Introduction
Spinal cord injury (SCI) is a worldwide medical
problem with an increasing number of incidences. SCI results in
necrosis and apoptosis of neurons and glial cells following the
primary and secondary sequential injuries, and the latter may be
the main cause for the loss of sensory and motoric function
(1). Following SCI, syringomyelia
and glial scars constitute a mechanical barrier impeding the growth
of axons (2), and neuronal
apoptosis decreases the self-repair capacity of the spinal cord and
shortage of neurotrophic factors (3). Therefore, despite advances in the
elucidation of the pathophysiological processes of SCI, no
treatment is currently available to restore the injury-induced loss
of function, and further investigation is required to determine
therapeutic strategies for SCI (4).
Schwann cells (SCs) are the major cells constituting
the peripheral nerve structure and function, and also secret a
variety of neurotrophic factors, including nerve growth,
brain-derived neurotrophic, neurite-promoting and axon-inducing
factors (5,6). The transplantation of SCs may
potentially improve axon growth and myelination, inhibit glial scar
formation and promote the formation of endometrial nerves (7,8).
Previous studies reporting the transplantation of SCs in rodent and
primate models have provided significant evidence of their repair
potential for SCI (9,10). The development of in vitro
systems to harvest and expand human SCs has made them attractive
candidates for autologous transplantation in the clinical setting
(11). Therefore, SCs are regarded
as ideal seed cells for the repair of SCI.
Glial cell line-derived neurotrophic factor (GDNF)
was originally identified and cloned from conditioned media derived
from the rat glial cell line B49 and demonstrated survival and
differentiation effects on cultured midbrain dopaminergic neurons
(12). GDNF is a glycosylated
homodimer with a molecular mass of ~33–45 kDa and is a distant
member of the transforming growth factor-β (TGF-β) superfamily
(4). This trophic agent has been
shown to inhibit cell death of various central and peripheral
neuronal cell types under different conditions (13–16).
Its neuroprotective activities include enhancing nerve
regeneration, promoting neuronal survival and trophic effects on
various neuronal populations (17). Administration of GDNF has been
shown to promote recovery of SCI (18–21)
and peripheral nerve injuries (22) in animal models, and has also been
tested for the treatment of Parkinson’s disease in clinical trials
(23). Moreover, GDNF also
negatively regulates the effects of substance abuse and presents a
potential target for the treatment of drug addiction (24).
Based on these previous studies, the present study
sought to investigate whether genetically modified SCs producing
GDNF are able to promote spinal cord repair in a rat model of
SCI.
Materials and methods
Establishment of SCs expressing GDNF
The rat SC line RSC96 was purchased from the
Shanghai Institute of Biochemistry and Cell Biology (Shanghai,
China). The GDNF-expressing plasmid, pEGFP-N1/GDNF, and empty
vector, pEGFP-N1, were kindly provided by the Bio-engineering
Laboratory of the School of Stomatology, Jilin University
(Changchun, China). The cells were routinely cultured at 37°C in
Dulbecco’s modified Eagle medium (DMEM; Gibco-BRL, Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal calf
serum (FCS; Gibco-BRL), penicillin (100 U/ml) and streptomycin (100
μg/ml) in a CO2 incubator (Heraeus; Thermo Fisher
Scientific Inc., Hanau, Germany). Cells (2×105/ml) were
seeded in 6-well plates (Costar Cell Culture plate; Corning
Incorporated, Lowell, MA, USA) and grown to ~90% confluence at
which point they were transfected with 5 μg plasmid (pEGFP-N1/GDNF
or pEGFP-N1) using Lipofectamine™ (Gibco-BRL). Cells were detached
with 0.25% trypsin (Sigma-Aldrich, St. Louis, MO, USA) following
transfection at 10 multiplicity of infection for 72 h and seeded at
a dilution of 1:4 in selection medium containing 1.2 mg/ml neomycin
G418 (Gibco-BRL). The SCs transfected with pEGFP-N1/GDNF or
pEGFP-N1 were selected after 3 weeks of culture, and named
SCs-pEGFP-N1/GDNF or SCs-pEGFP-N1, respectively.
ELISA
The SCs were seeded into 24-well plates
(1×106/ml), and cultured in serum-free DMEM at 37°C.
After 24, 48 and 72 h, respectively, the supernatants were
collected and subjected to the ELISA to measure the levels of GDNF
with a GDNF assay kit (Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) according to the manufacturer’s instructions.
Preparation of primary spinal
neurons
All the animal experimental protocols were approved
by the Animal Ethics Committee of Jilin University College of
Pharmacy (Changchun, China). Inbred Wistar rats at 14–15 days of
gestation were provided by the Experimental Animal Center of Jilin
University (Changchun, China). Primary culture of spinal cord
neurons was performed according to the methods previously described
(25). Briefly, spinal cords were
quickly removed from the fetuses, washed and minced in Hank’s
Balanced Salt Solution, and then enzymatically digested with 0.25%
trypsin for 20 min at 37°C. The cell suspension was triturated and
sieved through a 200 μm mesh. Following centrifugation (Sigma
Laborzentrifugen GmbH, Ostrode, Germany) at 1,000 × g for 10 min,
the supernatant was discarded and cells were harvested.
In vitro experimental design
The primary spinal cord neurons were suspended in
DMEM at 1×106/ml and seeded into a 24-well plate. When
the cells reached ~80% confluency, they were exposed to radiation
at 100 cGy/min with a distance of 100 cm for 24 h. The total dose
of radiation was 2 Gy. The cells were then co-cultured with SCs at
a ratio of 3:1. A transwell membrane (0.45 μm pore size; BD
Biosciences, San Jose, CA, USA) was used to separate neurons and
SCs. Intact neurons and irradiated neurons served as controls. The
cells were cultured for 24 h and then harvested for the apoptosis
assay as described below.
Apoptosis assay
Cells (1×105) were suspended in 100 μl
binding buffer prior to addition of 5 μl Annexin V and 5 μl
propidium iodide (PI) and incubation for 15 min at room temperature
in the dark according to the manufacturer’s instructions (BD
Biosciences). Cells were then subjected to flow cytometric analysis
to measure the rate of apoptosis (%) with a Beckman Coulter EPICS
Altra II cytometer (Beckman Coulter Inc., Brea, CA, USA).
