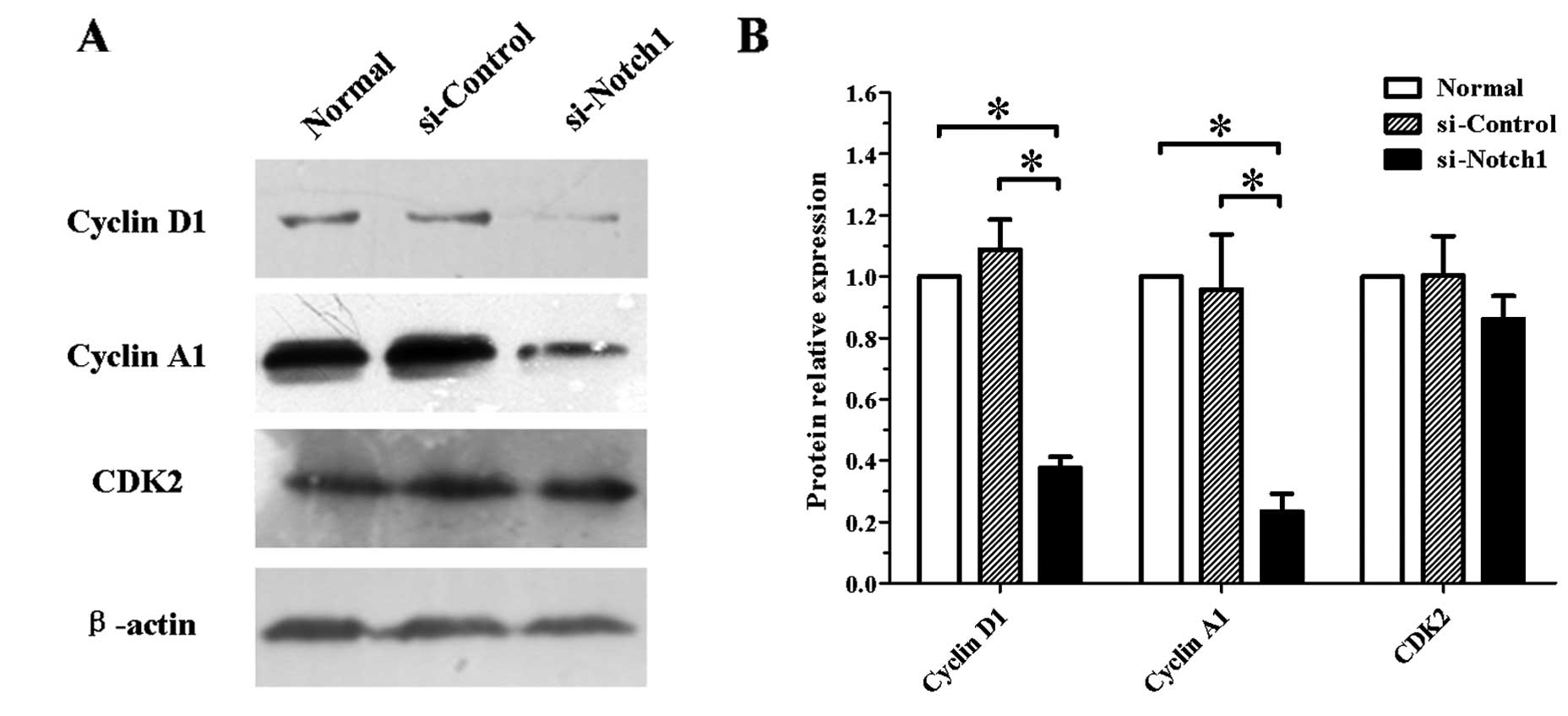
Effect of Notch1 silencing on the
expression of cell cycle-related proteins in SGC-7901 cells
The effect of Notch1 silencing on the
expression of cell cycle-related proteins in SGC-7901 cells was
assessed. Following transfection with siRNA for 48 h, total cell
protein extracts were subjected to western blotting. As shown in
Fig. 3, Notch1-silenced
SGC-7901 cells showed reduced expression of cyclin D1 and A1. By
contrast, the protein expression of CDK2 remained unchanged.
