Introduction
Bronchial asthma is an allergic disease manifesting
with symptoms such as wheezing, breathlessness, chest tightness and
coughing (1). Cardinal features of
the disease are airway hyper-responsiveness (AHR), inflammation and
remodeling (2). Airway remodeling
refers to structural changes of the airway wall arising from
unresolved inflammation, including epithelial denudation,
subepithelial fibrosis, mucus gland hypertrophy, myofibroblast and
smooth muscle proliferation and angiogenesis (3–5).
These structural changes play important roles in the
pathophysiology of asthma. In patients with severe asthma, airway
remodeling is associated with airway thickening, airway flow
limitation and AHR (6).
Naringenin is a naturally occurring flavonoid
possessing anti-inflammatory and antiproliferative activities
(7,8). We have previously demonstrated that
naringenin attenuates acute inflammation and AHR in
allergen-induced murine models of asthma (9). There are also reports that naringenin
can inhibit neointimal hyperplasia and smooth muscle cell
proliferation (10,11). Therefore, we hypothesized that
naringenin may interfere with the structural changes occurring in
chronic airway diseases. In the present study, we investigated the
effects of naringenin on airway remodeling in a murine model of
asthma.
Materials and methods
Antigen sensitization, challenge and
naringenin treatment
Female BALB/c mice (6–8 weeks old) were purchased
from the Shanghai Laboratory Animal, Inc. (Shanghai, China). The
experimental procedures involving these mice were approved by the
Nanjing Medical University and followed the guidelines of the
Institutional Animal Care and Use Committee. Naringenin
(Sigma-Aldrich, St. Louis, MO, USA) was dissolved in
dimethylsulfoxide to obtain a final concentration of 0.1 mg/μl (367
mM), used as the stock solution.
Mice were randomly divided into four groups
according to the difference of antigen in sensitization and
treatment before challenge, namely control group, ovalbumin (OVA)
group, dexamethasone group and naringenin group. Dexamethasone was
purchased from Tianyao Company (Hubei, China) and was used at a
concentration of 1 mg/ml. On days 0, 7 and 14, mice of the OVA,
dexamethasone and naringenin groups were immunosensitized by
intraperitoneal injection of 100 μg chicken egg OVA (grade V;
Sigma-Aldrich) adsorbed to 100 μl of Imject Alum adjuvant (Pierce
Biotechnology, Inc., Rockford, IL, USA). From day 21, the mice were
challenged with 1% OVA aerosolized for 30 min/day, 3 days/week for
8 weeks. Mice of the dexamethasone and naringenin groups received
intraperitoneal injections of dexamethasone (5 mg/kg body weight)
and naringenin (50 mg/kg body weight) 1 h prior to each OVA
challenge. The dose of naringenin was selected based on our
previous study (9). Mice of the
negative control group were sham-sensitized and -challenged using
phosphate-buffered saline (PBS).
Airway physiology
Airway responsiveness was measured 24 h after the
last OVA challenge. The mice were anesthetized, tracheostomized and
placed in a whole-body plethysmograph (Synol High-Tech, Beijing,
China). Then, mice were ventilated via an intratracheal tube at 90
breaths/min. Methacholine was administered through the caudal vein.
The maximal values of lung resistance were measured after the
administration of increasing doses of methacholine (10–200
μg/kg).
Analysis of bronchoalveolar lavage fluid
(BALF) and serum
The airway lumina were washed 3 times with 0.5 ml of
PBS. The BALF was centrifuged and the upper fluid sample was
retained for IL-4 and IL-13 detection. The total number of
different cell types in the BALF was estimated using Wright’s
staining and a haemocytometer; at least 200 cells were counted.
Serum IgE levels were measured with the OptEIA™ ELISA kit (BD
Biosciences, San Diego, CA, USA).
Lung histology and periodic acid schiff
(PAS) staining
The left lungs, collected 24 h after the last OVA
challenge, were fixed in formalin, embedded in paraffin, sectioned
and stained with hematoxylin and eosin solution and PAS.
Blind-scoring histological analyses of the four groups was
performed by pathologists. To quantify the mucus production in the
airways, the number of PAS-negative and -positive epithelial cells
(goblet cells) in individual bronchioles was counted as previously
described (12). The percentage of
PAS-positive cells was calculated as the number of PAS-positive
epithelial cells per bronchiole divided by the total number of
epithelial cells.