Animal experiments
Adult female inbred Wistar rats (weight, 250±20 g)
were provided by the Experimental Animal Center of the Jilin
University. The rats were anesthetized by intraperitoneal injection
of pentobarbital (40 mg/kg), a midline incision was made on the
back region and a laminectomy was performed aseptically at the
T8-T12 level. The spinal cord was subjected to a 10 g weight-drop
impact from a height of 5 cm, which was repeated six times. The
rats demonstrating severe loss of locomotor movement in the hind
legs were randomly assigned to three treatment groups (n=20 per
group). Saline (5 μl) or an identical volume of saline containing
1×105 SCs or SCs-pEGFP-N1/GDNF was slowly injected into
the site of the SCI, respectively. At each of the indicated
time-points (seven and 15 days following cell injection), 10 rats
(per group) were randomly sacrificed, and a 1-cm long segment of
the spinal cord was dissected between the cranial (1 cm) and caudal
(1 cm) area of the injury epicenter. Each sample was split into two
sections: One section was fixed with 10%-buffered formalin and the
other one was stored at −80°C for further analysis.
Histological, immunocytochemical and
immunohistochemical analyzes
Cells were fixed with 4% paraformaldehyde.
Formalin-fixed spinal cord specimens were transferred to 70%
ethanol and subsequently paraffin-embedded and sectioned. The
sections (5 μm) were stained with hematoxylin and eosin (H&E)
and examined under light microscopy (Eclipse TE2000-U equipped with
an attached SXM1200F digital camera; Nikon Corporation, Tokyo,
Japan). Cells or tissue sections were blocked with 3% biotinylated
bovine serum albumin (BSA) and incubated with anti-rat Bcl-2 and
Bax antibodies (Abs; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), and then subsequently incubated for 30 min with appropriate
secondary Abs (SP kit; Zhongshan Biotechnology Co., Beijing,
China), and developed with Sigma FAST 3,3′-diaminobenzidine and
CoCl2 enhancer tablets (Sigma-Aldrich). The slides were
mounted and examined under a microscope.
Western blot analysis
Western blot analysis was performed as previously
described (26). Briefly, cells or
tissues were homogenized in protein lysate buffer and debris was
removed by centrifugation. Protein samples (20 μg) were resolved on
polyacrylamide SDS gels and electrophoretically transferred to
polyvinylidene difluoride membranes (Pierce PVDF membranes, Thermo
Fisher Scientific Inc., Rockford, IL USA). The membranes were
blocked with 3% BSA overnight, incubated with primary Abs (anti-rat
Bax, Bcl-2 and β-actin; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), then with an alkaline phosphatase-conjugated secondary Ab (AP
kit; Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China), and immunoreactivity was visualized with
5-bromo-4-chloro-3-indolyl phosphate/nitro-blue tetrazolium
chloride (BCIP/NBT Substrate Kit; Tiangen Biotech Co., Ltd.,
Beijing, China) according to the manufacturer’s instructions.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was extracted from spinal cord homogenates
using TRIzol® (Life Technologies) according to the
manufacturer’s instructions. The quality and amount of RNA were
determined. RNA (1 μg) was reverse-transcribed using a cDNA
Synthesis kit (Takara Bio, Inc., Shiga, Japan) at 30°C for 5 min,
50°C for 30 min, followed by 95°C for 5 min. The cDNA was used as a
template for PCR amplification under the following conditions: 30
cycles at 94°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec,
with the following pairs of primers: 5′-CCCTGGCATCTTCTCCTTCC-3′ and
5′-CAT CCCAGCCTCCGTTATCC-3′ to generate a 446 bp product of Bcl-2
mRNA; 5′-TTGTTACAGGGTTTCATCCAGG-3′ and 5′-GAGTCCGTGTCCACGTCAG-3′ to
generate a 185 bp product of Bax mRNA; and 5′-GTCAGGTCATCACTATCGG
CAAT-3′ and 5′-AGAGGTCTTTACGGATGTCAACGT-3′ to generate a 147 bp
product of β-actin (served as an internal control). The products of
the PCR were subjected to 2% agarose gel electrophoresis, and the
density of each band was evaluated using the gel image analyzer
(ultraviolet ChemiDOC; Bio-Rad, Hercules, CA, USA). The relative
density of mRNA was calculated using the following formula: Band
density/β-actin band density.
