Introduction
Inflammation is a process associated with numerous
diseases, which is regulated by a variety of immune cells and
effector molecules. Nitric oxide (NO), prostaglandin E2
(PGE2), and pro-inflammatory cytokines are important
mediators of macrophage-mediated inflammation (1,2).
Various in vitro and in vivo models have been used to
measure the inhibitory effects of natural products on inflammatory
cytokines and other inflammatory mediators (3,4).
Lipopolysaccharide (LPS) is a potent activator of macrophage cells,
which produce a variety of pro-inflammatory mediators such as NO,
prostaglandins, and cytokines (5,6).
Among these molecules, inducible nitric oxide synthase (iNOS) and
cyclooxygenase-2 (COX-2) are two pro-inflammatory enzymes that play
a critical role in inflammation. They produce the pro-inflammatory
mediators NO and PGE2, respectively, thereby enhancing
expression of pro-inflammatory cytokines, including interleukin-6
(IL-6) and IL-1β (7–9).
Expression of inflammatory cytokines and the genes
that encode them can be regulated by activation of the
transcription factor nuclear factor-κB (NF-κB), which is crucially
involved in chronic inflammatory diseases (10). Phosphorylation of mitogen-activated
protein kinases (MAPKs), including MAP kinase kinase (MEK),
extracellular signal-related kinase (ERK), p38, and C-Jun
N-terminal kinase (JNK) is also a key factor in the inflammatory
response (11). These events lead
to the activation of macrophages, which consequently express genes
encoding pro-inflammatory proteins such as iNOS and COX-2, and
inflammatory cytokines (12).
Ardisia tinctoria (AT) is a plant of the Myrsinaceae
family, which has traditionally been used as a natural black dye.
Although some anti-inflammatory activities from other species of
the genus Ardisia have been reported (13,14),
the biological activity of AT has yet not been studied.
In this study, to explore the anti-inflammatory
properties of AT, we investigated the effect of an AT extract on
the production of inflammatory mediators in a macrophage cell line,
as well as in an in vivo model of carrageenan-induced paw
edema.
Materials and methods
Preparation of the AT extract from
Aldisia tinctoria
Samples of the plant were collected from the Dak
Glei District of Kon Tum Province, Dakman, Vietnam in 2009. The
samples were identified as Aldisia tinctoria by Dr Tran The
Bach at the Institute of Ecology and Biological Resources, Hanoi,
Vietnam. A voucher specimen (KRIBB 0027029) was deposited in the
herbarium of the Korea Research Institute of Bioscience and
Biotechnology. AT (113 g) was treated with MeOH and sonicated
several times at room temperature for 3 days to produce the extract
(total, 10.3 g).
Cell cultures
The RAW 264.7 murine macrophage cell line was
cultured in Dulbecco’s modified Eagle’s medium (Gibco-BRL, Grand
Island, NY, USA) supplemented with 10% (v/v) heat-inactivated fetal
bovine serum (HyClone, Logan, UT, USA) and 1% (w/v) of 100X
antibiotic-antimycotic solution (cat. no. 15240–062; Invitrogen
Life Technologies, Carlsbad, CA, USA), at 37°C, in a 95% air and 5%
CO2 (v/v) atmosphere.
Cell viability
The viability of cells treated with various
concentrations of AT was monitored with a
3-(4,5-dimethylthiaxol-2yl)-2,5-diphenyltetrazolium bromide (MTT)
(CAS#298-93-1; Amresco, Solon, OH, USA) assay. MTT solution (5
mg/ml) was added to the cell supernatant at a final concentration
of 0.5 mg/ml. After 4 h of incubation at 37°C, the medium was
removed and dimethylsulfoxide (DMSO) was added to the 96-well
plates. The optical density of formazan was measured at 570 nm
using a Benchmark microplate reader (Bio-Rad Laboratories,
Hercules, CA, USA). The level of formazan generated by untreated
cells was used to define the 100% value.
Nitric oxide assay
RAW 264.7 cells were plated at a density of
5×105 cells/ml in 96-well plates, and incubated with or
without LPS (0.5 μg/ml) in the absence or presence of various
concentrations of AT extract for 24 h. Nitrite accumulation in
supernatants was assessed using the Griess reaction (15). Aliquots (100 μl) of culture
supernatants were mixed with equal volumes of Griess reagent [0.1%
(w/v) N-(1-naphthyl)-ethylenediamine, with 1% (w/v) sulfanilamide
in 5% (v/v) phosphoric acid] and incubated at room temperature for
10 min. Absorbance was measured at 540 nm using a microplate
reader, and a series of known sodium nitrite concentrations served
as standards.
Enzyme-linked immunosorbent assay
(ELISA)
PGE2, IL-6, and IL-1β levels were
quantified in the supernatants using the PGE2 EIA kit
(Cayman Chemical Co., Ann Arbor, MI, USA), and the IL-6- and
IL-1β-sensitive Quantikine Biosource™ ELISA kits (Invitrogen Life
Technologies, Camarillo, CA, USA) according to the manufacturer’s
instructions.