Immunohistochemical detection of α-smooth
muscle actin (α-SMA)
Sections were deparaffinized and rehydrated in
graduated alcohol solutions. Endogenous peroxidase activity was
blocked by incubation with 3% H2O2. Specimens
were flooded with 5% normal goat serum to prevent non-specific
absorption of immunoglobulin. Specimens were then incubated with
mouse anti-human α-SMA moloclonal antibody (Dako, Glostrup,
Denmark). For the negative control, the primary antibody was
replaced with normal rabbit IgG. The slides were incubated
overnight and rinsed with PBS, then incubated with
peroxidase-labeled goat anti-mouse IgG (Dako) for 30 min at 37°C,
washed again with PBS, and stained with diaminobenzidine (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The sections were
counterstained with hematoxylin and eosin, dehydrated and observed
under a light microscope. At least 10 bronchioles were counted in
each of the slides. Data were expressed as the immunostained α-SMA
area per length of the basement membrane of bronchioles (internal
diameter, 150–200 μm2).
Assessment of peribronchial fibrosis
The total collagen content of the lung was
determined according to the protocol of a commercial hydroxyproline
detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). The peribronchial area was stained with Masson’s trichrome
staining and quantified using a light microscope attached to an
image-analysis system (Simple PCI, version 5.2; Leica, Mannheim,
Germany). At least 10 bronchioles were counted in each of the
slides. Data were expressed as the stained area per length of the
basement membrane of bronchioles.
Statistical analysis
Data were presented as means ± SEM. Statistical
comparisons were performed with one-way ANOVA using the SPSS 16.0
software (IBM SPSS Statistics, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Naringenin attenuates chronic airway
inflammation
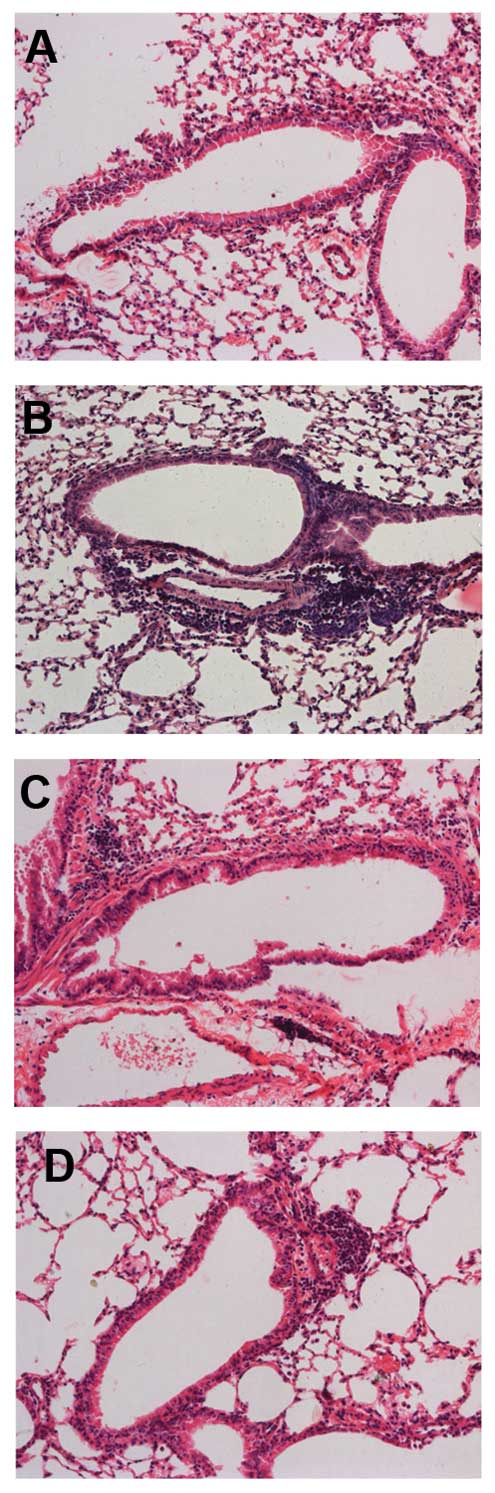
Marked influxes of inflammatory cells into the
airway and around the blood vessels were observed in the
OVA-sensitized and -challenged mice, but not in the control mice.
Mice treated with naringenin and dexamethasone showed marked
reductions in the infiltration of inflammatory cells compared to
the OVA-treated group (Fig.
1).
