Introduction
Ovarian cancer is the fourth leading cause of
cancer-related mortality in females in the world and the leading
cause of mortality from a gynecological cancer. The standard
first-line treatment for ovarian cancer has not markedly altered
since 1996 and includes the intravenous administration of a
platinum agent (carboplatin or cisplatin) and paclitaxel (Taxol)
(1). Initially, the majority of
patients respond, however, the disease usually recurs within 5
years. Thus, fewer than one in 10 patients survive beyond 5 years
following standard salvage chemotherapy (2). Consequently, there is an urgent need
to identify new and improved therapeutic approaches that are able
to target this malignancy and improve long-term patient
survival.
Cancer progression and development is predominantly
dependent on the cancer stem cells (CSCs) residing or populating
within the cancer. The self-renewing, near infinite proliferative
capacity and potential for differentiation of CSCs is of vital
importance in the occurrence, development and metastasis of cancer.
It is believed that targeting CSCs may offer important and perhaps
revolutionary advances in the targeting of cancer. Several dietary
compounds, including curcumin (3,4),
quercetin (5,6), genistein (7–9),
sulforaphane (10–14) and epigallocatechin-gallate
(15,16), may have potential therapeutic
utility against CSC self-renewal. Previously, studies by our
laboratory have demonstrated that the novel synthetic genistein
analogue, 7-difluoromethoxyl-5,4′-di-n-octyl genistein (DFOG),
induced cellular apoptotic death of ovarian and gastric cancer
cells (17,18). However, whether DFOG inhibits the
self-renewing capacity of ovarian cancer stem cells (OCSCs) has not
been previously demonstrated.
The current study demonstrated that sphere-forming
cells (SFCs) derived from the ovarian cancer cell-line SKOV3
possessed ovarian cancer stem-like cell (OCSLC) properties,
including self-renewal and high tumorigenicity. For the first time,
to the best of our knowledge, DFOG has been demonstrated to
significantly inhibit the self-renewal capacity of OCSCs through a
mechanism partly dependent on the activation of FOXO3a.
Materials and methods
Cell culture and reagents
The human ovarian cancer cell-lines, SKOV3, A2780
and OVCAR-3 were obtained from the cell bank of the Chinese Academy
of Sciences (Shanghai, China). The cells were maintained as a
monolayer in high glucose DMEM supplemented with 10% vol/vol fetal
bovine serum (FBS), 100 IU/ml of penicillin G and 100 μg/ml of
streptomycin at 37°C in a fully-humidified 5% CO2
incubator. For sphere-forming culture, cells were collected and
washed to remove serum, then suspended in serum-free DMEM/F12
supplemented with 100 IU/ml of penicillin, 100 μg/ml of
streptomycin, 20 ng/ml of human recombinant epidermal growth
factor, 10 ng/ml of human recombinant basic fibroblast growth
factor, 2% wt/vol B27 supplement without vitamin A and 1% wt/vol N2
supplement (Invitrogen Life Technologies, Carlsbad, CA, USA). The
cells were subsequently cultured in ultra low attachment 6-well
plates (Corning Inc., Corning, NY, USA) at a density of 1,000
cells/well. The culture medium was replaced or supplemented with
additional growth factors twice a week. To propagate spheres in
vitro, the cells were collected by gentle centrifugation,
dissociated into single-cell suspensions and cultured to allow the
regeneration of spheres. Third-generation spheres were used for all
subsequent experiments.
In order to investigate the percentage of single
cells capable of regenerating new spheres, cells were plated at a
density of 1,000 cells/ml in a 6-well plate to obtain new spheres.
The total number of tumor spheres was counted following 8 days of
culture. The efficiency of sphere formation was calculated by
dividing the total number of spheres formed by the total number of
viable cells seeded and multiplying that result by a factor of
100.
In vivo tumorigenicity experiments
All mice were cared for in accordance with the
institutional guidelines of Hunan Normal University (Changsha,
Hunan, China). The study was approved by the Ethics Committee of
Hunan Normal University. Parental SKOV3 cells and the same cells at
the third passage of SFC formation were used in tumorigenicity
experiments. Trypan blue staining was used to assess cell viability
and various numbers of single viable cells were subcutaneously
injected into 4 week-old male BALB/c-nu mice (Shanghai Laboratory
Animal Center, Chinese Academy of Sciences, Shanghai, China) in
serum-free DMEM/Matrigel (at a 1:1 ratio) using a 100 μl
microsyringe. The mice were sacrificed 8 weeks following injection
and tumors were harvested for further study.
MTT assay
The SFCs that were derived from the SKOV3 cell-line
and parental cells were seeded at a density of 5,000 cells per well
in 96-well plates that had been previously coated with Matrigel.
The cells were treated with increasing concentrations of DFOG as
indicated. Following 48 h, MTT reagent (Sigma-Aldrich, St. Louis,
MO, USA) was then added to each well according to the
manufacturer’s instructions. Absorbance values of each well were
measured at 570 nm.
Western immunoblot analysis
Western blot analysis was performed as previously
described (18). The following
primary antibodies were used: anti-FOXO3a, anti-p-FOXO3a,
anti-Bmi1, anti-Nagon, anti-Sox2, anti-CD133, anti-CD44, anti-ALDH1
and anti-β-actin. The cells were lysed in a lysis buffer for 20 min
at 4°C. The protein concentration was determined using the Bio-Rad
assay system (Bio-Rad, Hercules, CA, USA). Total proteins were
fractionated by SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (Millipore, Billerica, MA, USA). The protein
signals were detected by an ECL advance western blot system
(Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA).