Reverse transcription (RT)-PCR
analysis
Total RNA was isolated from RAW 264.7 cells using
TRIzol (Invitrogen Life Technologies), according to the
manufacturer’s instructions. The purified RNA was resuspended in
diethylpyrocarbonate-treated water, and its integrity was confirmed
by electrophoresis on an agarose-formaldehyde gel. cDNA was
synthesized from total RNA that was previously treated with
DNA-free DNase (Ambion Inc., Austin, TX, USA) according to
indications of the supplier. RNA (1 μg) was incubated with
oligo(dT)18-mer (Bioneer Corp., Daejeon, Korea), RNase OUT
(Invitrogen Life Technologies) and the Omniscript RT kit reagents
(Qiagen, Hilden, Germany) for 60 min at 37°C, following the
indications of the supplier. The amplification of genes coding for
iNOS (forward primer, 5′-GGA GCG ACT TGT GGA TTG TC-3′ and reverse,
5′-GTG AGG GCT TGG CTG AGT GAG-3′) and COX-2 (forward, 5′-GAA GTC
TTT GGT CTG GTG CCT G-3′ and reverse, 5′-GTC TGC TGG TTT GGA ATA
GTT GC-3′) was performed in the GeneAmp® PCR System 9700
(Applied Biosystems, Foster City, CA, USA) at 94°C for 30 sec, at
56°C for 30 sec, and at 72°C for 30 sec. As an internal control,
the gene coding for β-actin was amplified using forward primer,
5′-AGG CTG TGC TGT CCC TGT ATG C-3′ and reverse, 5′-ACC CAA GAA GGA
AGG CTG GAA A-3′. The amplified products were resolved on a 1.2%
(w/v) agarose gel, stained with RedSafe (Intron Biotechnology,
Seongnam-si, Korea), and photographed under ultraviolet light.
Western blot analysis
AT-treated and untreated RAW 264.7 cells were
scraped and lysed in 100 μl of lysis buffer containing protease
inhibitors [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.5%
(v/v) NP-40, 0.1% (w/v) SDS, 1 mM EGTA, 100 μg/ml PMSF, 10 μg/ml
pepstatin A, and 100 μM Na3VO3]. Protein
concentrations in supernatants were determined using the Bradford
reagent (Bio-Rad Laboratories). Proteins (20 μg for each sample)
were separated by electrophoresis at 100 V on a SDS-polyacrylamide
gel for 90 min, and transferred to PVDF membranes (Amersham
Biosciences, Piscataway, NJ, USA). The membranes were blocked with
5% (w/v) non-fat dry milk dissolved in TBST buffer [10 mM Tris-HCl
(pH 7.5), 150 mM NaCl, 0.1% (v/v) Tween 20] overnight at 4°C and
incubated with primary antibodies that recognize iNOS (1:1,000
dilution; Enzo Clinical Labs Inc., Farmingdale, NY, USA); COX-2
(1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA);
β-actin (1:1,000; Cell Signaling Technology, Inc., Boston, MA,
USA); poly (ADP-ribose) polymerase (PARP), NF-κB subunit 65 (p65),
ERK 2, p38 MAPK and JNK1/3 (1:1,000; all from Santa Cruz
Biochemicals); phosphorylated forms of p38 MAPK and JNK1/2 (1:1000;
Enzo Clinical Labs Inc.) and phosphorylated forms of the NF-κB
inhibitor IκB-α, MEK, ERK and total IκB-α (1:1,000; Cell Signaling
Technology, Inc.). Immunoreactive bands were visualized using the
ECL reagent (Amersham Pharmacia Biotech, Uppsala, Sweden) on a
RAS-4000 mini film (Fujifilm, Tokyo, Japan). Densitometric values
for each band were determined using the ImageJ software version
1.43 (National Institutes of Health), and were statistically
analyzed.
Immunofluorescence
To analyze the nuclear localization of NF-κB, RAW
264.7 cells were maintained on Permanox plastic chamber slides
(Nunc, Rochester, NY, USA) for 24 h. Cells treated with AT for 1 h
were incubated with LPS for 1 h as described by Park et al
(16). Cells were fixed in ethanol
at 4°C for 30 min, and slides were washed three times with
phosphate-buffered saline (PBS) and blocked with 3% (w/v) bovine
serum albumin (BSA) in PBS for an additional 30 min. The slides
were then incubated for 24 h at 4°C with rabbit polyclonal IgG
antibodies targeting iNOS (1:200; Santa Cruz Biotechnology, Inc.)
and NF-κB p65 (1:500; Assay Designs, Ann Arbor, MI, USA). After
washing to remove the excess primary antibody, the slides were
further incubated with anti-rabbit Alexa Fluor 488-conjugated or
Texas Red-conjugated secondary antibodies (Santa Cruz Biotechnology
Inc.) for 2 h at room temperature, washed with PBS, and mounted
using ProLong® Gold Antifade reagent containing
4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen Life Technologies)
for 5 min. Subsequently, the slides were coverslipped and
visualized on a confocal laser scanning microscope (LSM510; Carl
Zeiss, Jena, Germany), to localize the proteins of interest. The
samples were photographed under the same exposure conditions and
nuclei were quantified from the obtained images.
Animal care and carrageenan-induced paw
edema
Pathogen-free female BALB/c mice (6 weeks old) were
purchased from Koatech Co. (Seoul, Korea) and used after 1 week of
quarantine and acclimatization. The mice were given sterilized tap
water and standard rodent food. The experimental procedures were
carried out in accordance with the NIH Guidelines for the Care and
Use of Laboratory Animals and were approved by the institutional
Animal Care and Use Committee of the Korea Research Institute of
Bioscience and Biotechnology. The animals were handled in
accordance with principles of the National Animal Welfare Law of
Korea as previously described.
Carrageenan-induced paw inflammation was established
based on a previously described method (18). Mice (~20 g) were randomly selected
and divided into groups of 5–6. AT was dissolved in PBS and
administered at 40 mg/kg doses. The other groups were either orally
administered with 5 mg/kg indomethacin (positive control) or
injected with PBS (negative control). After 30 min, the edema was
induced by the injection of 20 μl of 1% w/v carrageenan solution
(Sigma-Aldrich, St. Louis, MO, USA) in PBS into the animal’s left
hind paw. Measurements of the paw volume were performed by means of
a caliper immediately prior to the carrageenan injection and 4 h
later. The paw thickness was determined by the difference between
the final and initial thickness.