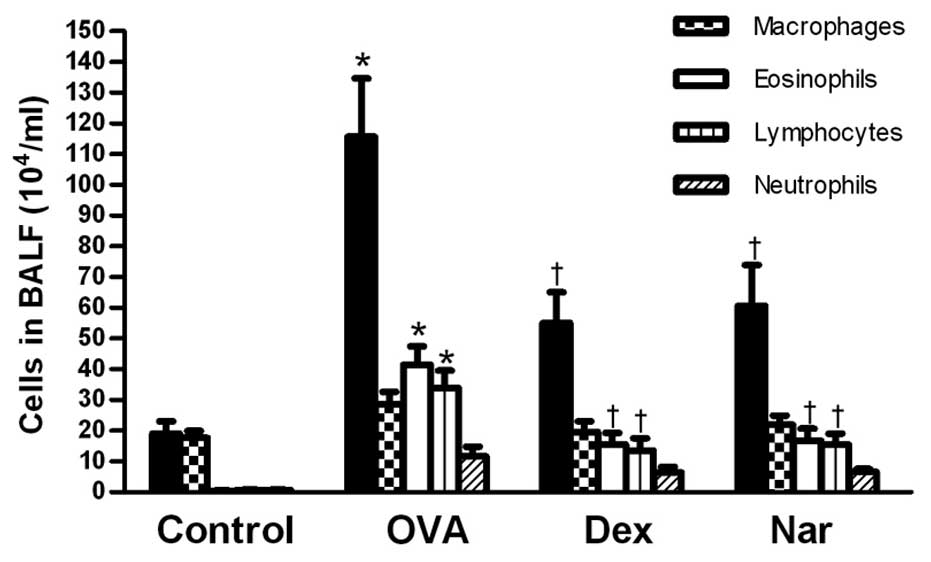
Following sensitization and challenge with OVA, the
number of total leukocytes, eosinophils and lymphocytes were
significantly increased compared to those of the control mice.
Treatment with naringenin and dexamethasone significantly reduced
the number of eosinophils and total inflammatory cells in the BALF
(Fig. 2).
Naringenin decreases AHR in a model of
chronic asthma
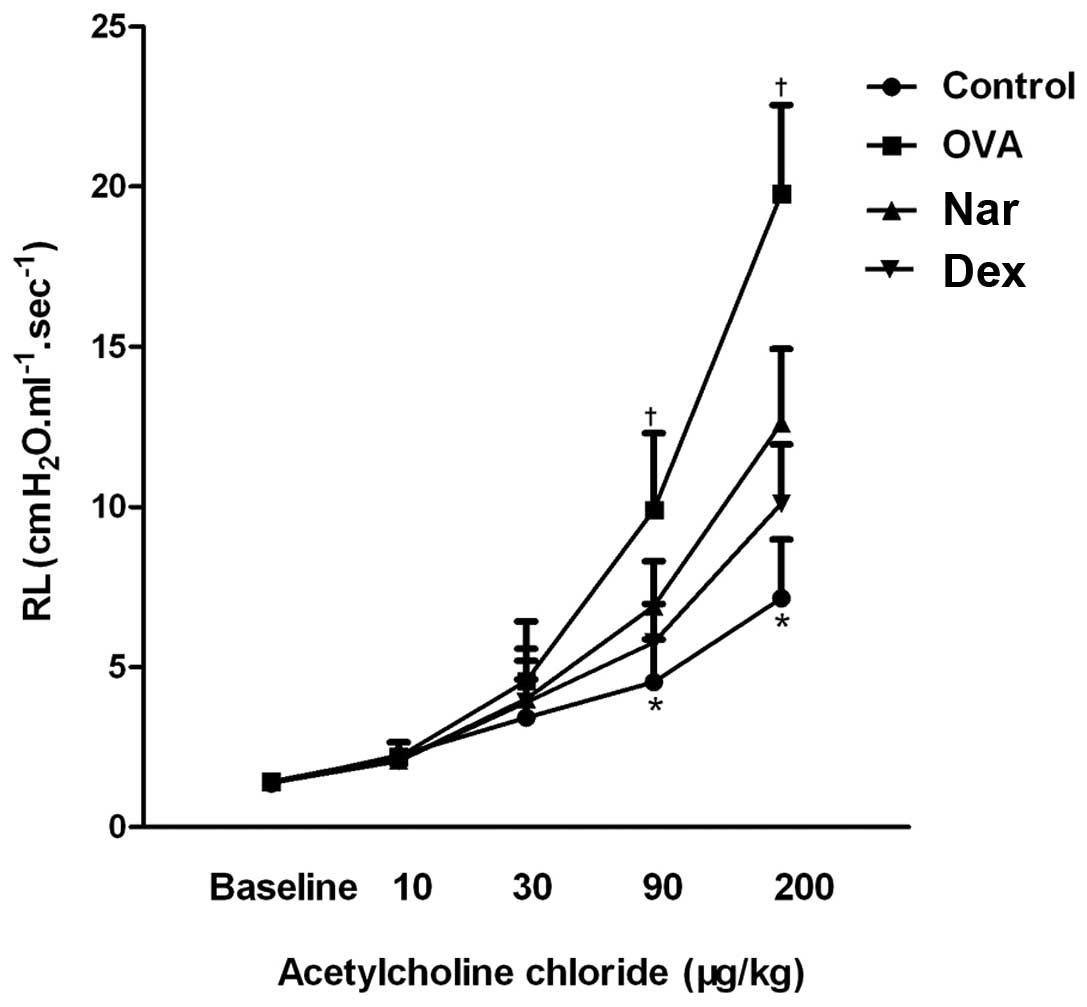
OVA treatment resulted in a marked increase in lung
resistance values relative to those of the control group. This
increase was reverted by treatment with dexamethasone and
naringenin, especially at the 90 and 200 μg/kg methacholine doses
(Fig. 3).
