Introduction
Vestibular schwannomas (VSs) are benign,
slow-growing neoplasms that develop in the cerebellopontine angle
(CPA) area of the brain (1). VSs may
be subdivided into cystic and solid according to their morphology
(2,3).
Cystic VSs are relatively less common than solid VSs, with a
reported incidence rate ranging between 5.7 and 24% (2). Furthermore, cystic VSs are more
aggressive than solid VSs due to the rapid growth and unpredictable
expansion of their cystic component (2,3).
Fluid-fluid levels in tumors display a radiological
appearance of two fluid levels in the cystic section of tumors
(4–10). This level is apparent on computed
tomography (CT) and magnetic resonance imaging (MRI) scans,
particularly on T2-weighted MRI scans (4–11).
Previous studies have reported fluid-fluid levels in non-neurogenic
tumors (4,5), as well as a small number of cranial
nerve schwannomas, including trigeminal, glossopharyngeal and
hypoglossal schwannomas (6–8). However, cystic VSs with fluid-fluid
levels are fairly rare (3,6,9,10).
The current study presented three cases of
multicystic VS with fluid-fluid levels, introducing their clinical
manifestations, imaging features and surgical findings. In
addition, the possible mechanism of fluid-fluid level formation,
the effect of fluid-fluid levels and the therapeutic strategy
employed were discussed. This study was approved by the ethics
committees of Laiwu Hospital (Laiwu, Shandong, China) and Beijing
Tiantan Hospital (Beijing, China), and written informed consent was
obtained from each of the patients.
Case reports
Case one
In December 2013, a 65-year-old male patient
presented to Beijing Tiantan Hospital with a six-month history of
right-sided facial numbness and sialorrhea, with no tinnitus,
hearing loss, headache or other symptoms. Neurological examination
demonstrated impaired sensation to light touch and pinprick testing
in the maxillary division of the right trigeminal nerve. The
preoperative right-sided facial nerve function was diagnosed as
House-Brackmann grade II (11).
CT and MRI scans identified a predominately
multicystic mass with two apparent fluid-fluid levels in the right
CPA extending to an enlarged internal acoustic meatus, measuring
4.3×2.9×3.3 cm (Fig. 1). The right
cerebellum and brainstem were markedly distorted by the lesion;
however, the fourth ventricle was not clearly compressed and there
was no evidence of hydrocephalus.
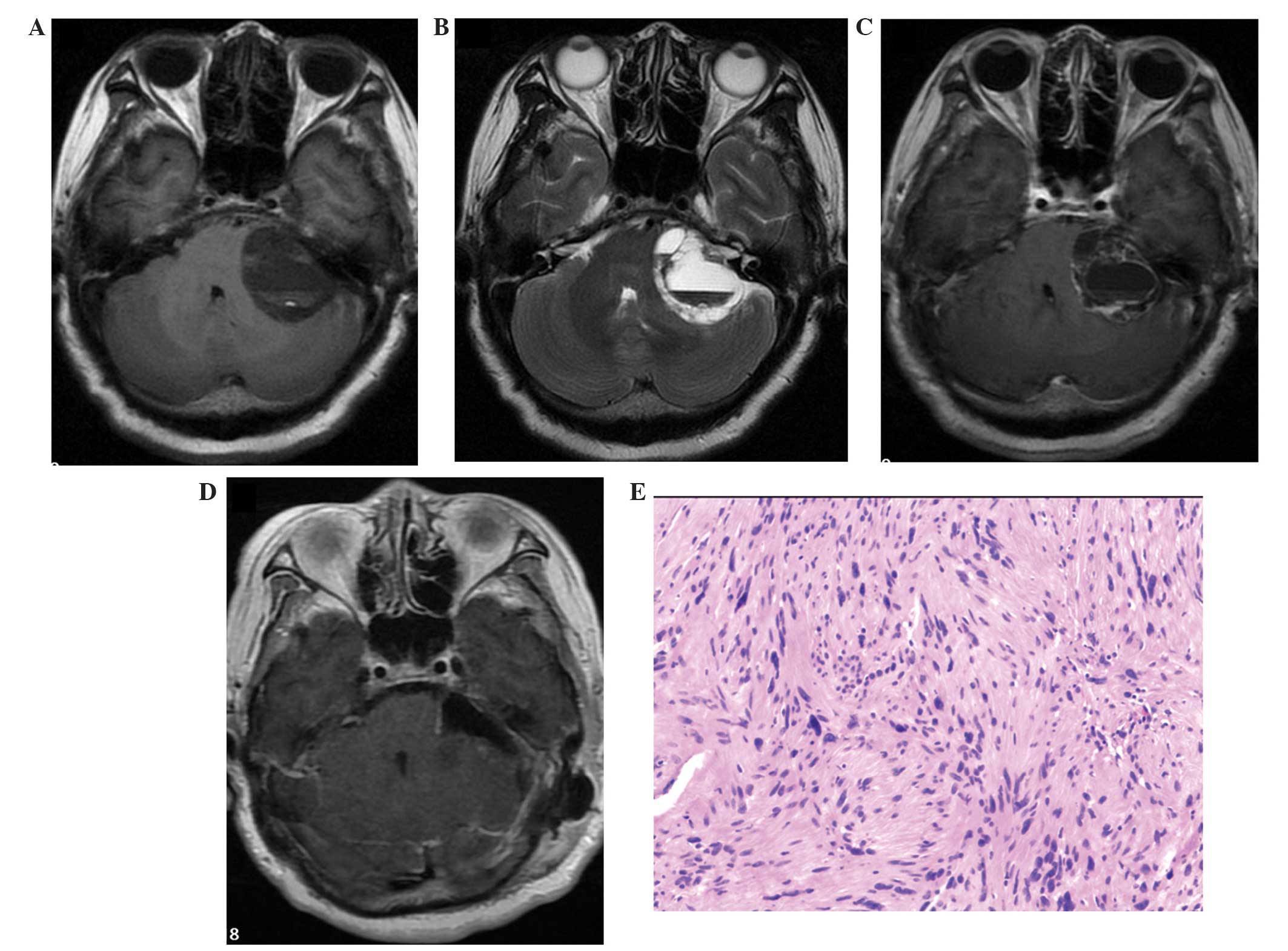