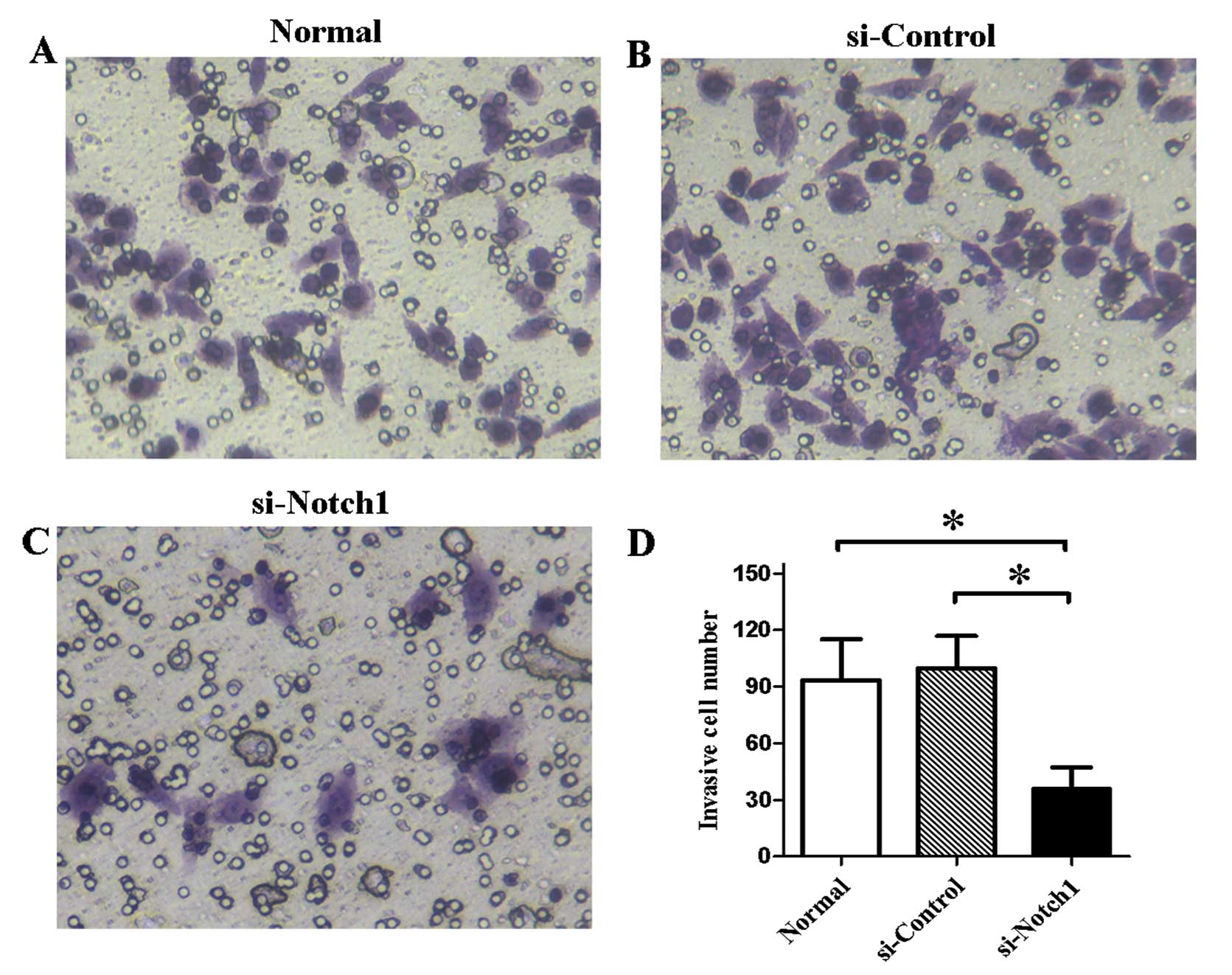
Invasive ability of SGC-7901 cells is
inhibited by Notch1 silencing
We tested the effect of Notch1 silencing on
the invasive ability of SGC-7901 cells in vitro. The results
of the Transwell assay showed that the number of invasive cells was
significantly reduced (P<0.05) compared to the non-transfected
cells or the control siRNA-treated cells (Fig. 4).