Naringenin reduces the level of T helper
2 (Th2) cytokines in BALF and that of total serum IgE
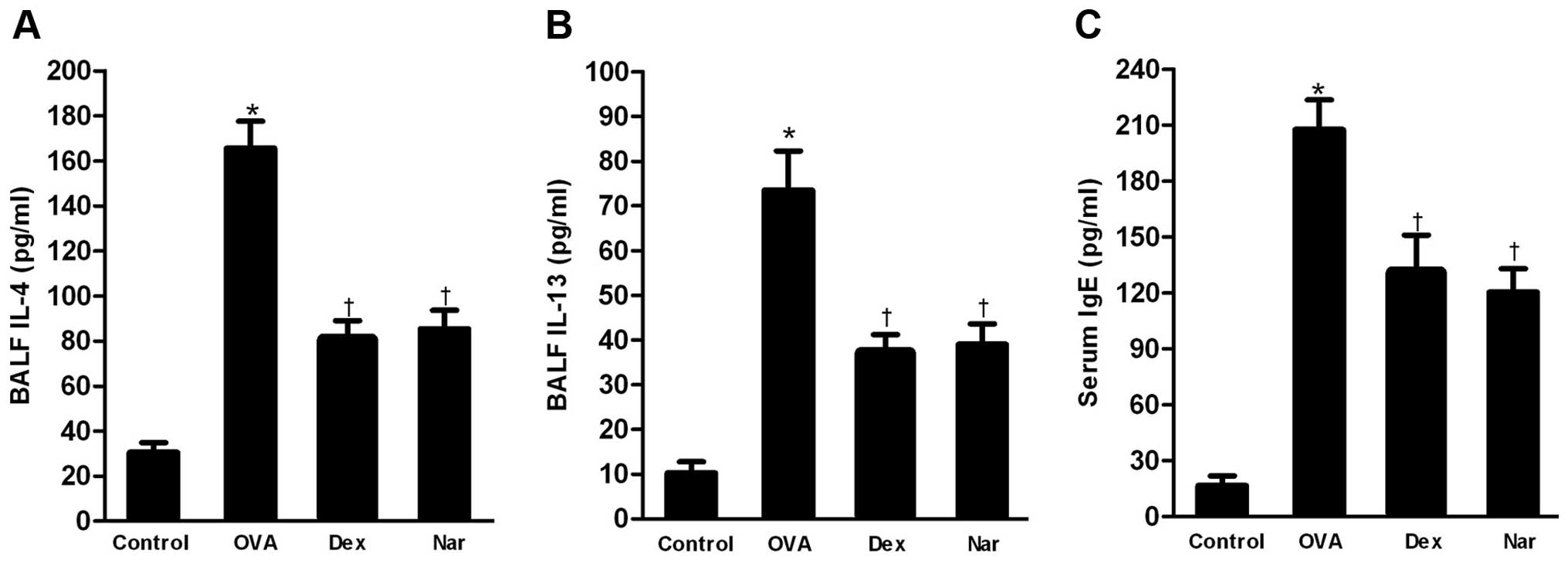
The levels of Th2 cytokines IL-4 and IL-13 in BALF
and that of total serum IgE were markedly increased in the
OVA-sensitized and -challenged mice compared to those in the
control group. The administration of naringenin and dexamethasone
significantly reduced the levels of Th2 cytokines (Fig. 4A and B) in BALF and total serum IgE
(Fig. 4C) relative to those in the
OVA group.