Plasmids and transfections
A non-specific control siRNA
(5′-UUCUCCGAACGUGUCACGUdTdT-3′) was obtained from Qiagen (Hilden,
Germany). FOXO3a siRNA (5′-ACUCCGGGUCCAGCUCCAC-3′) was synthesized
by Shanghai GenePharma Co., Ltd. (Shanghai, China). The
transfection of cells with siRNA was performed using Lipofectamine
2000 (Invitrogen Life Technologies) according to the manufacturer’s
instructions. The cells were exposed to DMSO (control) or 10 μmol/l
of DFOG for 24 h, 48 h after transfection. The cells were then
collected and processed for western blot analysis and tumorsphere
formation assay.
Statistical analysis
The database was set up with the SPSS 15.0 software
package (SPSS, Inc., Chicago, IL, USA) for analysis. Data are
presented as the mean ± SD. The means of multiple groups were
compared by one-way ANOVA, following confirmation of equal variance
and pairwise comparisons among the means by the LSD method.
Statistical comparisons were also made by the two-tailed t-test
when appropriate. An α value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Sphere formation and self-renewal in
ovarian cancer cell lines
To determine if a population of self-renewing CSCs
exists in ovarian cell-lines, SKOV3, OVCAR-3 and A2780 ovarian
cancer cell-lines were grown in serum-free sphere-forming
conditions. Following 8 days of culture, ball-like spheres ranging
from 50 to 100 cells per sphere were observed. The cells from three
cell lines formed spheres, however, at different sizes (Fig. 1A) and efficiencies (Fig. 1B), suggesting that each cell line
contained a different number of cancer stem-like cells. SKOV3 cells
exhibited a higher sphere-forming efficiency than OVCAR-3 and A2780
cells (Fig. 1B). Therefore, SKOV3
cells were selected for all further studies.
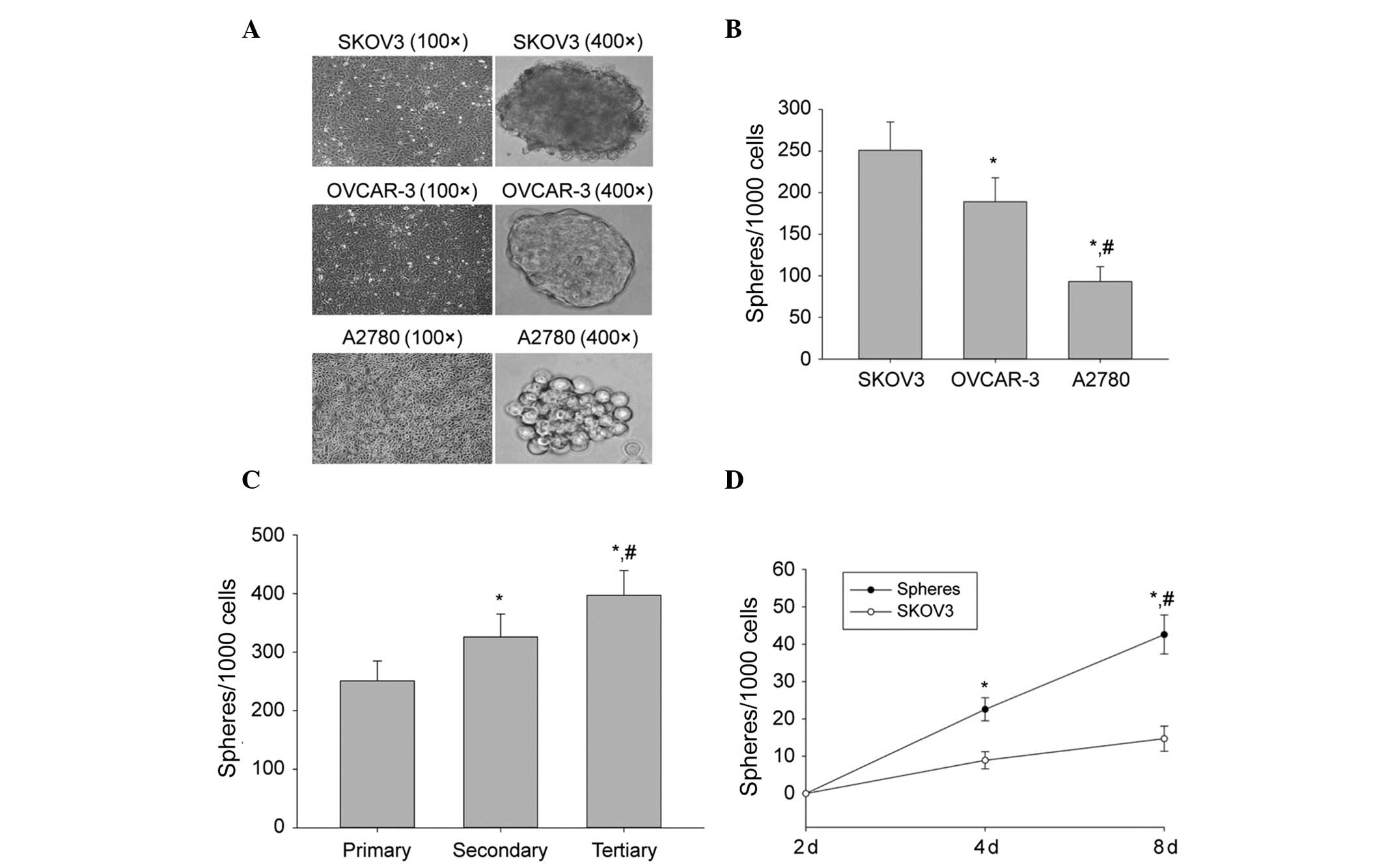 | Figure 1Sphere formation and self-renewal. (A)
In a conditioned stem cell culture system, the human ovarian cancer
cell lines SKOV3, OVCAR-3 and A2780 formed spheres and the volume
of SFCs derived from SKOV3 cells was greater in size than that of
either the OVCAR-3 or the A2780 cell-line. (B) Sphere-forming
efficiency of SKOV3, OVCAR-3 and A2780 cells. The number of
first-generation spheres formed on day 8 from 1,000 cells is shown.