Statistical analysis
All values are expressed as the mean ± standard
deviation. One-way analysis of variance was used to evaluate
statistical significance. Data were analyzed using SPSS 15.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of GDNF by genetically
modified SCs
The RS96 SCs were transfected with pEGFP-N1 or
pEGFP-N1/GDNF expression vectors and selected by co-culturing with
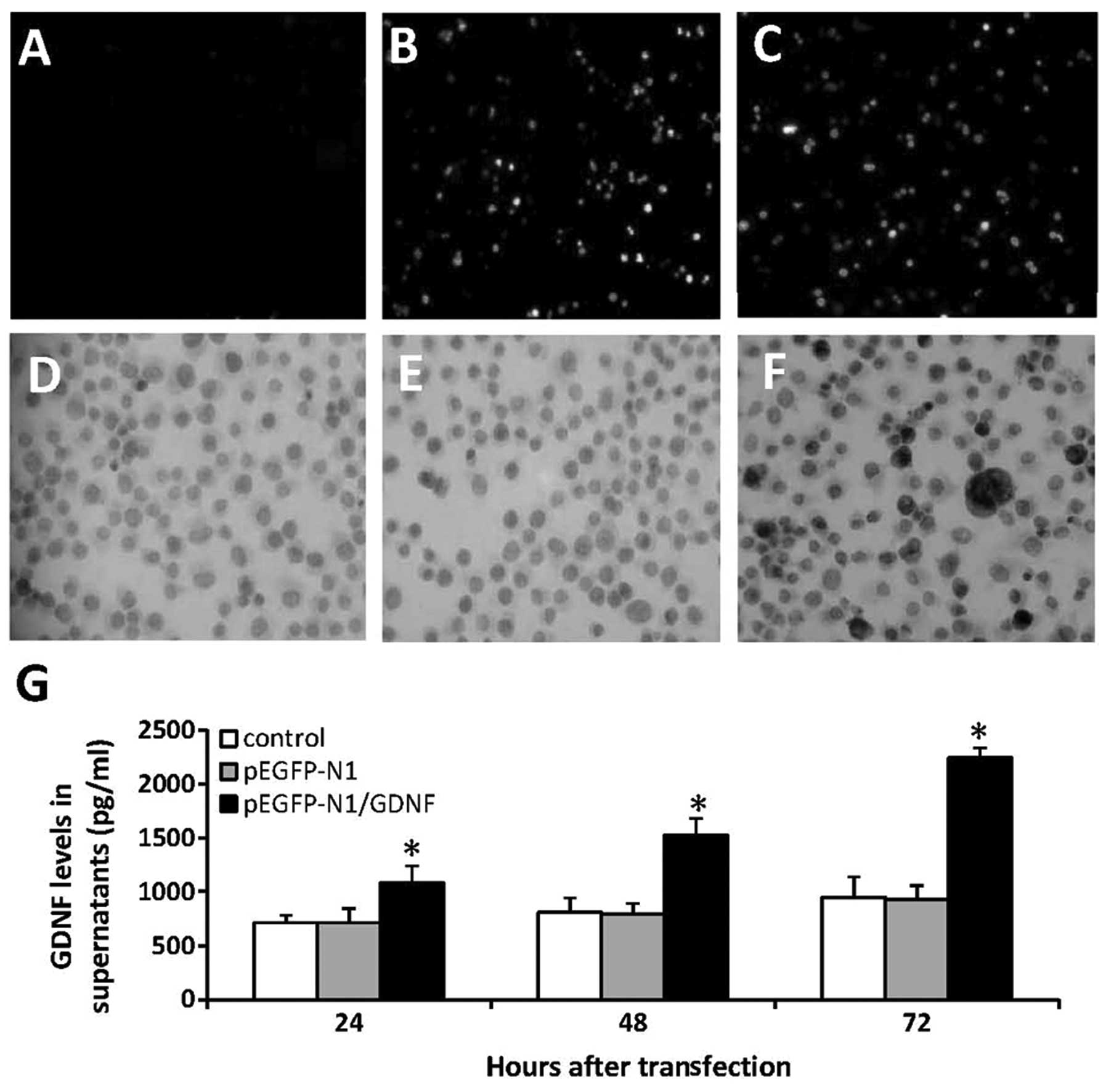
G418. The untreated SCs exhibited no green fluorescence (Fig. 1A), whereas SCs transfected with
pEGFP-N1 (Fig. 1B) or
pEGFP-N1/GDNF (Fig. 1C) exhibited
positive fluorescence under a fluorescence microscope.
Immunocytochemical analysis performed with an anti-GDNF Ab revealed
that untreated cells (Fig. 1D) and
cells transfected with pEGFP-N1 (Fig.
1E) showed weak expression levels of GDNF, while cells
transfected with pEGFP-N1/GDNF (Fig.
1F) showed a higher expression of GDNF. The above 3 types of
cells were further cultured and the supernatants were collected and
harvested to measure the concentrations of GDNF using ELISA. As
shown in Fig. 1G, following
culture for 24, 48 and 72 h the levels of GDNF in the supernatant
of SCs-pEGFP-N1/GDNF were significantly higher than those of
unmodified SCs or SCs-pEGFP-N1. In addition, the levels of GDNF in
the supernatant of SCs-pEGFP-N1/GDNF increased in a time-dependent
manner, while GDNF levels remained similar in the supernatants of
unmodified SCs or SCs-pEGFP-N1.
Genetically modified SCs inhibit
apoptosis of rat primary spinal neurons induced by radiation
Primary spinal neuronal cells were collected from
Wistar rats and exposed to radiation followed by co-culturing with
unmodified SCs, SCs-pEGFP-N1 or SCs-pEGFP-N1/GDNF for 24 h. Flow
cytometric analysis was used to measure the rate of apoptosis. It
was found that exposure to radiation significantly increased the
rate of apoptosis in neurons (53.2±9.5%) compared with that of the
untreated neurons (9.8±1.1%) (P<0.001) (Fig. 2A). In addition, exposure to
radiation followed by co-culture with unmodified SCs or
SCs-pEGFP-N1 significantly attenuated apoptosis (31.9±5.0 or
32.3±5.1%, respectively; P<0.05). However, exposure to radiation
followed by co-culture with SCs-EGFP-N1/GDNF significantly reduced
the rate of apoptosis (20.1±2.9%), which was lower than that of the
controls (P<0.001) and significantly lower than that of cells
co-cultured with unmodified SCs or SCs-pGEFP-N1 (P<0.05)
(Fig. 2A).