Naringenin inhibits airway remodeling in
a model of chronic asthma
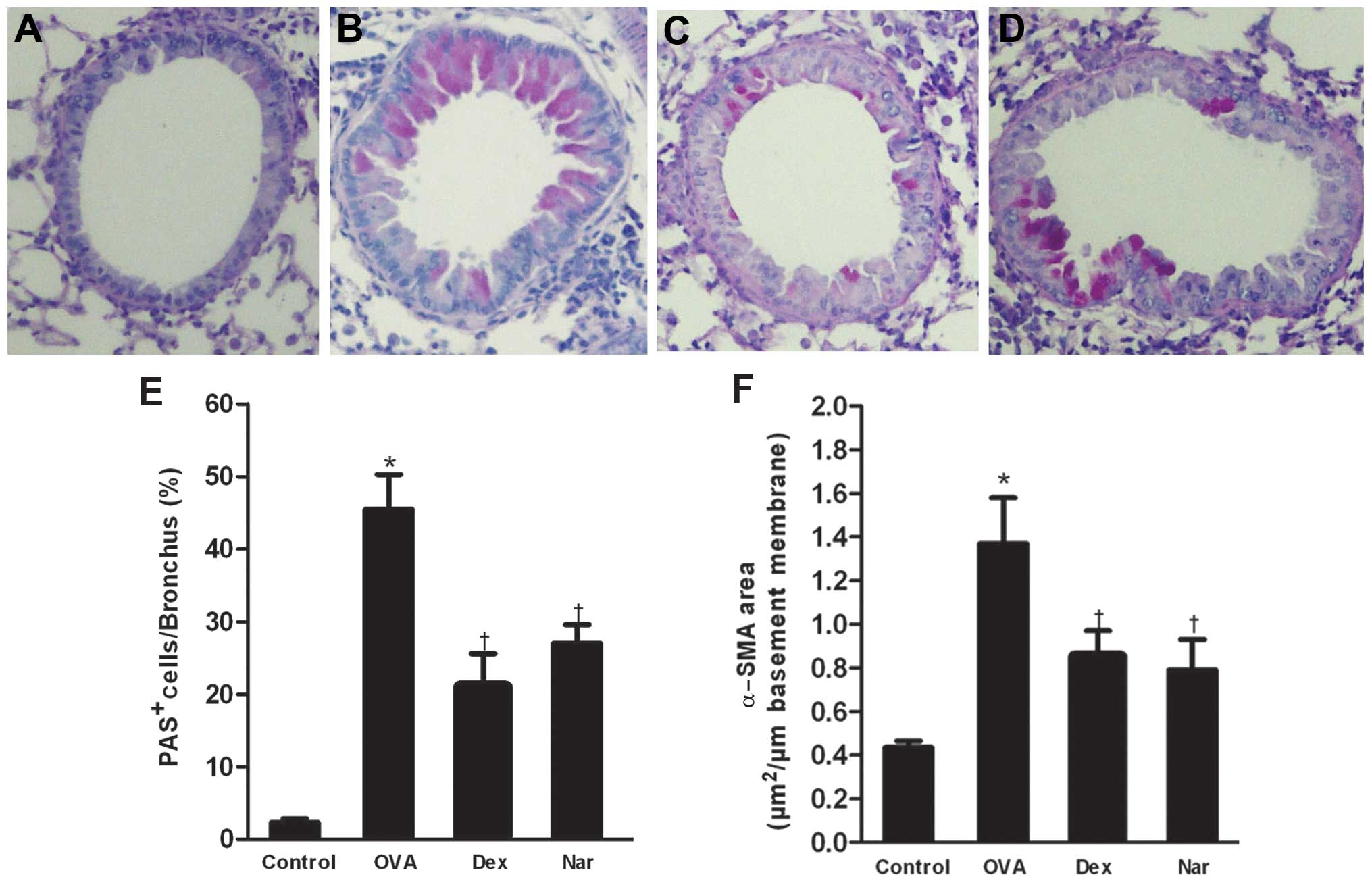
The level of airway metaplasia and mucus production
was assessed by PAS staining of lung tissues and the percentage of
PAS-positive cells was determined. Overproduction of mucus and
goblet cell hyperplasia were observed in the bronchial airways of
mice in the OVA group. By contrast, dexamethasone- and
naringenin-treated mice showed a reduction in the number of
PAS-stained goblet cells (Fig.
5).