The data are presented as the mean ± SD of three independent
experiments. *P<0.05, compared with SFCs derived from
SKOV3 cells; #P<0.05, compared with SFCs of OVCAR-3
cells. (C) Sphere-forming efficiency of SKOV3 during three serial
passages. The number of primary, secondary (generated from
dissociated primary spheres) and tertiary (generated from
dissociated secondary spheres) spheres obtained from 1,000 cells is
shown. The data are presented as the mean ± SD of three independent
experiments. *P<0.05, compared with primary spheres;
#P<0.05, compared with secondary spheres. (D) Number
of spheres generated from tertiary spheres and from SKOV3 parental
cells on the indicated days. Data are presented as the mean ± SD of
three independent experiments. *P<0.05, compared with
day 0; #P<0.05, compared with SKOV3 parental cells.
SFCs, sphere-forming cells. |
To assess the capability of SKOV3 cells to initiate
self-renewal, they were subjected to several serial passages. SKOV3
spheres were dissociated into single cells and grown at a clonal
density of 1,000 cells/ml. The dissociated first-generation spheres
were able to generate second-generation spheres, which subsequently
generated third-generation spheres (Fig. 1C). The sphere cultures were
maintained for >12 passages, suggesting that SFCs that were
derived from SKOV3 cells were fully capable of self-renewal. Thus,
SKOV3 third-generation spheres were used in all subsequent
experiments.
To determine the frequency of CSCs in SKOV3 third
generation spheres, a limiting dilution assay was used to examine
the ability of single cells from third-generation spheres to
produce new spheres. Following 8 days of culture, 39.4% of the
single cells had generated new spheres. These spheres were able to
be passaged >12 times. By contrast, a lower frequency of single
cells derived from the parental cells was capable of regenerating
spheres compared with single cells derived from third generation
spheres (Fig. 1D). These results
demonstrated that a considerable frequency of single cells that
were derived from third generation spheres were self-renewing cells
that were able to be expanded and maintained in culture as tumor
spheres.
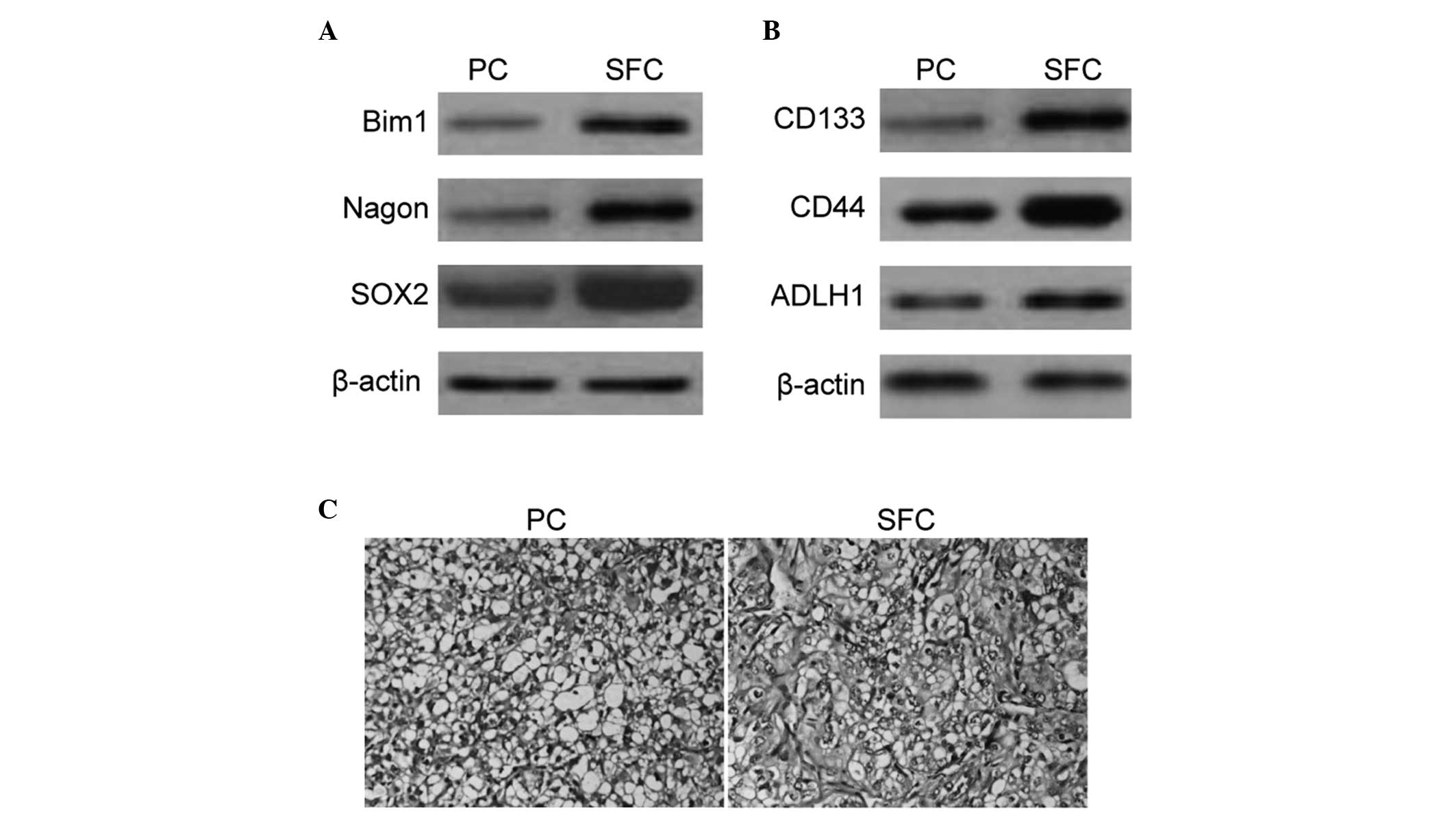
Enhanced expression of the self-renewal
associated markers and tumorigenicity in SFCs derived from the
SKOV3 cell line
To further characterize the expression profile of
cell surface markers belonging to the SFCs, western blot analysis
of several candidate self-renewal and stem cell associated markers
was performed. BMI1, Nagon and SOX2 populations were enriched in
tumor spheroids derived from SKOV3 cells (Fig. 2A). The present study also
demonstrated enrichment of CD133+, CD44+ and
ALDHhigh populations in SFCs derived from SKOV3 cells
(Fig. 2B).