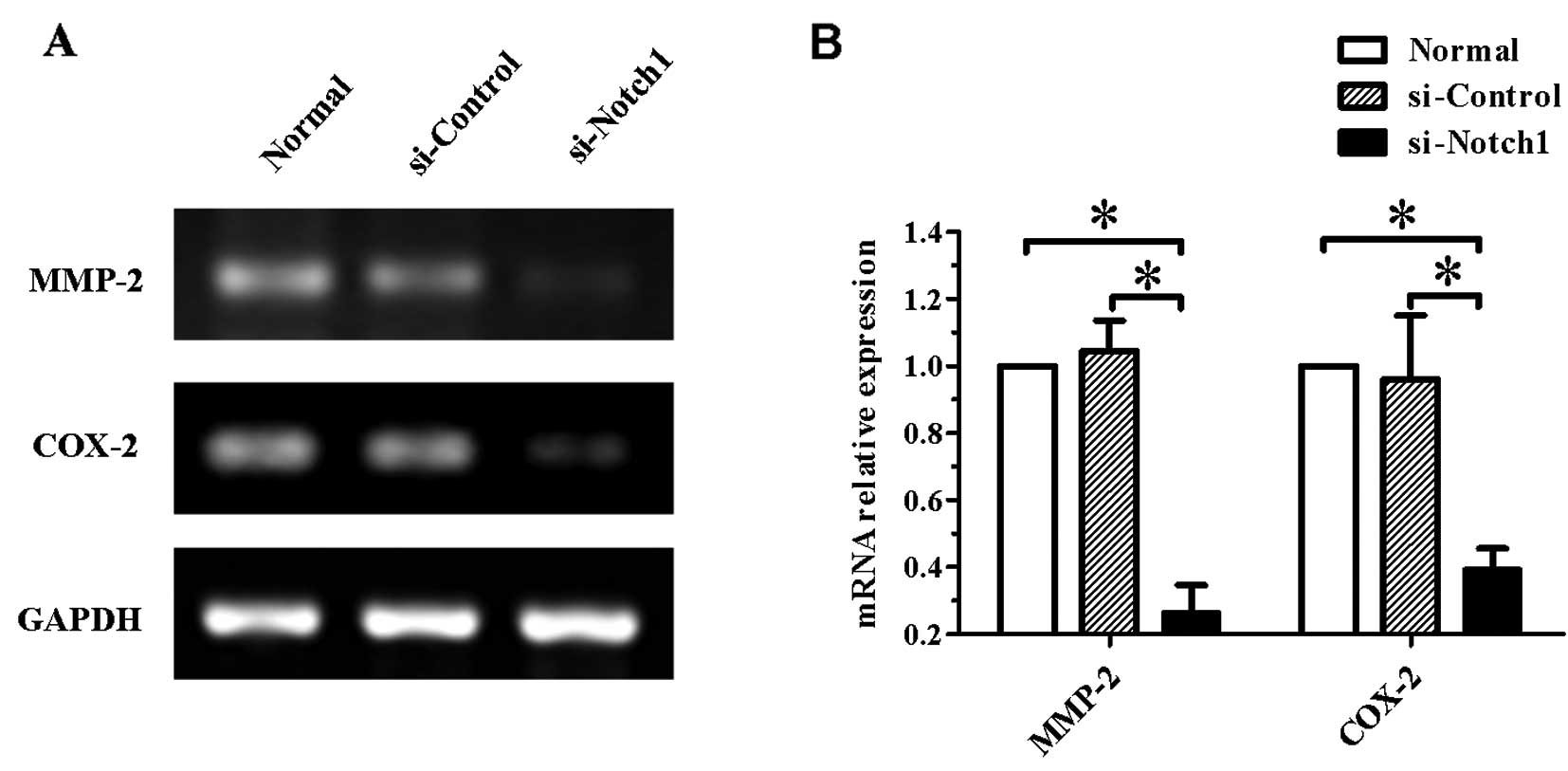
Gene expression of MMP-2 and COX-2 is
decreased in Notch1-silenced SGC-7901 cells
Since the impairment of the cell invasive ability is
commonly due to the modulation of expression of invasion-related
genes, to confirm whether the expression of such genes was affected
by Notch1 silencing, we examined the expression of
MMP-2 and COX-2 genes. As shown in Fig. 5, the mRNA levels of the two genes
were significantly reduced in Notch1-silenced cells
(P<0.05).