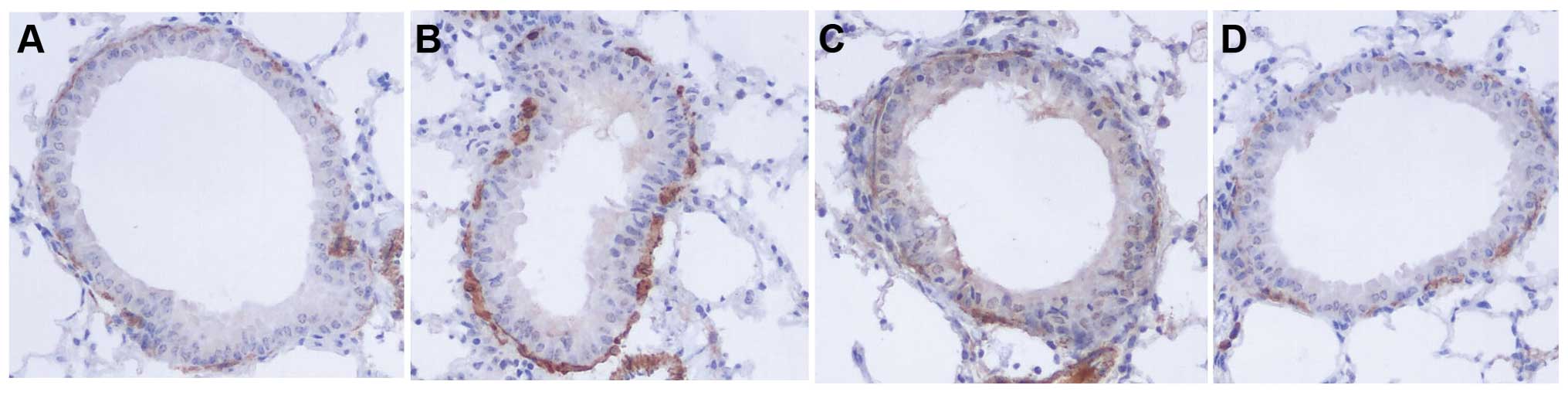
The areas of α-SMA-stained smooth muscle layers in
OVA mice were significantly larger compared to control mice.
Administration of naringenin and dexamethasone reduced the areas of
α-SMA compared to the OVA group (Figs.
5F and 6).
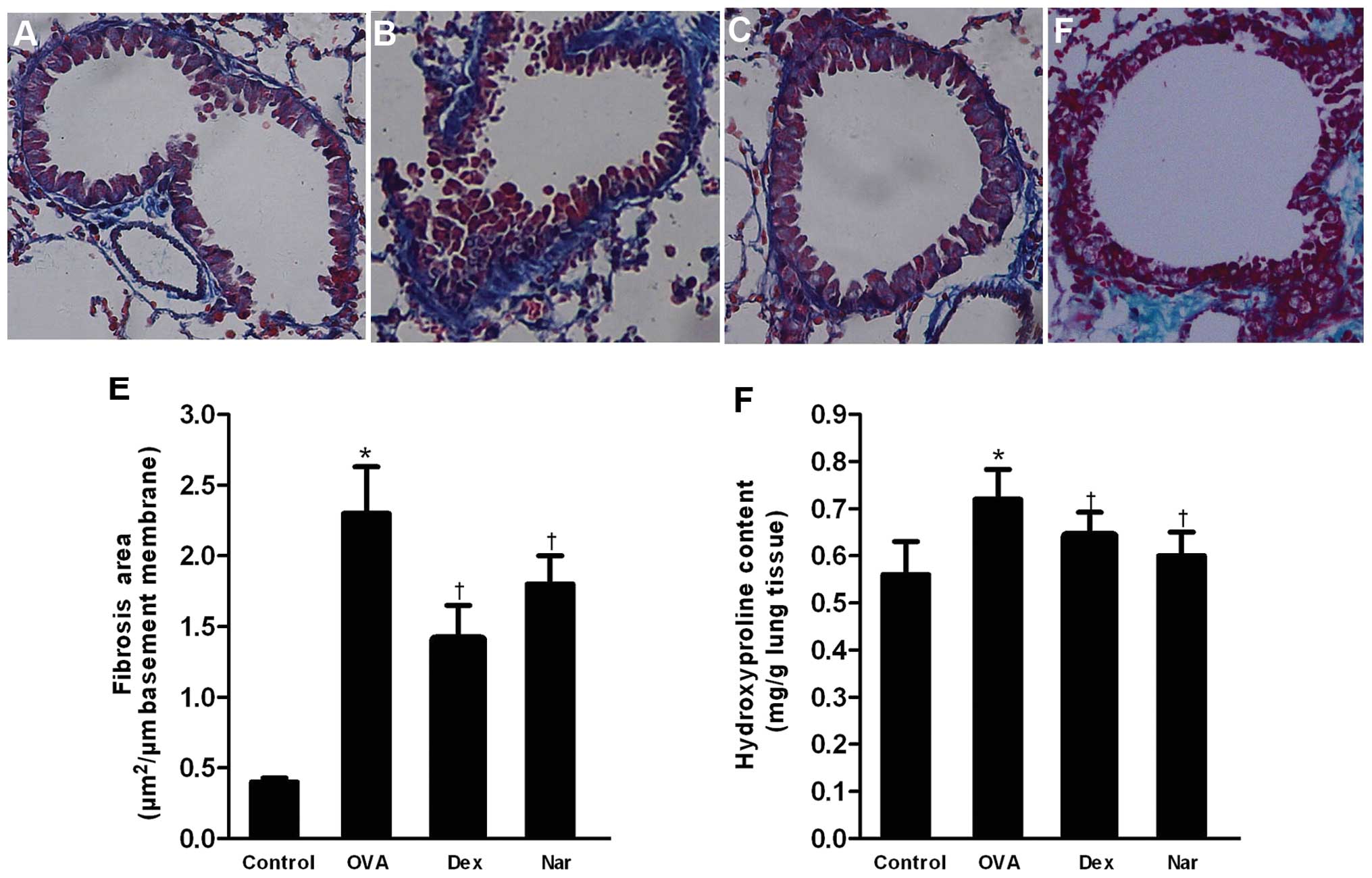
Masson’s trichrome staining revealed collagen
deposition (fibrosis). The mean area of airway fibrosis in the OVA
group was markedly enhanced compared to the control group.