To test the tumorigenic potential of cells grown
under sphere-forming conditions with enriched stem cells, SFCs of
the SKOV3 cell line and parental cells at varying cell numbers were
subcutaneously implanted in the flanks of nude mice. Tumors were
able to be formed with only 103 sphere cells, however, a
minimum of 106 parental cells was required to form
xenograft tumors (Table I). The
tumor nodules formed by SFCs of the SKOV3 cell line exhibited a
similar histology to that observed by the parental cells (Fig. 2C). The results suggested that the
tumorigenic efficacies of SFCs were enhanced compared with the
parental cells and non-adherent tumor spheres that were derived
from ovarian cancer SKOV3 cells cultured in stem cell conditioned
medium, which also possessed properties of OCSLCs.
 | Table ITumorigenicity experiments using SFCs
derived from the SKOV3 cell line and parental cells in BALB/c-nu
mice. |
Table I
Tumorigenicity experiments using SFCs
derived from the SKOV3 cell line and parental cells in BALB/c-nu
mice.
| Cell type | Cell number | Incidence | Latency (days) |
|---|
| Parental cells | 5×104 | 0/4 | - |
| 1×105 | 0/4 | - |
| 2×105 | 3/4 | 35 |
| 5×105 | 4/4 | 29 |
| 1×106 | 4/4 | 12 |
| CD133+
cells | 5×102 | 0/4 | - |
| 1×103 | 4/4 | 25 |
|
5×103 | 4/4 | 13 |
|
1×104 | 4/4 | 9 |
|
5×104 | 4/4 | 6 |
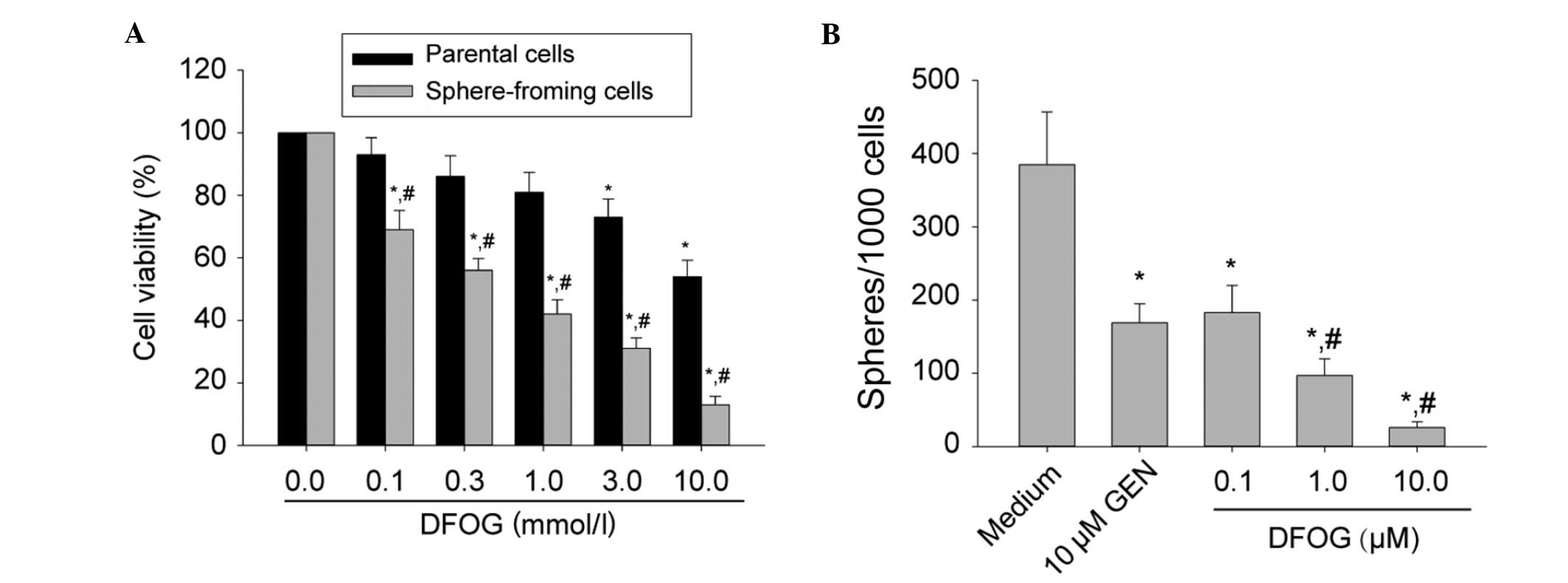
DFOG significantly reduces the formation
of primary and secondary tumor spheroids in SFCs of the SKOV3 cell
line
It has previously been reported that CSCs are
capable of extensive proliferation. Genistein inhibited the
proliferative activity of pancreatic CSCs (8,9). The
present study sought to examine whether DFOG inhibits SKOV3
cell-derived SFCs. Parental cells or third-generation spheres
derived from SKOV3 cells were treated with various doses of DFOG
(0.0–10.0 μM) for 48 h. Cell viability was measured by an MTT
assay. DFOG preferentially inhibited cell viability of SKOV3
cell-derived SFCs in a dose-dependent manner (Fig. 3A) and the IC50 values of
DFOG against parental cells and SKOV3-derived SFCs were 10.90 and
0.47 μmol/l, respectively. This observation suggested that DFOG was
capable of preferentially suppressing the proliferation activity of
OCSLCs.