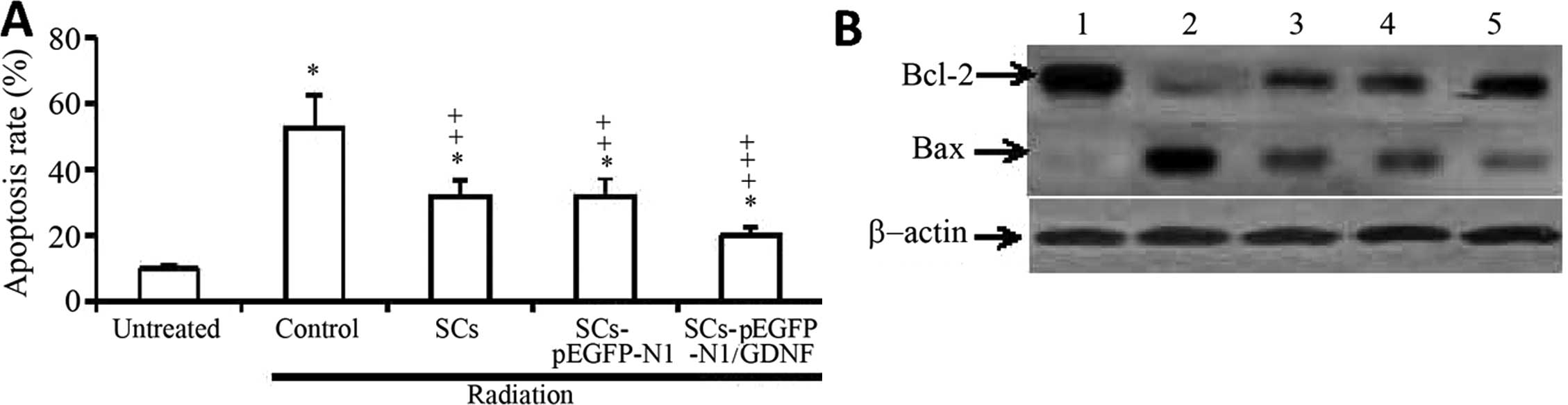 | Figure 2Co-culture with genetically modified
SCs attenuates apoptosis of rat primary spinal neurons induced by
radiation. Neuronal cells were exposed to radiation at 100 cGy/min
for 24 h, then co-cultured without SCs (control) or with SCs,
SCs-pEGFP-N1 or SCs-pEGFP-N1-GDNF for 24 h. (A) Untreated cells
served as the negative control. The apoptosis of neurons was
detected by flow cytometry. *Indicates a significant
increase compared with untreated cells; ‡significant
reduction compared with the control cells; or
†significant reduction compared with the co-cultured SCs
with SCs-pEGFP-N1. (B) Expression of Bcl-2 and Bax in untreated
cells (lane 1), control cells exposed to radiation (lane 2), cells
co-cultured with SCs (lane 3), SCs-pEGFP-N1 (lane 4) and
SCs-pEGFP-N1-GDNF (lane 5). SCs, Schwann cells; GDNF, glial cell
line-derived neurotrophic factor; EGFP, enhanced green fluorescent
protein; pEGFP-N1/GDNF, GDNF-expressing plasmid; pEGFP-N1, empty
vector; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated x. |
Western blot analysis was used to examine the
changes of Bcl-2 and Bax expression in the abovementioned cells. As
shown in Fig. 2B, exposure to
radiation downregulated the expression of Bcl-2 and upregulated the
expression of Bax, and thus reduced the ratio of Bcl-2 to Bax.
However, co-culture with unmodified SCs, SCs-pEGFP-N1 or
SCs-pEGFP-N1/GDNF inhibited the changes in expression levels of
Bcl-2 and Bax induced by radiation. Furthermore, co-culture with
SCs-pEGFP-N1/GDNF showed the strongest effects in attenuating the
alteration of Bcl-2 and Bax expression induced by radiation.
Transplantation of SCs attenuates spinal
cord injury
The survival rates of SCs that were injected into
the spinal cord of rats were examined. Normal Wistar rats were
injected with unmodified SCs or SCs-pEGFP-N1/GDNF into the spinal
cord. The unmodified SCs or SCs-pEGFP-N1/GDNF were harvested after
15 days, sectioned and examined by fluorescence microscopy; the
adjacent sections were stained with H&E and examined by light
microscopy. The images captured through the fluorescence and light
microscopes were merged using Photoshop software (Adobe Systems,
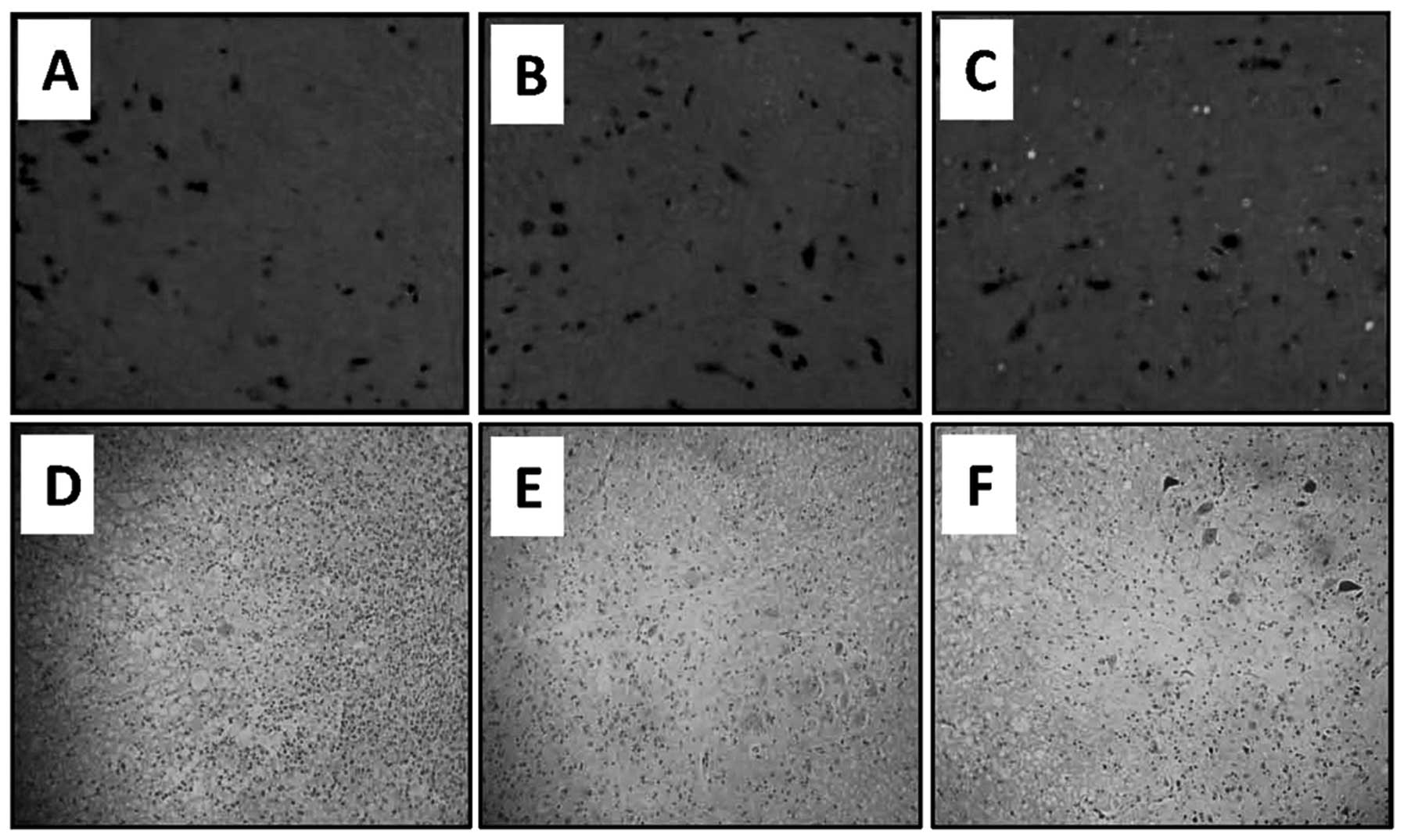
San Jose, CA, USA). Representative images obtained from the spinal
cord sections of normal control rats (Fig. 3A) and rats injected with unmodified
SCs (Fig. 3B) demonstrated no
fluorescence. However, the analysis of the spinal cord sections
from rats injected with SCs-pEGFP-N1/GDNF revealed the presence of
fluorescent cells (Fig. 3C),
indicating that following transplantation, SCs survived in the
spinal cords for ~15 days.