Administration of naringenin and dexamethasone caused a reduction
in collagen deposition compared to the OVA group (Fig. 7A–E). The total hydroxyproline
content in lungs of the OVA group was significantly higher compared
to that of the control group. Treatment with naringenin and
dexamethasone reduced the hydroxyproline content compared to the
OVA group (Fig. 7F).
Discussion
A previous study showed that naringenin, a naturally
occurring flavonoid, attenuates acute airway inflammation and AHR,
in association with NF-κB repression and reduced cytokine
production (9). Consequently, we
evaluated the effect of naringenin on airway remodeling using a
long-term exposure murine model of asthma. The results of our study
clearly demonstrate the inhibitory effect of naringenin on airway
remodeling.
Sensitization and chronic challenge of mice with OVA
resulted in inflammation of the airway, evidenced here by the
recruitment of inflammatory cells, the production of Th2 cytokines
and an increase in the total IgE level. The inflammation was
effectively attenuated by naringenin treatment. As a natural
anti-inflammatory flavonoid, naringenin displayed anti-inflammatory
action in a long-term asthmatic process.
Marked goblet cell hyperplasia and mucus
hypersecretion were previously observed in the small airway under
chronic airway inflammatory conditions (13), consistent with results on our
murine model of asthma. In this study, we demonstrated that
naringenin significantly reduced the number of mucus-producing
goblet cells within the epithelium after OVA challenge. It has been
shown that IL-4 and IL-13 induce goblet cell hyperplasia in the
lungs of mice (14,15). Therefore, the ability of naringenin
to reduce mucus production in chronic asthma might relate to its
inhibitory effect on Th2 cytokine production.
Another important aspect of airway remodeling is
airway smooth muscle (ASM) hyperplasia, which has been linked to
the severity of asthma (16).
Consistent with previous reports, the areas of α-SMA were
significantly increased after exposure to OVA in our animal model,
while ASM hyperplasia was greatly reduced by treatment with
naringenin In addition, we found that airway resistance was reduced
following naringenin treatment. Since ASM contributes to airway
narrowing and AHR in response to a variety of stimuli during
asthma, we hypothesize that the ability of naringenin to reduce the
thickness of the ASM layer may partially account for the observed
decrease in AHR.
Collagen deposition in the airways is also
associated with a decrease in lung function and with the severity
of asthma in humans. We demonstrated for the first time that
naringenin significantly inhibits the deposition of subepithelial
collagen in a chronic asthma model. In the asthmatic process,
epithelial damage and subsequent Th2-mediated inflammation are
expected to cause an increase in the production of a few potent
profibrotic cytokines, such as transforming growth factor β and
tissue inhibitors of metalloproteinases (17,18).
Therefore, we assume that the naringenin-mediated reduction in the
Th2 cytokine levels may account for the anti-fibrotic activity of
naringenin through inhibition of the production of profibrotic
cytokines.
Additional mechanisms by which naringenin may
modulate airway remodeling exist. Naringenin is a naturally
occurring antioxidant (19).
Oxidative stress is closely linked to the pathogenesis of chronic
airway disorders, such as the increase in microvascular
permeability, excessive mucus secretion and remodeling of the
extracellular matrix and blood vessels (20). Therefore, naringenin may prevent
airway remodeling by reducing the production of reactive oxygen
species.