In order to determine whether DFOG suppressed the
formation of tumor spheres in vitro, SKOV3 cell-derived SFCs
were exposed to varying concentrations of DFOG or genistein (10.0
μM; Fig. 3B). It was demonstrated
that DFOG and genistein inhibited the formation of spheres. It is
worth noting that the concentrations of DFOG that were capable of
suppressing tumorsphere formation (IC50 around 0.5 μM
for the SKOV3 spheres) were ~10-fold lower than those exhibiting
anti-proliferative effects by the MTT assay (with an
IC50 around 10 μM for parental SKOV3 cells). These data
suggest that DFOG may be effective in inhibiting the self-renewal
capacity of OCSLCs.
DFOG decreases the phosphorylation level
of FOXO3a and stem cell surface markers of SKOV3 cell-derived
SFCs
FOXO3a has been reported to be pivotal in the
control of the tumorigenicity of glioblastoma CSLCs (19). Thus, the present study sought to
investigate if DFOG inhibited the self-renewal capacity of SFCs
derived from the SKOV3 cell-line and whether DFOG was involved in
the modulation of FOXO3a activity. SKOV3-derived third generation
spheres elevated the protein expression levels of phosphorylated
FOXO3a, indicating the presence of FOXO3a inactivation (Fig. 4A). In addition, DFOG decreased the
level of phosphorylated FOXO3a protein in SKOV3 cell-derived SFCs
(Fig. 4B). These data suggest that
DFOG decreased the activity of FOXO3a and that it may participate
in inhibiting the self-renewal capacity of OCSLCs.
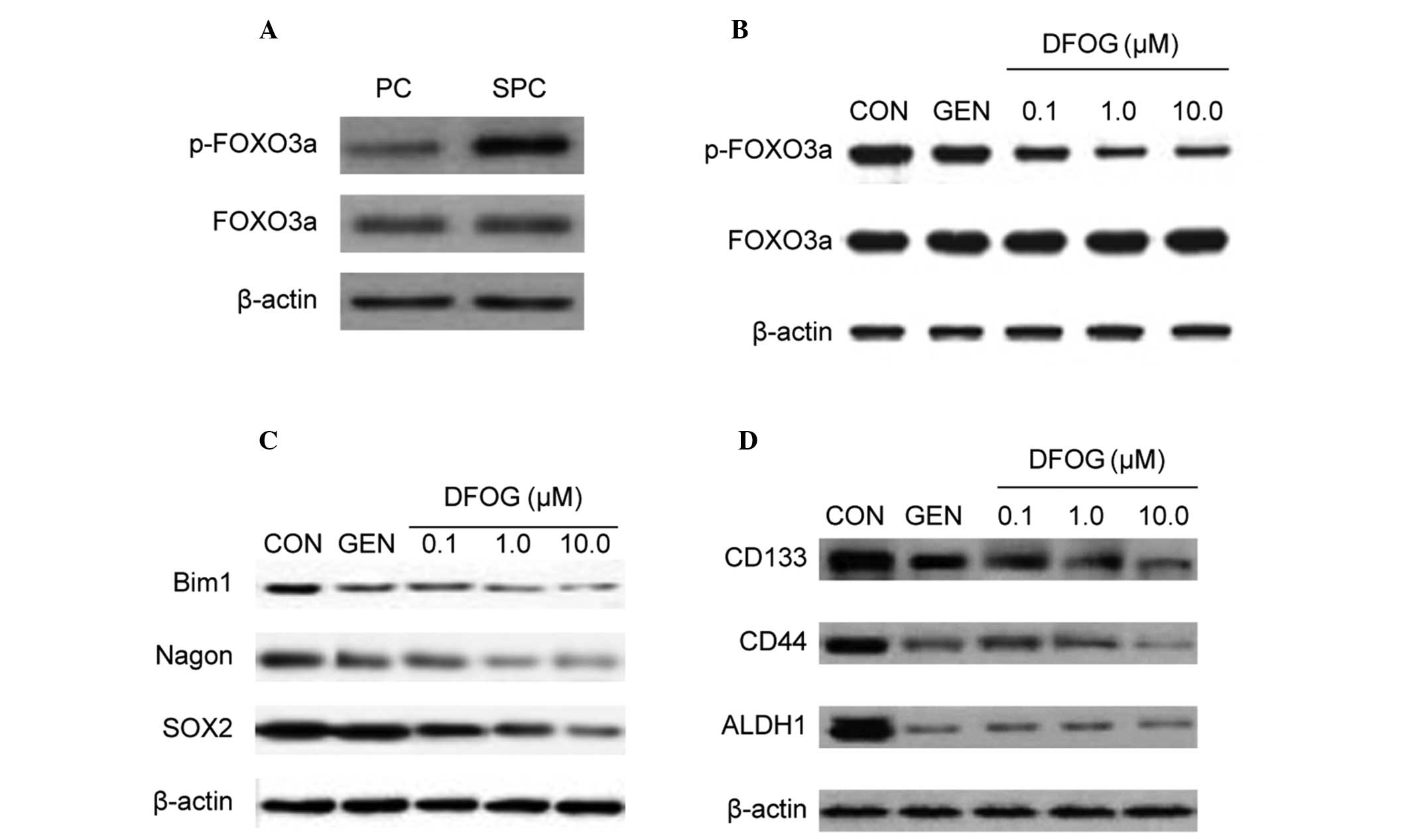 | Figure 4DFOG reduces FOXO3a phosphorylation
and CSC marker expression of OCSLCs derived from SKOV3 cells. (A)
The phosphorylated form of the FOXO3a protein was highly expressed
in SFCs derived from SKOV3 cells compared with corresponding PCs.