The effects of SC-transplantation on SCI were
examined. The rats with SCI were randomly injected with saline
(control) or 1×105 SCs or SCs-pEGFP-N1/GDNF into the
spinal cords, and then randomly sacrificed after seven and 15 days.
The spinal cords were harvested and subjected to histological
analysis. Histological analysis of the central gray matter of SCI
demonstrated hemorrhage, cell swelling, blurring Nissl bodies,
pyknotic nuclei in several cells and infiltration of inflammatory
cells. However, transplantation of SCs attenuated the histological
changes, and SCs-pEGFP-N1/GDNF further inhibited the histological
alterations. Representative images were captured from
H&E-stained spinal cord sections in untreated rats with SCI
(Fig. 3D), rats with SCI treated
with SCs (Fig. 3E) and rats with
SCI treated with SCs-pEGFP-N1/GDNF (Fig. 3F) 15 days following cell
transplantation.
Regulation of Bcl-2 and Bax expression in
the spinal cords
The spinal cords of rats were subjected to
immunohistochemical analysis with anti-Bcl-2 and anti-Bax Abs. As
shown in Fig. 4A–H, SCI induced
downregulation of Bcl-2 and upregulation of Bax, thus resulting in
a reduced ratio of Bcl-2 to Bax. However, injection of SCs or
SCs-pEGFP-N1/GDNF attenuated this change, resulting in increased
expression of Bcl-2 and decreased expression of Bax, compared with
that of untreated rats with SCI. This altered expression of Bcl-2
and Bax was further confirmed by western blot analysis detecting
protein expression (Fig. 4I and J)
and by RT-PCR detecting mRNA expression (Fig. 4K and L). Furthermore,
quantification of the density of the bands in the western blots
indicated that there was a significant difference in Bcl-2 and Bax
at the protein and mRNA levels in SCs and SCs-pEGFP-N1/GDNF-treated
SCI rats. This suggests that SCs-pEGFP-N1/GDNF may have marked
anti-apoptotic activity against SCI-induced apoptosis compared with
that of unmodified SCs due to its effects on the
apoptosis-associated proteins Bcl-2 and Bax.
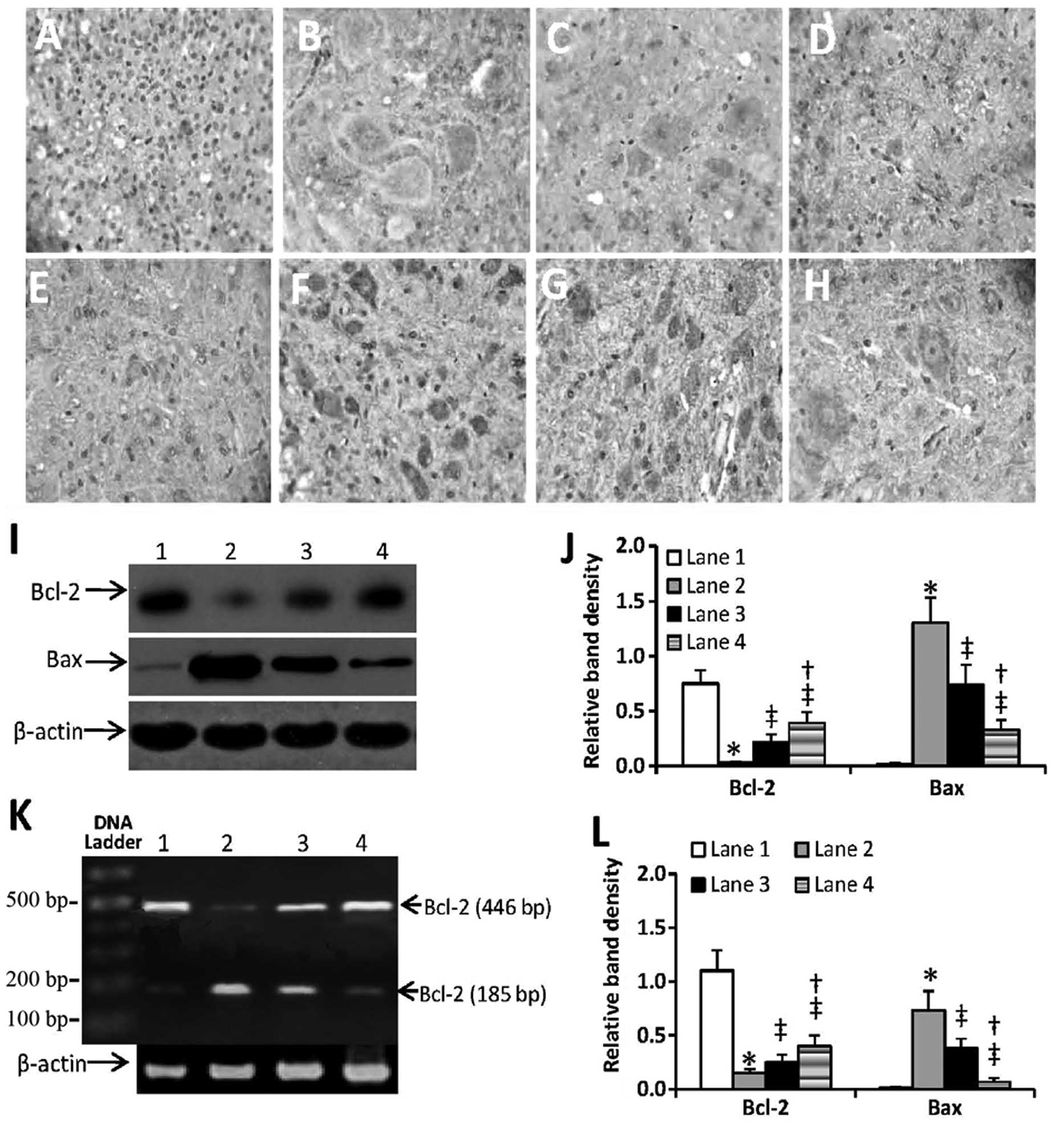 | Figure 4Injection of genetically modified SCs
regulated Bcl-2 and Bax expression in the spinal cords. (A–H)
Spinal cords were obtained from (A and E) normal rats, (B and F)
untreated rats with SCI, (C and G) rats with SCI injected with SCs
and (D and H) rats with SCI injected with SCs-pEGFP-N1-GDNF 7 days
following SCI. Spinal cords were sectioned, immunostained with
(A–D) anti-Bcl-2 and (E–H) anti-Bax antibodies and examined under a
microscope. (I and J) Expression of Bcl-2 and Bax protein were
detected using western blot analysis in the spinal cords of (lane
1) normal rats, (lane 2) untreated rats with SCI, (lane 3) rats
with SCI injected with SCs and (lane 4) rats with SCI injected with
SCs-pEGFP-N1-GDNF. (J) The density of each band of protein was
measured and compared with that of the internal control, β-actin.
(K and I) Expression of Bcl-2 and Bax mRNA was detected with
reverse transcription-polymerase chain reaction in spinal cords of
(lane 1) normal rats, (lane 2) untreated rats with SCI, (lane 3)
rats with SIC injected with SCs and (lane 4) rats with SIC injected
with SCs-pEGFP-N1-GDNF. (L) The density of each band of mRNA was
measured and compared with that of the internal control, β-actin.
*Indicates significant difference from the normal rats;
‡significant difference from the untreated rats with
SCI, and †significant difference from the rats with SCI
treated with SCs. SCs, Schwann cells; GDNF, glial cell line-derived
neurotrophic factor; EGFP, enhanced green fluorescent protein;
pEGFP-N1/GDNF, GDNF-expressing plasmid; pEGFP-N1, empty vector;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated x. |
Discussion
The combination of SC transplantation and gene