Statistical analysis
For statistical analysis, values were expressed as
means ± SEMs. Statistical significance was determined using the
two-tailed Student’s t-test for independent samples. P<0.05 was
considered to indicate statistically significant differences.
Results
Effects of AT extract on NO production in
LPS-treated RAW 264.7 cells
To determine the inhibitory effect of AT on NO
production, measured as nitrite levels, RAW 264.7 cells were
treated with LPS. The AT extract markedly reduced nitrite levels in
LPS-induced cells in a dose-dependent manner (Fig. 1A). As shown in Fig. 1B–D, expression levels of iNOS were
determined by western blot analysis, immunofluorescence and RT-PCR.
These assays showed that LPS treatment increased the expression of
iNOS protein and mRNA; however, pretreatment of cells with the AT
extract attenuated the LPS-induced iNOS expression in a
dose-dependent manner. This finding suggested that the AT extract
inhibits NO production by inhibiting iNOS gene expression in
LPS-stimulated RAW 264.7 cells.
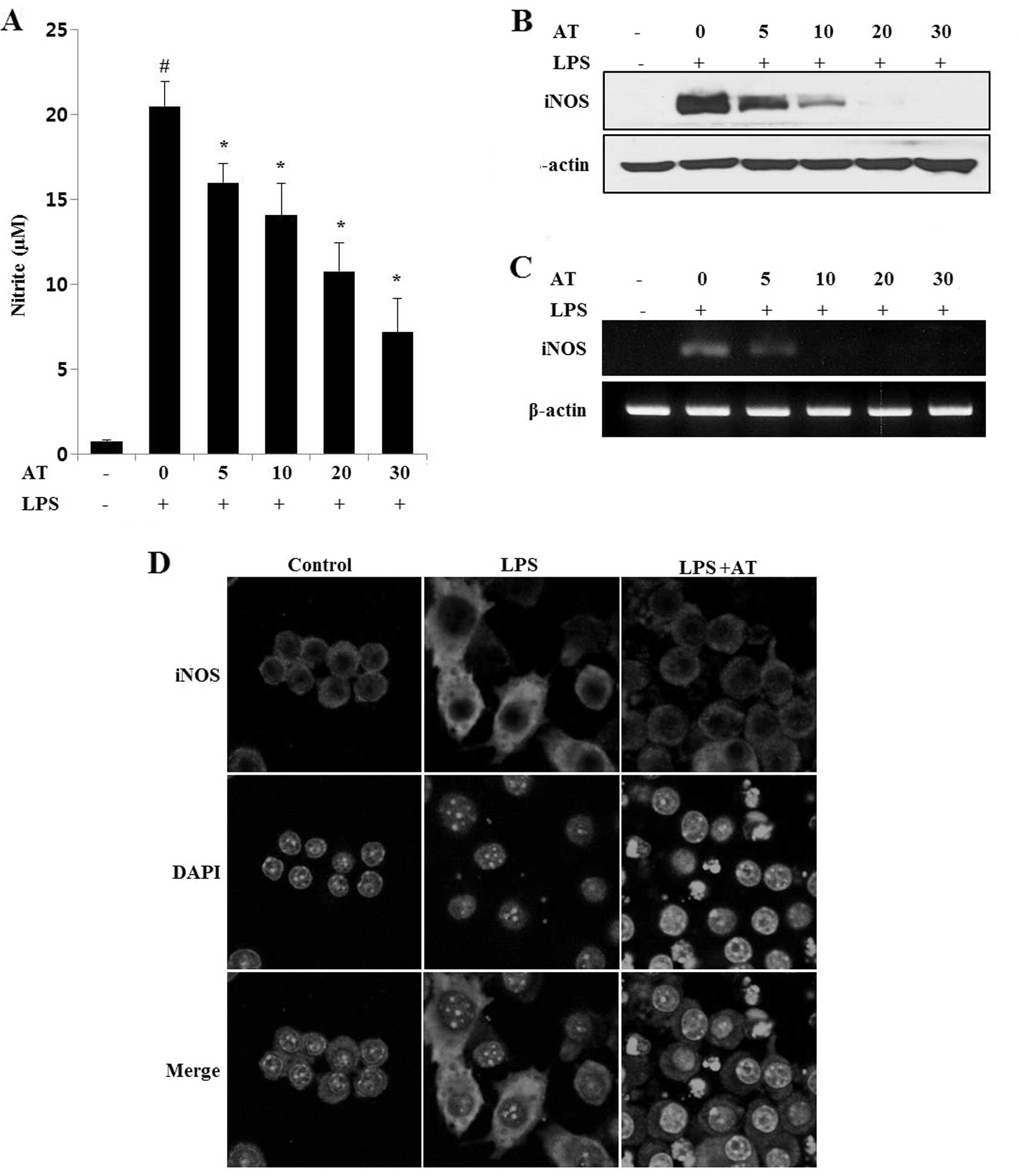 | Figure 1Lipopolysaccharide (LPS)-induced
nitric oxide (NO) production and nitric oxide synthase (iNOS)
expression are inhibited by Ardisia tinctoria (AT) extract
in RAW 264.7 cells. (A) RAW 264.7 cells were treated with AT