(B) Treatment with DFOG downregulated the expression of
phosphorylated FOXO3a in SFCs derived from SKOV3 cells. (C)
Treatment with DFOG downregulated the expression of the
self-renewal associated proteins, including BMI1, Nagon and SOX2 in
SFCs derived from SKOV3 cells. (D) Treatment with DFOG
downregulated the expression of CSC markers, including CD133, CD44
and ALDH1 in SFCs derived from SKOV3 cells. DFOG,
7-difluoromethoxyl-5,4′-di-n-octyl genistein; CSC, cancer stem
cell; OCSLCs, ovarian cancer stem-like cells; SFCs, sphere-forming
cells; PCs, parental cells. |
The study by Shiota et al demonstrated that
FOXO3a regulates the motility of urothelial cancer cells through
negative regulation of Twist1 (20). Previous demonstration of Twist1
directly regulating the expression of BMI1 provides a mechanistic
explanation of the association between EMT and cancer stemness
(21). The present study aimed to
investigate whether DFOG affected the expression of BMI1, Nagon and
SOX2 proteins in OCSLCs. Fig. 4C
shows that DFOG reduced the protein expression levels of BMI1,
Nagon and SOX2 in SKOV3-derived SFCs.
Since inactivation of FOXO3a led to an increase in
SFC capacity and expression of the CSC surface marker CD44 in
prostate cancer stem-like cell populations (22), the present study next analyzed
whether DFOG inhibited the protein expression levels of CD133, CD44
and ALDH1. DFOG inhibited the protein expression levels of CD133,
CD44 and ALDH1 in SKOV3-derived SFCs (Fig. 4D). These results illustrated that
DFOG downregulated the expression of CD133, CD44 and ALDH1 in
OCSLCs.
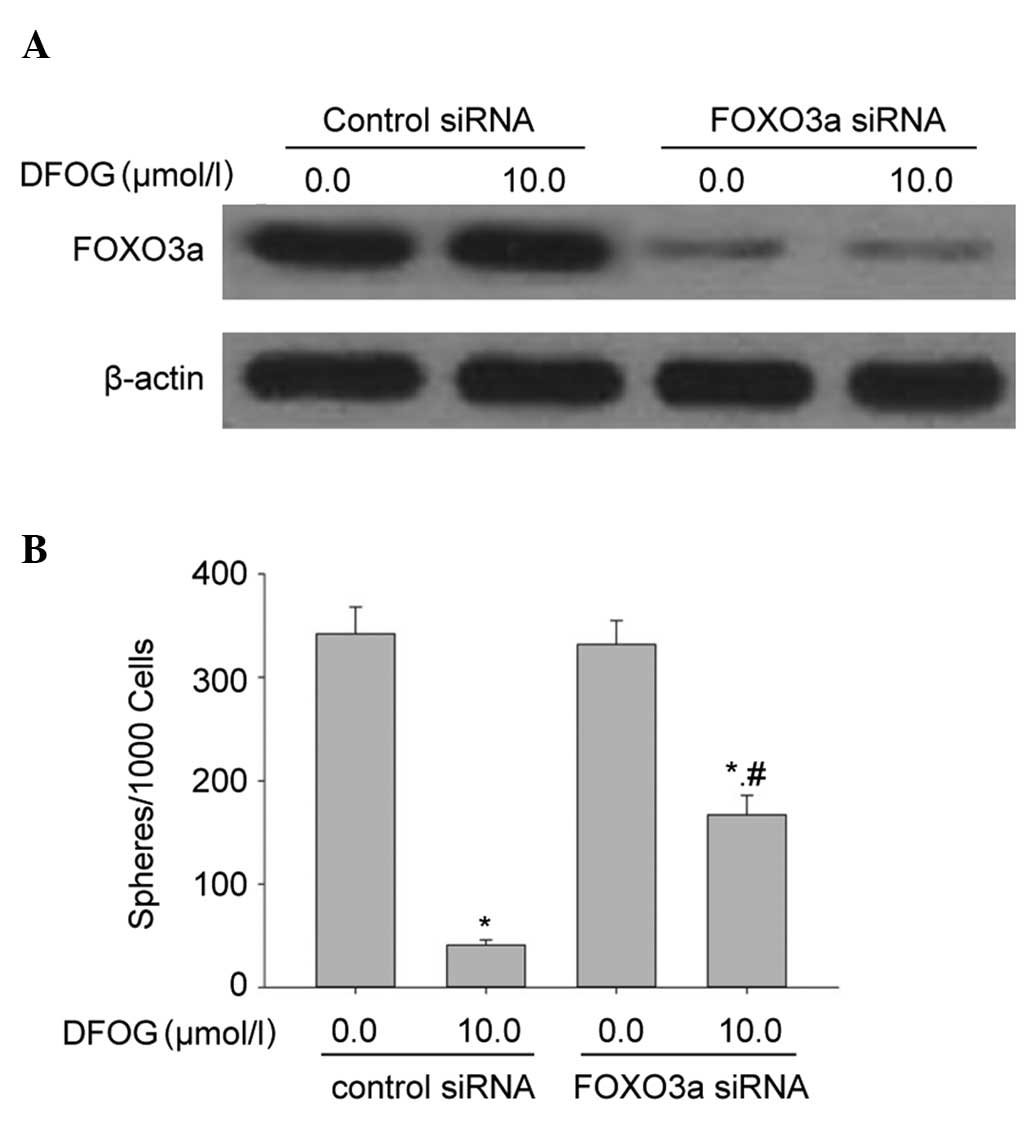
Knockout of FOXO3a attenuates the
inhibitory effects of DFOG on the self-renewal capacity of
SKOV3-derived SFCs
The present study examined whether activation of
FOXO3a affected DFOG-inhibited self-renewal capacity of
SKOV3-derived SFCs. DFOG and genistein inhibited the self-renewal
capacity in SFCs/control siRNA cells that were derived from SKOV3
cells. The inhibition of FOXO3a expression by siRNA significantly
attenuated the ability of DFOG to inhibit the self-renewal capacity
of SKOV3-derived SFCs (Fig. 5A and
B). These data suggest that the silencing of FOXO3a contributed
to the self-renewal capacity of OCSLCs.