therapy is potentially one of the most powerful strategies to
promote the recovery of the central nervous system (CNS), as SCs
are involved in the repair of traumatic injury and demyelination in
the spinal cord and other sections of the CNS. For example, SCs
support axonal regeneration by secreting growth factors (27), providing a permissive extracellular
matrix, secreting growth-promoting adhesion molecules and filling
cystic cavities following SCI (28). Even when a biological conduit is
used to bridge a new defect, the conduit is soon colonized by a
number of SCs that form a pathway for regrowing axons. SC
transplantation has been performed in various models of SCI,
resulting in functional recovery (29,30),
and has more recently been applied in clinical trials of SCI
(31). The present study
demonstrated that injection of unmodified SCs inhibited SCI-induced
apoptosis and attenuated SCI.
In addition, there is increasing interest in
utilizing the regenerative properties of SCs, as SCs are a
potential vector to introduce therapeutic genes for nerve repair. A
promising option to foster nerve repair entails the genetic
modification of autologous SCs either in vivo or in
vitro, followed by reimplantation into the same individual for
clinical application. Although viral expression vectors are the
most efficient method to introduce transgenes into sites of SCI,
their immunological response, safety issues and toxicity preclude
their use in humans (32). Plasmid
DNA has low immunogenicity, viral infection risks and is easy to
propagate on a large scale at a high quality. The major limitation
of the use of plasmid DNA as a vector is its low efficiency for
gene delivery. The present study genetically modified rat RS96 SCs
in vitro by transfection with the GDNF gene. The genetically
modified SCs, which were selected with antibiotics, expressed high
levels of GDNF in a time-dependent manner. Notably, following
transplantation, SCs survived for ~15 days in the spinal cords of
rats. Therefore, the SCs were able to support the recovery of SCI
by guiding regenerating axons and providing trophic factors, in
particular, high levels of GDNF.
GDNF was originally identified as a potent trophic
factor for midbrain dopaminergic neurons, and was subsequently
found to have the ability to support the survival of motor neurons
both in vitro and in vivo. When applied to the spinal
cord, GDNF exerts trophic effects on corticospinal neurons and
promotes their long-term survival following axotomy (33). Administration of recombinant GDNF
has been shown to improve behavioral outcome, neuronal survival and
sprouting of fibers following SCI (18,20).
However, currently recombinant human GDNF protein is made from
E. coli, insect cells or Pichia pastoris systems,
which are unable to produce a mammalian glycosylation pattern
(34). The clinical application of
the GDNF protein has been hindered by high dose requirements,
manufacturing constraints and its relative instability. However,
transplantation of genetically modified cells producing GDNF
represents an alternative method.
It has been demonstrated that GDNF inhibited
retinoic acid-induced apoptosis in CHP134 neuroblastoma cells
(35), attenuated ethanol-induced
apoptosis and necrosis in SK-N-SH neuroblastoma cells (36), prevented ethanol-induced B92 glial
cell death via the phosphoinositide-3-kinase/serine threonine
protein kinase and mitogen-activated protein kinase/extracellular
signal-regulated kinase signaling pathways (37), and protected against
aluminum-induced apoptosis in rabbit brains by upregulating Bcl-2
and B-cell lymphoma extra large protein expression (38). The members of the Bcl-2 family are
the most prominent regulators of apoptosis in various cell types,
including cancer cells (39).