extract (5, 10, 20 and 30 μg/ml) for 1 h, and with LPS (0.5 μg/ml)
for 6 or 24 h. The supernatant was collected at 24 h, and nitrite
concentrations were measured using the Griess reaction. Three
independent experiments were performed, and the data are presented
as means ± SEM. #P<0.05; *P<0.05;
**P<0.01; and ***P<0.001 compared to
control (#) or cells treated with LPS alone (*, **,
***). (B) iNOS mRNA and (C) iNOS protein levels were measured
with reverse transcription (RT)-PCR and western blotting assays,
respectively, using β-actin as an internal control. (D)
Immunocytochemical analysis of iNOS expression. After fixation,
cells were stained with Alexa 488. Nuclei were visualized using
DAPI, and observed at ×400 magnification. Merge, superposition of
the DAPI and iNOS fields; Control, untreated cells; LPS, LPS (0.5
μg/ml) treatment; AT, AT extract (40 μg/ml) and LPS treatment. |
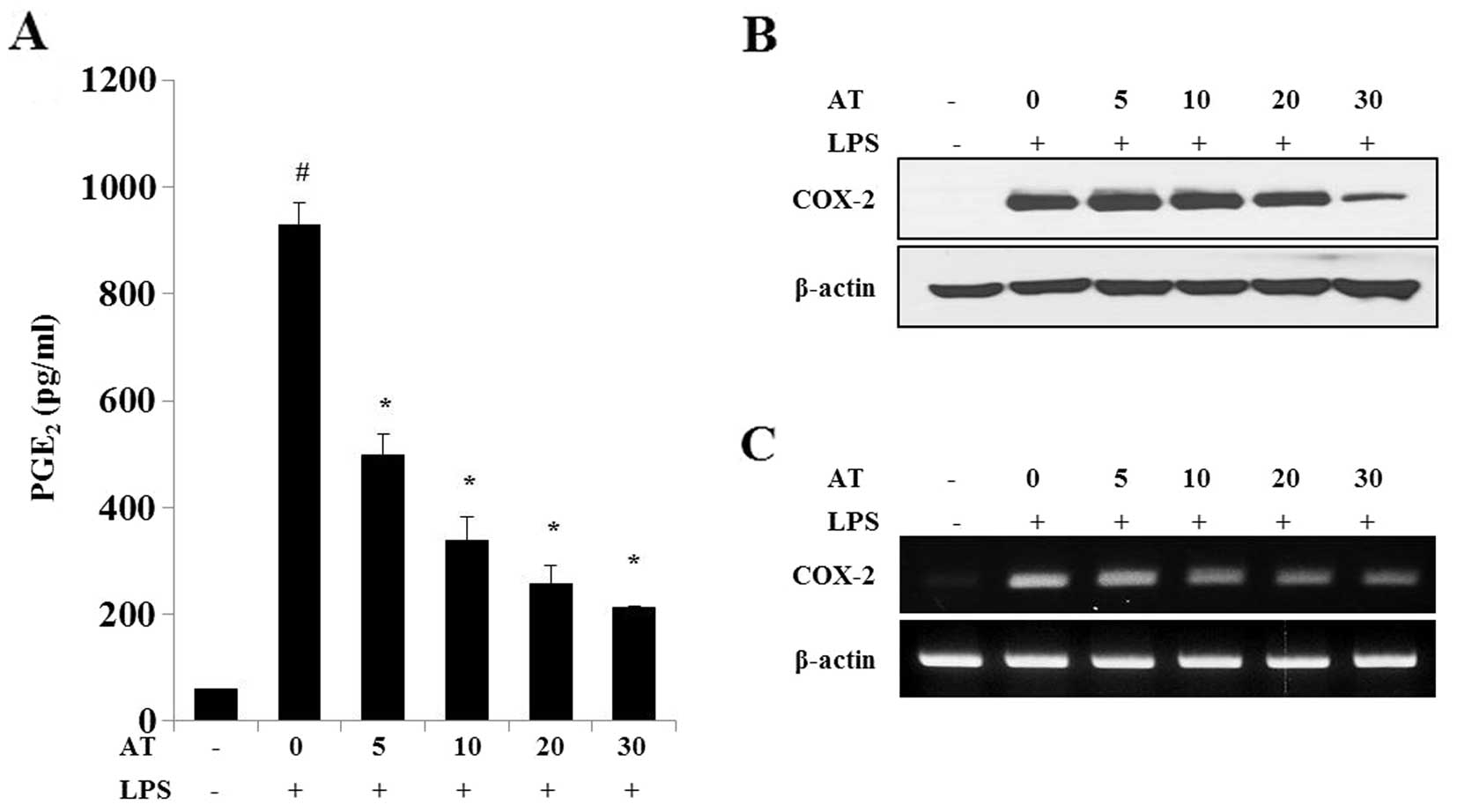
Effects of AT extract on PGE2
production in LPS-treated RAW 264.7 cells
The AT extract strongly inhibited the LPS-induced
increase in PGE2 production (Fig. 2A) in a dose-dependent manner, while
COX-2 protein (Fig. 2B) and mRNA
(Fig. 2C) levels were reduced by
treatment with the AT extract. Our results indicated that the AT
extract has the potential to inhibit LPS-induced PGE2
and COX-2 expression in RAW 264.7 cells.