In conclusion, we have shown that treatment with
naringenin effectively inhibits allergen-induced airway
inflammation and remodeling. The ability of naringenin to modulate
chronic asthma pathology renders it a promising target for asthma
therapy.
References
|
1
|
Bousquet J, Clark TJ, Hurd S, Khaltaev N,
Lenfant C, O’Byrne P and Sheffer A: GINA guidelines on asthma and
beyond. Allergy. 62:102–112. 2007. View Article : Google Scholar
|
|
2
|
Bousquet J, Jeffery PK, Busse WW, Johnson
M and Vignola AM: Asthma. From bronchoconstriction to airways
inflammation and remodeling. Am J Respir Crit Care Med.
161:1720–1745. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vignola AM, Gagliardo R, Siena A,
Chiappara G, Bonsignore MR, Bousquet J and Bonsignore G: Airway
remodeling in the pathogenesis of asthma. Curr Allergy Asthma Rep.
1:108–115. 2001. View Article : Google Scholar
|
|
4
|
Tagaya E and Tamaoki J: Mechanisms of
airway remodeling in asthma. Allergol Int. 56:331–340. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Warner SM and Knight DA: Airway modeling
and remodeling in the pathogenesis of asthma. Curr Opin Allergy
Clin Immunol. 8:44–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benayoun L and Pretolani M: Airway
remodeling in asthma: mechanisms and therapeutic perspectives. Med
Sci (Paris). 19:319–326. 2003.(In French).
|
|
7
|
Park HY, Kim GY and Choi YH: Naringenin
attenuates the release of pro-inflammatory mediators from
lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear
factor-κB and inhibiting mitogen-activated protein kinases. Int J
Mol Med. 30:204–210. 2012.PubMed/NCBI
|
|
8
|
Anand K, Sarkar A, Kumar A, et al:
Combinatorial antitumor effect of naringenin and curcumin elicit
angioinhibitory activities in vivo. Nutr Cancer. 64:714–724. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi Y, Dai J, Liu H, et al: Naringenin
inhibits allergen-induced airway inflammation and airway
responsiveness and inhibits NF-κB activity in a murine model of
asthma. Can J Physiol Pharmacol. 87:729–735. 2009.PubMed/NCBI
|
|
10
|
Cayci C, Wahlquist TC, Seckin SI, et al:
Naringenin inhibits neointimal hyperplasia following arterial
reconstruction with interpositional vein graft. Ann Plast Surg.
64:105–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen S, Ding Y, Tao W, Zhang W, Liang T
and Liu C: Naringenin inhibits TNF-α induced VSMC proliferation and
migration via induction of HO-1. Food Chem Toxicol. 50:3025–3031.
2012.
|
|
12
|
Cho JY, Miller M, Baek KJ, et al:
Inhibition of airway remodeling in IL-5-deficient mice. J Clin
Invest. 113:551–560. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rogers DF: Airway goblet cell hyperplasia
in asthma: hypersecretory and anti-inflammatory? Clin Exp Allergy.
32:1124–1127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuperman DA, Huang X, Koth LL, et al:
Direct effects of interleukin-13 on epithelial cells cause airway
hyperreactivity and mucus overproduction in asthma. Nat Med.
8:885–889. 2002.PubMed/NCBI
|
|
15
|
Kuperman DA, Huang X, Nguyenvu L, Holscher
C, Brombacher F and Erle DJ: IL-4 receptor signaling in Clara cells
is required for allergen-induced mucus production. J Immunol.
175:3746–3752. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Black JL, Panettieri RA Jr, Banerjee A and
Berger P: Airway smooth muscle in asthma: just a target for
bronchodilation? Clin Chest Med. 33:543–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bosse Y and Rola-Pleszczynski M:
Controversy surrounding the increased expression of TGFβ1 in
asthma. Respir Res. 8:662007.
|
|
18
|
Mohamed SA, Noack F, Schoellermann K, et
al: Elevation of matrix metalloproteinases in different areas of
ascending aortic aneurysms in patients with bicuspid and tricuspid
aortic valves. Scientific World Journal. 2012:8062612012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Li Q, Zhou XD, Kolosov VP and
Perelman JM: Naringenin attenuates mucous hypersecretion by
modulating reactive oxygen species production and inhibiting NF-κB
activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway
epithelial cells. Mol Cell Biochem. 351:29–40. 2011.PubMed/NCBI
|
|
20
|
de Boer WI, Yao H and Rahman I: Future
therapeutic treatment of COPD: struggle between oxidants and
cytokines. Int J Chron Obstruct Pulmon Dis. 2:205–228.
2007.PubMed/NCBI
|