Discussion
To reveal the effect of the Notch1 protein on the
proliferative and invasive ability of gastric cancer SGC-7901
cells, we examined the expression of cyclin D1, cyclin A1, CDK2,
MMP-2 and COX-2 after silencing of the Notch1 gene by siRNA.
We found that the Notch1-specific siRNA significantly reduced the
expression of the Notch1 gene and decreased the expression
of cell cycle-related proteins (cyclin D1, cyclin A1 and CDK2) and
invasion-related genes (MMP-2 and COX-2), thus
attenuating the proliferation and invasion rates of SGC-7901
cells.
The MTT assays demonstrated that silencing of
Notch1 attenuates the rate of SGC-7901 cell proliferation.
We found that the expression of cyclin D1, cyclin A1 and CDK2 was
significantly decreased after silencing of the Notch1 gene
by siRNA, which suggests that Notch signaling mediates the
proliferation and differentiation of SGC-7901 cells by directly or
indirectly regulating the expression of cell cycle-related genes. A
recent study has shown that Notch signaling is involved in the
differentiation from gastric epithelium to foveolar glands in
normal gastric mucosa (25). It is
noteworthy that Notch signaling is associated with glandular
differentiation not only in normal gastric mucosa, but also in
gastric carcinoma cells. Notch1, 2 and 3 were also detected in
human gastric cancer tissue (25).
A previous study indicated that Notch1 functions as a
tumor-suppressor gene in mammalian skin tissue and that
Notch1 silencing leads to epidermal and corneal hyperplasia,
followed by the development of skin tumors, while it can also
promote chemical-induced skin carcinogenesis (26). Furthermore, activation of the
Notch1 receptor was shown to facilitate the colony-forming ability
and xenografted tumor growth of human pancreatic adenocarcinoma
(8).
In this study, inhibition of Notch1 gene
expression by a specific siRNA led to a significant decrease in the
invasive ability of gastric cancer cells, accompanied by the
downregulation of MMP-2 and COX-2 genes, suggesting
that the Notch/MMP-2/COX-2 signaling pathway regulates the invasive
ability of gastric cancer cells by adjusting the expression levels
of invasion-related genes. Previous studies suggested that MMPs
degrade the extracellular matrix of tumor cells to allow them to
invade the surrounding tissue (27,28)
and that COX-2 promotes angiogenesis, inhibits apoptosis, increases
tumor invasiveness and suppresses immune responses to cause
tumorigenesis (19). However,
COX-2 expression is an independent prognostic factor of gastric
cancer (29).
In conclusion, the silencing of Notchl
significantly inhibited the proliferative and invasive ability of
the gastric cancer cell line SGC-7901, indicating that the Notch
signaling pathway plays an important role in the proliferation and
invasion of gastric cancer. Our findings provide a basis for
developing new therapies targeting gastric cancer.