Bcl-2, located on the mitochondrial membrane, is a proapoptotic
protein, while Bax directly binds to Bcl-2 and inhibits its
function (40). The present study
demonstrated that genetically modified SCs producing GDNF inhibited
the downregulation of Bcl-2 and the upregulation of Bax, which had
been induced by radiation in vitro and by SCI in
vivo. In the in vitro assays, a transwell membrane was
used to prevent direct contact of SCs and neurons, which further
confirms the protective effects of GDNF.
In conclusion, the present study demonstrated that
genetically modified SCs producing high levels of GDNF attenuated
SCI through anti-apoptotic activity. The engineered SCs were
characterized by their ability to express and secrete biologically
active GDNF, which inhibited radiation-induced apoptosis of primary
spinal neurons by upregulating the expression of Bcl-2 and
downregulating the expression of Bax in vitro. Following SC
implantation into the spinal cord of adult rats with SCI induced by
weight-drop impact, the modified SCs survived in the spinal cords
and expressed high protein and mRNA levels of GDNF for ~15 days.
Transplantation of modified SCs attenuated SCI and altered the
expression of the apoptosis-associated proteins Bcl-2 and Bax,
which was in accordance with the in vitro observations. The
present study suggested that the growth-promoting properties of SCs
were able to be significantly improved when these cells were
genetically modified to secrete high levels of GDNF. The genetic
engineering of SCs may provide novel possibilities for future
clinical applications in SCI.
References
|
1
|
Qiao F, Atkinson C, Song H, Pannu R, Singh
I and Tomlinson S: Complement plays an important role in spinal
cord injury and represents a therapeutic target for improving
recovery following trauma. Am J Pathol. 169:1039–1047. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karimi-Abdolrezaee S, Eftekharpour E, Wang
J, Morshead CM and Fehlings MG: Delayed transplantation of adult
neural precursor cells promotes remyelination and functional
neurological recovery after spinal cord injury. J Neurosci.
26:3377–3389. 2006. View Article : Google Scholar
|
|
3
|
Fawcett J: Repair of spinal cord injuries:
where are we, where are we going? Spinal Cord. 40:615–623. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nandoe Tewarie RS, Hurtado A, Bartels RH,
Grotenhuis A and Oudega M: Stem cell-based therapies for spinal
cord injury. J Spinal Cord Med. 32:105–114. 2009.
|
|
5
|
Lankford KL, Imaizumi T, Honmou O and
Kocsis JD: A quantitative morphometric analysis of rat spinal cord
remyelination following transplantation of allogenic Schwann cells.
J Comp Neurol. 443:259–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schaal SM, Kitay BM, Cho KS, Lo TP Jr,
Barakat DJ, Marcillo AE, Sanchez AR, Andrade CM and Pearse DD:
Schwann cell transplantation improves reticulospinal axon growth
and forelimb strength after severe cervical spinal cord contusion.
Cell Transplant. 16:207–208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Golden KL, Pearse DD, Blits B, Garg MS,
Oudega M, Wood PM and Bunge MB: Transduced Schwann cells promote
axon growth and myelination after spinal cord injury. Exp Neurol.
207:203–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lavdas AA, Franceschini I, Dubois-Dalcq M
and Matsas R: Schwann cells genetically engineered to express PSA
show enhanced migratory potential without impairment of their
myelinating ability in vitro. Glia. 53:868–878. 2006. View Article : Google Scholar
|
|
9
|
Bunge MB: Bridging the transected or
contused adult rat spinal cord with Schwann cell and olfactory
ensheathing glia transplants. Prog Brain Res. 137:275–282. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lavdas AA, Papastefanaki F, Thomaidou D
and Matsas R: Schwann cell transplantation for CNS repair. Curr Med
Chem. 15:151–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Levi AD, Bunge RP, Lofgren JA, Meima L,
Hefti F, Nikolics K and Sliwkowski MX: The influence of heregulins
on human Schwann cell proliferation. J Neurosci. 15:1329–1340.
1995.PubMed/NCBI
|
|
12
|
Lin LF, Doherty DH, Lile JD, Bektesh S and
Collins F: GDNF: a glial cell line-derived neurotrophic factor for
midbrain dopaminergic neurons. Science. 260:1130–1132. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nandoe Tewarie RS, Hurtado A, Bartels RH,
Grotenhuis A and Oudega M: Stem cell-based therapies for spinal
cord injury. J Spinal Cord Med. 32:105–114. 2009.
|
|
14
|
Henderson CE, Phillips HS, Pollock RA,
Davies AM, Lemeulle C, Armanini M, Simpson LC, Moffet B, Vandlen
RA, Koliatsos VE and Rosenthal A: GDNF: a potent survival factor
for motoneurons present in peripheral nerve and muscle. Science.
266:1062–1064. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomac A, Lindqvist E, Lin LF, Ögren SO,
Young D, Hoffer BJ and Olson L: Protection and repair of the
nigrostriatal dopaminergic system by GDNF in vivo. Nature.
373:335–339. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan Q, Matheson C and Lopez OT: In vivo
neurotrophic effects of GDNF on neonatal and adult facial motor
neurons. Nature. 373:341–344. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beck KD, Valverde J, Alexi T, Poulsen K,
Moffat B, Vandlen RA, Rosenthal A and Hefti F: Mesencephalic
dopaminergic neurons protected by GDNF from axotomy-induced
degeneration in the adult brain. Nature. 373:339–341. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ansorena E, Garbayo E, Lanciego JL,
Aymerich MS and Blanco-Prieto MJ: Production of highly pure human
glycosylated GDNF in a mammalian cell line. Int J Pharm. 385:6–11.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng H, Wu JP and Tzeng SF:
Neuroprotection of glial cell line-derived neurotrophic factor in
damaged spinal cords following contusive injury. J Neurosci Res.