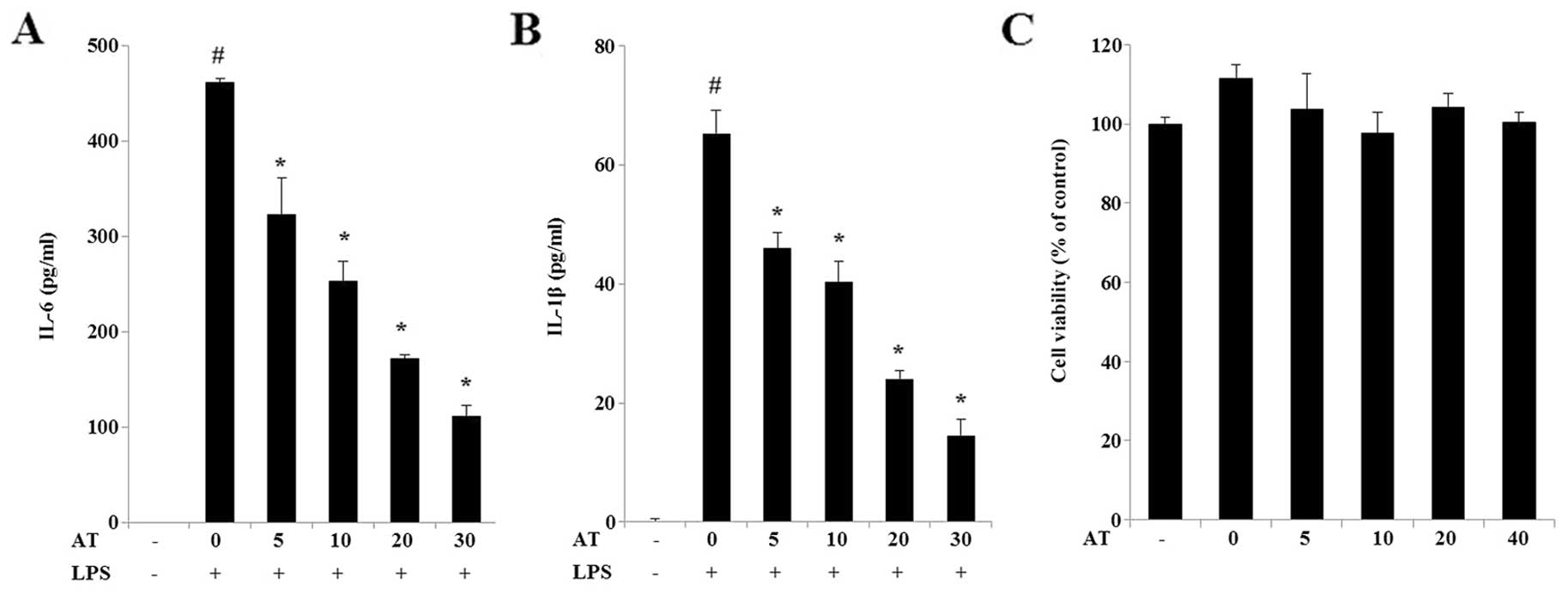
AT extract inhibits IL-6 and IL-1β
release in LPS-treated RAW 264.7 cells
Cytokines are produced during the inflammatory
process, and cytokine levels are indicative of the progression of
inflammation. We used IL-6- and IL-1β-sensitive ELISA kits to
assess the levels of these cytokines in RAW 264.7 cells incubated
with different concentrations of AT extract (5, 10, 20 and 30
μg/ml) for 24 h. LPS treatment caused an increase in the levels of
IL-6 (Fig. 3A) and IL-1β (Fig. 3B) in RAW 264.7 cells, while their
levels were reduced in a dose-dependent manner by exposure to AT.
Treatment with the AT extract did not affect the viability of
cultured RAW 264.7 cells (Fig.
3C).
Inhibitory effect of AT extract on NF-κB
activation in LPS-treated RAW 264.7 cells
We examined the effect of the AT extract on
LPS-induced NF-κB activation. NF-κB, which induces both iNOS and
COX-2, is translocated to the nucleus following phosphorylation and
subsequent degradation of IκB-α. Treatment of RAW 264.7 cells with
AT for 1 h markedly inhibited the nuclear level of the NF-κB p65
subunit induced by LPS (Fig. 4A).
Confocal microscopy showed NF-κB translocation to the nucleus in
response to LPS stimulation (Fig.
4B). Our results suggested that AT inhibits the expression of
pro-inflammatory enzymes such as iNOS and COX-2, by blocking the
translocation of NF-κB to the nucleus.