Acknowledgements
This work was financially supported by Grants from
the National Natural Science Foundation of China (No. 81172362 and
No. 81101874), and the Scientific and Technological Development
Research Project Foundation by Shaanxi Province (No.
2012K19-04-02). We are grateful to Dr Xuqi Li (Xi’an Jiaotong
University, Xi’an, China) for providing expert opinions on methods
of our study and his useful comments. We thank Medjaden Bioscience
Ltd. for assisting in the preparation of this manuscript.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu
L and He J: Report of incidence and mortality in China cancer
registries, 2009. Chin J Cancer Res. 25:10–21. 2013.
|
|
3
|
Oba K, Paoletti X, Bang YJ, Bleiberg H,
Burzykowski T, Fuse N, Michiels S, Morita S, Ohashi Y, Pignon JP,
et al: Role of chemotherapy for advanced/recurrent gastric cancer:
an individual-patient-data meta-analysis. Eur J Cancer.
49:1565–1577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borggrefe T and Liefke R: Fine-tuning of
the intracellular canonical Notch signaling pathway. Cell Cycle.
11:264–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
South AP, Cho RJ and Aster JC: The
double-edged sword of Notch signaling in cancer. Semin Cell Dev
Biol. 23:458–464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai Q, Andreu-Agullo C, Insolera R, Wong
LC, Shi SH and Lai EC: BEND6 is a nuclear antagonist of Notch
signaling during self-renewal of neural stem cells. Development.
140:1892–1902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mullendore ME, Koorstra JB, Li YM,
Offerhaus GJ, Fan X, Henderson CM, Matsui W, Eberhart CG, Maitra A
and Feldmann G: Ligand-dependent Notch signaling is involved in
tumor initiation and tumor maintenance in pancreatic cancer. Clin
Cancer Res. 15:2291–2301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bolos V, Mira E, Martinez-Poveda B, Luxan
G, Canamero M, Martinez-A C, Manes S and de la Pompa JL: Notch
activation stimulates migration of breast cancer cells and promotes
tumor growth. Breast Cancer Res. 15:R542013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piazzi G, Fini L, Selgrad M, Garcia M,
Daoud Y, Wex T, Malfertheiner P, Gasbarrini A, Romano M, Meyer RL,
et al: Epigenetic regulation of Delta-Like1 controls Notch1
activation in gastric cancer. Oncotarget. 2:1291–1301.
2011.PubMed/NCBI
|
|
11
|
Wang Z, Li Y and Sarkar FH: Notch
signaling proteins: legitimate targets for cancer therapy. Curr
Protein Pept Sci. 11:398–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Y, Gao X, Liu J, Kong QY, Wang XW,
Chen XY, Wang Q, Cheng YF, Qu XX and Li H: Differential Notch1 and
Notch2 expression and frequent activation of Notch signaling in
gastric cancers. Arch Pathol Lab Med. 135:451–458. 2011.PubMed/NCBI
|
|
13
|
Li DW, Wu Q, Peng ZH, Yang ZR and Wang Y:
Expression and significance of Notch1 and PTEN in gastric cancer.
Ai Zheng. 26:1183–1187. 2007.(In Chinese).
|
|
14
|
Li X, Ma Q, Xu Q, Duan W, Lei J and Wu E:
Targeting the cancer-stroma interaction: a potential approach for
pancreatic cancer treatment. Curr Pharm Des. 18:2404–2415. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shuman Moss LA, Jensen-Taubman S and
Stetler-Stevenson WG: Matrix metalloproteinases: changing roles in
tumor progression and metastasis. Am J Pathol. 181:1895–1899.
2012.PubMed/NCBI
|
|
16
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li T, Kon N, Jiang L, Tan M, Ludwig T,
Zhao Y, Baer R and Gu W: Tumor suppression in the absence of
p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell.