69:397–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharma HS: Post-traumatic application of
brain-derived neurotrophic factor and glia-derived neurotrophic
factor on the rat spinal cord enhances neuroprotection and improves
motor function. Acta Neurochir Suppl. 96:329–334. 2006. View Article : Google Scholar
|
|
21
|
Guzen FP, De Almeida Leme RJ, de Andrade
MS, de Luca BA and Chadi G: Glial cell line-derived neurotrophic
factor added to a sciatic nerve fragment grafted in a spinal cord
gap ameliorates motor impairments in rats and increases local
axonal growth. Restor Neurol Neurosci. 27:1–16. 2009.
|
|
22
|
Zhang L, Ma Z, Smith GM, Wen X, Pressman
Y, Wood PM and Xu XM: GDNF-enhanced axonal regeneration and
myelination following spinal cord injury is mediated by primary
effects on neurons. Glia. 57:1178–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Piquilloud G, Christen T, Pfister LA,
Gander B and Papaloizos MY: Variations in glial cell line-derived
neurotrophic factor release from biodegradable nerve conduits
modify the rate of functional motor recovery after rat primary
nerve repairs. Eur J Neurosci. 26:1109–1117. 2007. View Article : Google Scholar
|
|
24
|
Lang AE, Gill S, Patel NK, Lozano A, Nutt
JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA,
Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten
VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ
and Traub M: Randomized controlled trial of intraputamenal glial
cell line-derived neurotrophic factor infusion in Parkinson
disease. Ann Neuro. 59:459–466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carnicella S and Ron D: GDNF, a potential
target to treat addiction. Pharmacol Ther. 122:9–18. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moreels M, Vandenabeele F, Deryck L and
Lambrichts I: Radial glial cells derived from the neonatal rat
spinal cord: morphological and immunocytochemical characterization.
Arch Hictol Cytol. 68:361–369. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun X, Liu M, Wei Y, Liu F, Zhi X, Xu R
and Krissansen GW: Overexpression of von Hippel-Lindau tumor
suppressor protein and antisense HIF-1alpha eradicates gliomas.
Cancer Gene Ther. 13:428–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bampton ET and Taylor JS: Effects of
Schwann cell secreted factors on PC12 cell neuritogenesis and
survival. J Neurobiol. 63:29–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Afshari FT, Kwok JC, White L and Fawcett
JW: Schwann cell migration is integrin-dependent and inhibited by
astrocyte-produced aggrecan. Glia. 58:857–869. 2010.PubMed/NCBI
|
|
30
|
Pearse DD, Sanchez AR, Pereira FC, Andrade
CM, Puzis R, Pressman Y, Golden K, Kitay BM, Blits B, Wood PM and
Bunge MB: Transplantation of Schwann cells and/or olfactory
ensheathing glia into the contused spinal cord: Survival,
migration, axon association, and functional recovery. Glia.
55:976–1000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takami T, Oudega M, Bates ML, Wood PM,
Kleitman N and Bunge MB: Schwann cell but not olfactory ensheathing
glia transplants improve hindlimb locomotor performance in the
moderately contused adult rat thoracic spinal cord. J Neurosci.
22:6670–6681. 2002.PubMed/NCBI
|
|
32
|
Saberi H, Moshayedi P, Aghayan HR, Arjmand
B, Hosseini SK, Emami-Razavi SH, Rahimi-Movaghar V, Raza M and
Firouzi M: Treatment of chronic thoracic spinal cord injury
patients with autologous Schwann cell transplantation: an interim
report on safety considerations and possible outcomes. Neurosci
Lett. 443:46–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zabner J, Ramsey BW, Meeker DP, et al:
Repeat administration of an adenovirus vector encoding cystic
fibrosis transmembrane conductance regulator to the nasal
epithelium of patients with cystic fibrosis. J Clin Invest.
97:1504–1511. 1996. View Article : Google Scholar
|
|
34
|
Giehl KM, Schacht CM, Yan Q and Mestres P:
GDNF is a trophic factor for adult rat corticospinal neurons and
promotes their long-term survival after axotomy in vivo. Eur J
Neurosci. 9:2479–2488. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng H, Wu JP and Tzeng SF:
Neuroprotection of glial cell line-derived neurotrophic factor in
damaged spinal cords following contusive injury. J Neurosci Res.
69:397–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guzen FP, de Almeida Leme RJ, de Andrade
MS, de Luca BA and Chadi G: Glial cell line-derived neurotrophic
factor added to a sciatic nerve fragment grafted in a spinal cord
gap ameliorates motor impairments in rats and increases local
axonal growth. Restor Neurol Neurosci. 27:1–16. 2009.PubMed/NCBI
|
|
37
|
Gerngross TU: Advances in the production
of human therapeutic proteins in yeasts and filamentous fungi. Nat
Biotechnol. 22:1409–1414. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takada N, Isogai E, Kawamoto T, Nakanishi
H, Todo S and Nakagawara A: Retinoic acid-induced apoptosis of the
CHP134 neuroblastoma cell line is associated with nuclear
accumulation of p53 and is rescued by the GDNF/Ret signal. Med
Pediatr Oncol. 36:122–126. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McAlhany RE Jr, West JR and Miranda RC:
Glial-derived neurotrophic factor (GDNF) prevents ethanol-induced
apoptosis and JUN kinase phosphorylation. Brain Res Dev Brain Res.
119:209–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Villegas SN, Njaine B, Linden R and Carri
NG: Glial-derived neurotrophic factor (GDNF) prevents ethanol
(EtOH) induced B92 glial cell death by both PI3K/AKT and MEK/ERK
signaling pathways. Brain Res Bull. 71:116–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ghribi O, Herman MM, Forbes MS, DeWitt DA
and Savory J: GDNF protects against aluminum-induced apoptosis in
rabbits by upregulating Bcl-2 and Bcl-XL and inhibiting
mitochondrial Bax translocation. Neurobiol Dis. 8:764–773. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Walensky LD: BCL-2 in the crosshairs:
tipping the balance of life and death. Cell Death Differ.
13:1339–1350. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|