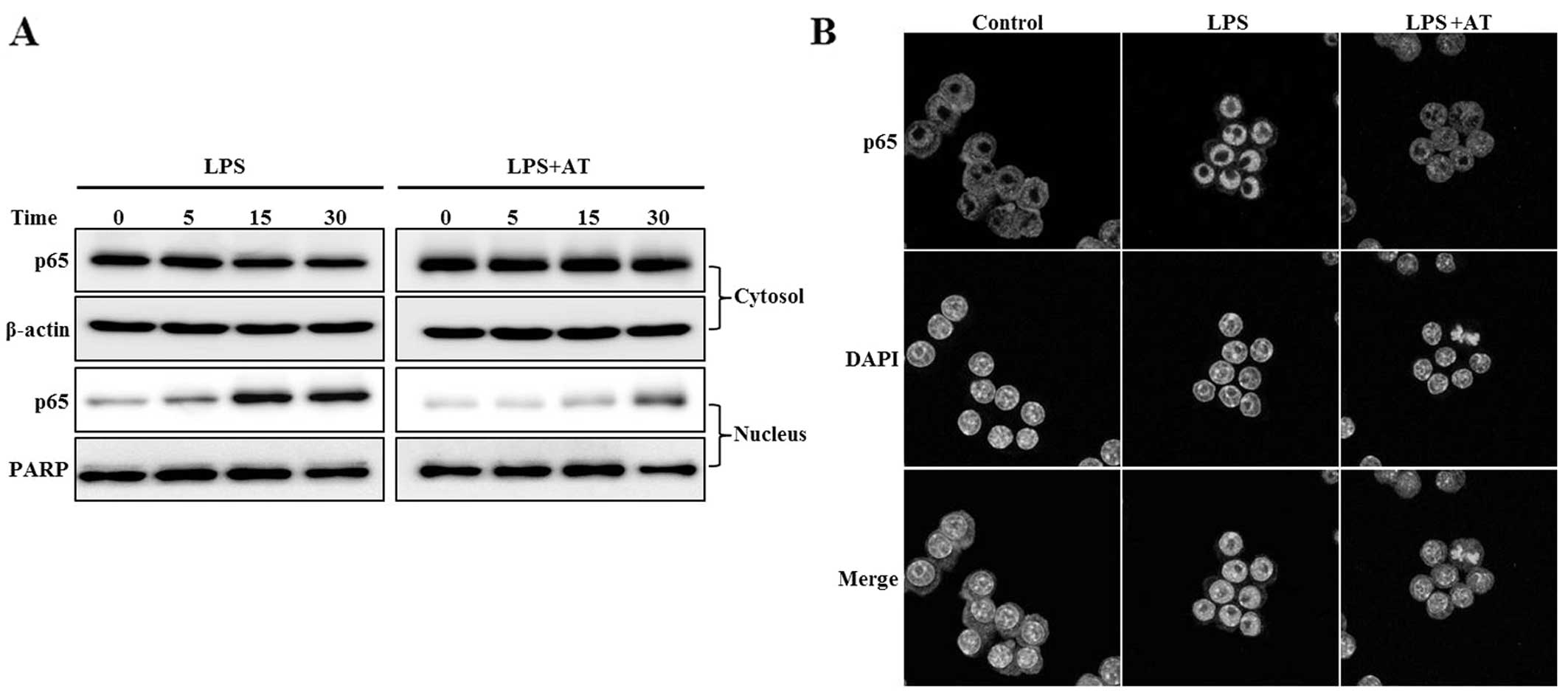 | Figure 4Ardisia tinctoria (AT) extract
inhibits the translocation of nuclear factor-κB (NF-κB) to the
nucleus in lipopolysaccharide (LPS)-treated RAW 264.7 cells. Cells
were treated with AT extract (30 μg/ml) for 1 h, then with LPS for
1 h hour, and the cytosol and nuclear fractions were separated. (A)
Translocation of NF-κB p65 to the nucleus, as determined by western
blot. Antibodies targeting β-actin and poly (ADP-ribose) polymerase
(PARP) were used as internal controls for the western blot analysis
of cytosolic and nuclear proteins, respectively. (B)
Immunocytochemical examination of NF-κB translocation. After
fixation, the cells were stained with Alexa 488. Nuclei were
visualized using DAPI, and observed at ×400 magnification. Merge,
superposition of the DAPI and iNOS fields; Control, untreated
cells; LPS, LPS (0.5 μg/ml) treatment; AT, AT extract (40 μg/ml)
and LPS treatment. Representative results of three independent
experiments are shown. |
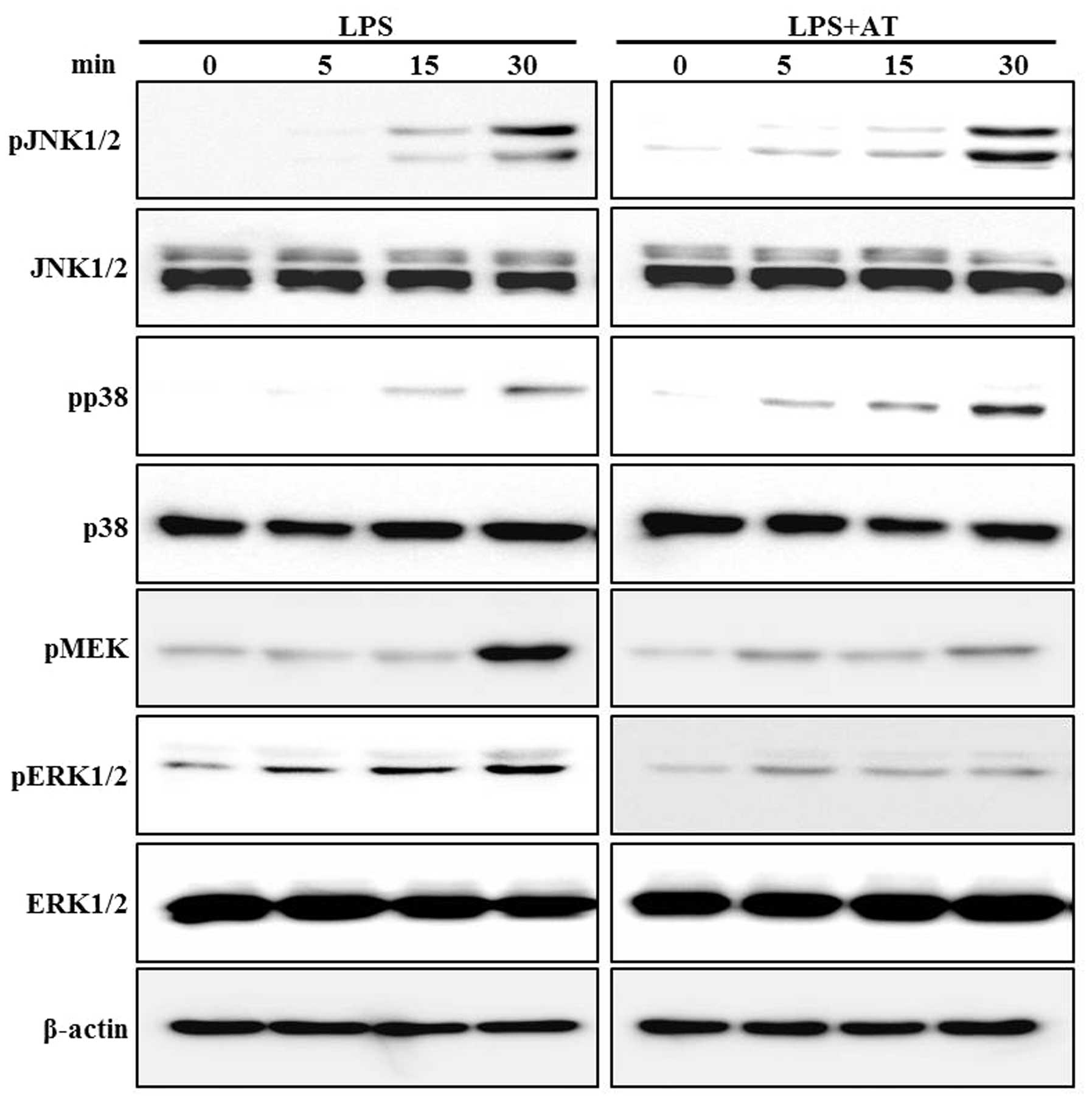
Inhibitory effect of AT extract on
phosphorylation of MEK and ERK in LPS-treated RAW 264.7 cells
We examined the effect of the AT extract on
LPS-induced phosphorylation of MEK, ERK, JNK and p38 MAPK. Western
blotting assays were performed after treating the cells with LPS
(0.5 μg/ml) for 0, 5, 15 and 30 min. Phosphorylation of these
proteins was clearly induced only at 30 min of LPS treatment. The
AT extract inhibited the LPS-induced phosphorylation of ERK in a
time-dependent manner, while reduced phosphorylation of MEK was
observed at 40 min of LPS treatment (Fig. 5).
Effect of AT extract on
carrageenan-induced paw edema in mice
Edema was induced by injection of carrageenan into
the paw of six mice. As shown in Fig.
6, the injection of carrageenan for 4 h significantly increased
the thickness of paw edema. Indomethacin, a non-steroidal
anti-inflammatory drug commonly used in the clinic, was used as a
positive control for the reduction in paw edema thickness.
Pre-treatment with 5 mg/kg of indomethacin effectively and
significantly reduced paw edema thickness. Similarly,
pre-administration of AT (40 mg/kg) markedly inhibited (by 58.8%)
carrageenan-induced paw edema thickness.
Discussion
In the present study, we evaluated the
anti-inflammatory effect of AT both in vivo and in vitro. To
understand the related molecular mechanisms, we investigated the
production of NO, PGE2, IL-1β and IL-6, the expression
of iNOS and COX-2, and the activation of the NF-κB and MAPK
signaling pathway proteins in response to AT.
Macrophages are an important component of the human
immune defense system. During the progress of inflammation,