149:1269–1283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng HH, Kuo CC, Yan JL, Chen HL, Lin WC,
Wang KH, Tsai KK, Guven H, Flaberg E, Szekely L, et al: Control of
cyclooxygenase-2 expression and tumorigenesis by endogenous
5-methoxytryptophan. Proc Natl Acad Sci USA. 109:13231–13236. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Moraes E, Dar NA, de Moura Gallo CV and
Hainaut P: Cross-talks between cyclooxygenase-2 and tumor
suppressor protein p53: balancing life and death during
inflammatory stress and carcinogenesis. Int J Cancer. 121:929–937.
2007.PubMed/NCBI
|
|
20
|
Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC,
Li AF, Wang AM, Kuo ML and Chi CW: The activated Notch1 signal
pathway is associated with gastric cancer progression through
cyclooxygenase-2. Cancer Res. 69:5039–5048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Portanova P, Notaro A, Pellerito O,
Sabella S, Giuliano M and Calvaruso G: Notch inhibition restores
TRAIL-mediated apoptosis via AP1-dependent upregulation of DR4 and
DR5 TRAIL receptors in MDA-MB-231 breast cancer cells. Int J Oncol.
43:121–130. 2013.PubMed/NCBI
|
|
22
|
Zhou H, Luo Y, Chen JH, Hu J, Luo YZ, Wang
W, Zeng Y and Xiao L: Knockdown of TRB3 induces apoptosis in human
lung adenocarcinoma cells through regulation of Notch 1 expression.
Mol Med Rep. 8:47–52. 2013.PubMed/NCBI
|
|
23
|
Kristoffersen K, Villingshoj M, Poulsen HS
and Stockhausen MT: Level of Notch activation determines the effect
on growth and stem cell-like features in glioblastoma multiforme
neurosphere cultures. Cancer Biol Ther. 14:625–637. 2013.
View Article : Google Scholar
|
|
24
|
Yabuuchi S, Pai SG, Campbell NR, de Wilde
RF, De Oliveira E, Korangath P, Streppel MM, Rasheed ZA, Hidalgo M,
Maitra A and Rajeshkumar NV: Notch signaling pathway targeted
therapy suppresses tumor progression and metastatic spread in
pancreatic cancer. Cancer Lett. 335:41–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang H, An HJ, Song JY, Kim TH, Heo JH,
Ahn DH and Kim G: Notch3 and Jagged2 contribute to gastric cancer
development and to glandular differentiation associated with MUC2
and MUC5AC expression. Histopathology. 61:576–586. 2012.PubMed/NCBI
|
|
26
|
Nicolas M, Wolfer A, Raj K, Kummer JA,
Mill P, van Noort M, Hui CC, Clevers H, Dotto GP and Radtke F:
Notch1 functions as a tumor suppressor in mouse skin. Nat Genet.
33:416–421. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fanjul-Fernandez M, Folgueras AR, Fueyo A,
Balbin M, Suarez MF, Fernandez-Garcia MS, Shapiro SD, Freije JM and
Lopez-Otin C: Matrix metalloproteinase Mmp-1a is dispensable for
normal growth and fertility in mice and promotes lung cancer
progression by modulating inflammatory responses. J Biol Chem.
288:14647–14656. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaimal R, Aljumaily R, Tressel SL, Pradhan
RV, Covic L, Kuliopulos A, Zarwan C, Kim YB, Sharifi S and Agarwal
A: Selective blockade of matrix metalloprotease-14 with a
monoclonal antibody abrogates invasion, angiogenesis, and tumor
growth in ovarian cancer. Cancer Res. 73:2457–2467. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi H, Xu JM, Hu NZ and Xie HJ: Prognostic
significance of expression of cyclooxygenase-2 and vascular
endothelial growth factor in human gastric carcinoma. World J
Gastroenterol. 9:1421–1426. 2003.PubMed/NCBI
|