Discussion
The anti-cancer efficacy of DFOG, which is a novel
synthetic analogue of genistein, has been evaluated in several
types of cancer, including ovarian cancer cell lines. For instance,
DFOG inhibited the growth and induced apoptosis of gastric cancer
and ovarian cancer cells (17,18).
Increasing evidence supports the cancer stem cell theory, which
hypothesizes that various types of cancer are driven and maintained
by a small proportion of CSCs (23). The concept of CSCs has profound
clinical implications for cancer therapeutics, management and
prevention (23,24). Previous studies indicated that CSCs
have the capacity to drive tumor resistance and relapse/recurrence
(25,26). Since ovarian cancer lacks efficacy
or sensitivity to the effects of current chemotherapies, metastatic
disease requires novel approaches to specifically target CSC
populations (23,27,28).
Numerous studies found that several dietary compounds are promising
chemopreventive agents against CSCs, including genistein (7–9).
Therefore, based on the chemopreventive activity of genistein and
DFOG, and the implications of the CSC theory, the present study
determined whether DFOG acted against OCSCs.
Numerous techniques have been developed to isolate
and characterize OCSCs in vitro. Tumorsphere cultures were
first used to isolate and expand ovarian cancer stem/progenitor
cells (29,30), and were based on the ability of
stem/progenitor cells to grow in serum-free suspension, while
differentiated cells failed to survive under the same conditions
(29,30). By employing this technique, the
present study demonstrated that SKOV3 cell-derived SFCs possessed
the properties of OCSLCs, including the self-renewal capacity and
higher tumorigenicity.
The well-known effects of genistein were also
demonstrated to regulate self-renewal associated transcription
factors in previous studies, including Nagon (31), Bmi1 (30) and Sox2 (32). The present study for the first
time, to the best of our knowledge, provided evidence that DFOG
preferentially inhibited the cellular viability and self-renewal
capacity of SFCs derived from ovarian cancer SKOV3 cells. This
observed effect was also accompanied by downregulation of the
self-renewal associated transcription factors, Bmi1, Nagon and Sox2
protein expression. The preference of DFOG in inhibiting CSCs may
be significant for chemopreventive effects.
The study by Sunayama et al demonstrated that
FOXO3a may function as a key integrator of these cellular signals
that regulate glioblastoma CSLCs and which may also be considered a
potential therapeutic target in the treatment of glioblastoma
(19). The present study
demonstrated that DFOG activated FOXO3a via inhibiting
phosphorylation of the FOXO3a protein in SFCs derived from SKOV3
cells. Silencing of the FOXO3a gene by transfection with FOXO3a
siRNA attenuated the ability of DFOG to inhibit the self-renewal
capacity of SKOV3 cell-derived SFCs. The significance of the
results indicates that not only is the DFOG-inhibitory effect of
the self-renewal capacity associated with the activation of FOXO3a,
but also the inactivation of FOXO3a contributes to the self-renewal
capacity of SKOV3 cell-derived SFCs.
In conclusion, the present study demonstrated that
DFOG was able to target OCSLCs as determined by the tumorsphere
formation assay. Furthermore, the present study identified the
activation of FOXO3a by DFOG as one of the possible mechanisms for
its efficacy. These studies support the use of DFOG for ovarian
cancer chemoprevention. These findings provide a persuasive and
supporting rationale for the preclinical and clinical evaluation of
DFOG targeted therapy of ovarian cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81301894), Guangdong Province
Science and Technique Department Item (no. 2012B031800271) of China
and Guangzhou Science and Information Bureau Item (20130000015) of
China.
References
|
1
|
Jemal A, Center MM, Ward E and Thun MJ:
Cancer occurrence. Methods Mol Biol. 471:3–29. 2009. View Article : Google Scholar
|
|
2
|
Schwartz PE: Current diagnosis and
treatment modalities for ovarian cancer. Cancer Treat Res.
107:99–118. 2002.PubMed/NCBI
|
|
3
|
Almanaa TN, Geusz ME and Jamasbi RJ:
Effects of curcumin on stem-like cells in human esophageal squamous
carcinoma cell lines. BMC Complement Altern Med. 12:1952012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quitschke WW: Curcuminoid binding to
embryonal carcinoma cells: reductive metabolism, induction of
apoptosis, senescence, and inhibition of cell proliferation. PLoS
One. 7:e395682012. View Article : Google Scholar
|
|
5
|
Chen SF, Nieh S, Jao SW, et al: Quercetin
suppresses drug-resistant spheres via the p38 MAPK-Hsp27 apoptotic
pathway in oral cancer cells. PLoS One. 7:e492752012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CH, Hong HM, Chang YY and Chang WW:
Inhibition of heat shock protein (Hsp) 27 potentiates the
suppressive effect of Hsp90 inhibitors in targeting breast cancer
stem-like cells. Biochimie. 94:1382–1389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Montales MT, Rahal OM, Kang J, et al:
Repression of mammosphere formation of human breast cancer cells by
soy isoflavone genistein and blueberry polyphenolic acids suggests
diet-mediated targeting of cancer stem-like/progenitor cells.