macrophages actively participate in inflammatory responses by
releasing the pro-inflammatory cytokines IL-1β and IL-6, as well as
other inflammatory factors, such as NO and PGE2, which
recruit additional immune cells to the sites of infection or tissue
injury (1). In this study, we
validated the effects of AT on the secretion of NO and
PGE2 in the supernatants of cultured RAW 264.7 cells
treated with LPS. Pre-treatment with AT reduced the LPS-induced
secretion of NO and PGE2. In addition, AT treatment
significantly inhibited the production of the cytokines IL-1β and
IL-6. AT also effectively inhibited the mRNA and protein expression
of iNOS, which triggers the effector molecule NO (19) and of COX-2, which catalyzes the
production of PGE2 (20).
LPS-induced secretion and expression of inflammatory
mediators in RAW 264.7 macrophage cells is activated by diverse
intracellular signals, including the NF-κB and MAP kinase pathways
(16). Two NF-κB pathways exist,
and it is believed that these play distinct and important roles in
the innate and the acquired immune response (21). NF-κB has been shown to be a key
transcription factor that activates several cellular signal
transduction pathways involved in the production of iNOS, COX-2 and
various cytokines (22,23). Immunofluorescence visualizations
suggested that AT inhibits iNOS and COX-2 expression through
reduction of the nuclear levels of translocated NF-κB p65 in
LPS-stimulated RAW 264.7 macrophage cells. MAPK family members,
including MEK, ERK, JNK and the MAPK p38 subunit, are also
activated and phosphorylated following LPS treatment (24–26).
In our study, LPS-stimulated phosphorylation of MEK and ERK was
inhibited by pretreatment with AT. Moreover, we tested the effects
of AT on acute inflammation in mice using the carrageenan-induced
paw edema model. The latter has been established as a valid model
for studying inflammatory states and screening of components for
anti-inflammatory activity. A reduction in edema thickness is a
good indicator of the protective action of anti-inflammatory agents
(27). AT (40 mg/kg) inhibited the
thickness of paw edema after 4 h of carrageenan treatment.
In summary, this study demonstrated that a methanol
extract of AT may possess anti-inflammatory effects, since it can
affect the production of NO, PGE2, IL-1β and IL-6, as
well as the mRNA and protein expression of iNOS and COX-2. These
effects may be mediated by the inhibition of LPS-induced NF-κB
activation and the phosphorylation of MEK and ERK, which were
observed in vitro in the RAW 264.7 macrophage cells.
Acknowledgements
This study was supported by grants from FGC (no.
1011231) and KGM (no. 1221312) awarded to the Korea Research
Institute of Bioscience and Biotechnology (KRIBB) of the Republic
of Korea.
References
|
1
|
Bosca L, Zeini M, Traves PG and Hortelano
S: Nitric oxide and cell viability in inflammatory cells: a role
for NO in macrophage function and fate. Toxicology. 208:249–258.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moncada S: Nitric oxide: discovery and
impact on clinical medicine. J R Soc Med. 92:164–169.
1999.PubMed/NCBI
|
|
3
|
Tripathi P, Tripathi P, Kashyap L and
Singh V: The role of nitric oxide in inflammatory reactions. FEMS
Immunol Med Microbiol. 51:443–452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winter CA, Risley EA and Nuss GW:
Carrageenin-induced edema in hind paw of the rat as an assay for
antiiflammatory drugs. Proc Soc Exp Biol Med. 111:544–547. 1962.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujihara M, Muroi M, Tanamoto K, Suzuki T,
Azuma H and Ikeda H: Molecular mechanisms of macrophage activation
and deactivation by lipopolysaccharide: roles of the receptor
complex. Pharmacol Ther. 100:171–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuroda E and Yamashita U: Mechanisms of
enhanced macrophage-mediated prostaglandin E2 production and its
suppressive role in Th1 activation in Th2-dominant BALB/c mice. J
Immunol. 170:757–764. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moncada S, Palmer RM and Higgs EA: Nitric
oxide: physiology, pathophysiology, and pharmacology. Pharmacol
Rev. 43:109–142. 1991.
|
|
8
|
Elder DJ, Halton DE, Hague A and Paraskeva
C: Induction of apoptotic cell death in human colorectal carcinoma
cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal
anti-inflammatory drug: independence from COX-2 protein expression.
Clin Cancer Res. 3:1679–1683. 1997.PubMed/NCBI
|
|
9
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:519–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Makarov SS: NF-kappaB as a therapeutic
target in chronic inflammation: recent advances. Mol Med Today.
6:441–448. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bresnihan B: Pathogenesis of joint damage
in rheumatoid arthritis. J Rheumatol. 26:717–719. 1999.
|
|
13
|
de Mejia EG and Ramirez-Mares MV: Ardisia:
health-promoting properties and toxicity of phytochemicals and
extracts. Toxicol Mech Methods. 21:667–674. 2011.PubMed/NCBI
|
|
14
|
Hamsin DE, Hamid RA, Yazan LS, Taib CN and
Ting YL: The hexane fraction of Ardisia crispa Thunb. A DC
roots inhibits inflammation-induced angiogenesis. BMC Complement
Altern Med. 13:52013.
|
|
15
|
Kim H, Lee HS, Chang KT, Ko TH, Baek KJ
and Kwon NS: Chloromethyl ketones block induction of nitric oxide
synthase in murine macrophages by preventing activation of nuclear
factor-kappa B. J Immunol. 154:4741–4748. 1995.PubMed/NCBI
|
|
16
|
Park JW, Kwon OK, Jang HY, et al: A leaf
methanolic extract of Wercklea insignis attenuates the
lipopolysaccharide-induced inflammatory response by blocking the
NF-κB signaling pathway in RAW 264.7 macrophages. Inflammation.
35:321–331. 2012.PubMed/NCBI
|
|
17
|
Yuk JE, Lee MY, Kwon OK, et al: Effects of
astilbic acid on airway hyperresponsiveness and inflammation in a
mouse model of allergic asthma. Int Immunopharmacol. 11:266–273.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Henriques MG, Silva PM, Martins MA, et al:
Mouse paw edema. A new model for inflammation? Braz J Med Biol Res.
20:243–249. 1987.PubMed/NCBI
|
|
19
|
Connelly L, Palacios-Callender M, Ameixa
C, Moncada S and Hobbs AJ: Biphasic regulation of NF-κB activity
underlies the pro- and anti-inflammatory actions of nitric oxide. J
Immunol. 166:3873–3881. 2001.
|
|
20
|
Cuccurullo C, Fazia ML, Mezzetti A and
Cipollone F: COX-2 expression in atherosclerosis: the good, the bad
or the ugly? Curr Med Chem. 14:1595–1605. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bonizzi G and Karin M: The two NF-κB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004.
|
|
22
|
Hayden MS and Ghosh S: Signaling to NF-κB.
Genes Dev. 18:2195–2224. 2004.
|
|
23
|
Marks-Konczalik J, Chu SC and Moss J:
Cytokine-mediated transcriptional induction of the human inducible
nitric oxide synthase gene requires both activator protein 1 and
nuclear factor κB-binding sites. J Biol Chem. 273:22201–22208.
1998.PubMed/NCBI
|
|
24
|
Geppert TD, Whitehurst CE, Thompson P and
Beutler B: Lipopolysaccharide signals activation of tumor necrosis
factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol
Med. 1:93–103. 1994.PubMed/NCBI
|
|
25
|
Hambleton J, Weinstein SL, Lem L and
DeFranco AL: Activation of c-Jun N-terminal kinase in bacterial
lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA.
93:2774–2778. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han J, Lee JD, Bibbs L and Ulevitch RJ: A
MAP kinase targeted by endotoxin and hyperosmolarity in mammalian
cells. Science. 265:808–811. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Handy RL and Moore PK: A comparison of the
effects of L-NAME, 7-NI and L-NIL on carrageenan-induced hindpaw
oedema and NOS activity. Br J Pharmacol. 123:1119–1126. 1998.
View Article : Google Scholar : PubMed/NCBI
|