Carcinogenesis. 33:652–660. 2012. View Article : Google Scholar
|
|
8
|
Bao B, Wang Z, Ali S, et al:
Over-expression of FoxM1 leads to epithelial-mesenchymal transition
and cancer stem cell phenotype in pancreatic cancer cells. J Cell
Biochem. 112:2296–2306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bao B, Wang Z, Ali S, et al: Notch-1
induces epithelial-mesenchymal transition consistent with cancer
stem cell phenotype in pancreatic cancer cells. Cancer Lett.
307:26–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodova M, Fu J, Watkins DN, Srivastava RK
and Shankar S: Sonic hedgehog signaling inhibition provides
opportunities for targeted therapy by sulforaphane in regulating
pancreatic cancer stem cell self-renewal. PLoS One. 7:e460832012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kallifatidis G, Labsch S, Rausch V, et al:
Sulforaphane increases drug-mediated cytotoxicity toward cancer
stem-like cells of pancreas and prostate. Mol Ther. 19:188–195.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rausch V, Liu L, Kallifatidis G, et al:
Synergistic activity of sorafenib and sulforaphane abolishes
pancreatic cancer stem cell characteristics. Cancer Res.
70:5004–5013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Zhang T, Korkaya H, et al:
Sulforaphane, a dietary component of broccoli/broccoli sprouts,
inhibits breast cancer stem cells. Clin Cancer Res. 16:2580–2590.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaudhuri D, Orsulic S and Ashok BT:
Antiproliferative activity of sulforaphane in Akt-overexpressing
ovarian cancer cells. Mol Cancer Ther. 6:334–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen D, Pamu S, Cui Q, Chan TH and Dou QP:
Novel epigallocatechin gallate (EGCG) analogs activate
AMP-activated protein kinase pathway and target cancer stem cells.
Bioorg Med Chem. 20:3031–3037. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nishimura N, Hartomo TB, Pham TV, et al:
Epigallocatechin gallate inhibits sphere formation of neuroblastoma
BE(2)-C cells. Environ Health Prev Med. 17:246–251. 2012.
View Article : Google Scholar
|
|
17
|
Xiang HL, Liu F, Quan MF, Cao JG and Lv Y:
7-difluoromethoxyl-5,4′-di-n-octylgenistein inhibits growth of
gastric cancer cells through downregulating forkhead box M1. World
J Gastroenterol. 18:4618–4626. 2012.
|
|
18
|
Ning Y, Li Q, Xiang H, Liu F and Cao J:
Apoptosis induced by 7-difluoromethoxyl-5,4′-di-n-octyl genistein
via the inactivation of FoxM1 in ovarian cancer cells. Oncol Rep.
27:1857–1864. 2012.PubMed/NCBI
|
|
19
|
Sunayama J, Sato A, Matsuda K, et al:
FoxO3a functions as a key integrator of cellular signals that
control glioblastoma stem-like cell differentiation and
tumorigenicity. Stem Cells. 29:1327–1337. 2011.PubMed/NCBI
|
|
20
|
Shiota M, Song Y, Yokomizo A, et al:
Foxo3a suppression of urothelial cancer invasiveness through
Twist1, Y-box-binding protein 1, and E-cadherin regulation. Clin
Cancer Res. 16:5654–5663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu KJ and Yang MH: Epithelial-mesenchymal
transition and cancer stemness: the Twist1-Bmi1 connection. Biosci
Rep. 31:449–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dubrovska A, Kim S, Salamone RJ, et al:
The role of PTEN/Akt/PI3K signaling in the maintenance and
viability of prostate cancer stem-like cell populations. Proc Natl
Acad Sci USA. 106:268–273. 2009.
|
|
23
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kakarala M and Wicha MS: Implications of
the cancer stem-cell hypothesis for breast cancer prevention and
therapy. J Clin Oncol. 26:2813–2820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakariassen PØ, Immervoll H and Chekenya
M: Cancer stem cells as mediators of treatment resistance in brain
tumors: status and controversies. Neoplasia. 9:882–892.
2007.PubMed/NCBI
|
|
26
|
Tang C, Chua CL and Ang BT: Insights into
the cancer stem cell model of glioma tumorigenesis. Ann Acad Med
Singapore. 36:352–357. 2007.PubMed/NCBI
|
|
27
|
Lippman ME: High-dose chemotherapy plus
autologous bone marrow transplantation for metastatic breast
cancer. N Engl J Med. 342:1119–1120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Williams SD, Birch R, Einhorn LH, Irwin L,
Greco FA and Loehrer PJ: Treatment of disseminated germ-cell tumors
with cisplatin, bleomycin, and either vinblastine or etoposide. N
Engl J Med. 316:1435–1440. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu T, Cheng W, Lai D, Huang Y and Guo L:
Characterization of primary ovarian cancer cells in different
culture systems. Oncol Rep. 23:1277–1284. 2010.PubMed/NCBI
|
|
30
|
Ma L, Lai D, Liu T, Cheng W and Guo L:
Cancer stem-like cells can be isolated with drug selection in human
ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin
(Shanghai). 42:593–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Chen H, Hardy TM and Tollefsbol TO:
Epigenetic regulation of multiple tumor-related genes leads to
suppression of breast tumorigenesis by dietary genistein. PLoS One.
8:e543692013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Regenbrecht CR, Jung M, Lehrach H and
Adjaye J: The molecular basis of genistein-induced mitotic arrest
and exit of self-renewal in embryonal carcinoma and primary cancer
cell lines. BMC Med Genomics. 1:492008. View Article : Google Scholar : PubMed/NCBI
|