 | Figure 1.Preoperative and postoperative images
of case one. (A) Axial CT scan revealing a mass in the right
cerebellopontine angle. Arrow indicates the fluid-fluid level (the
superior layer being of a lower density and the inferior layer of a
higher density). Arrow head indicates the enlarged internal
acoustic meatus. (B) Axial T1-weighted MRI scan, identifying two
apparent fluid-fluid levels (arrows) in the multicystic mass. The
inferior layer and the solid section of the mass produced an
isointense signal, while the superior layer produced a hypointense
signal. Furthermore, the mass compressed the cerebellum and the
brainstem, extending into the internal acoustic meatus. (C) Axial
T2-weighted MRI scan, revealing a hyperintense signal from the
superior layer of the fluid-fluid level and a hypointense signal
from the inferior layer. The basilar artery, and the left seventh
and eighth cranial nerves are distinctly visible in this image. (D)
Axial T1-weighted enhanced MRI scan, revealing enhancement in the
capsule and the solid section of the tumor, including the meatal
component. (E) Postoperative axial T1-weighted enhanced MRI scan,
demonstrating that the tumor was completely resected during
surgery. (F) Histological analysis, indicating that the tumor has
characteristics consistent with schwannoma (hematoxylin and eosin
staining; original magnification, x100). CT, computed tomography;
MRI, magnetic resonance imaging. |
Microsurgery was performed using a suboccipital
retrosigmoid approach (12) with
intraoperative neurophysiological monitoring and gross-total
resection was achieved. During the surgical procedure, a
yellow-green fluid and unclotted blood were observed in the cyst of
the tumor. Furthermore, the tumor adhered to the brainstem and
facial nerve. The brainstem was intact following tumor resection
and the facial nerve was anatomically preserved. Histopathological
findings revealed that the tumor was hypercellular and composed of
spindle-shaped cells with obvious palisade arrangement and regular
nuclei, which indicated a diagnosis of VS with Antoni type A.
Postoperatively, the patient developed House-Brackman grade V
facial nerve palsy and hearing loss, which did not resolve over the
six-month follow-up period. Hypoglossal-facial nerve anastomosis
was proposed; however, the patient did not consent to the
procedure. The tumor did not recur during the six-month follow-up
period.
Case two
A 59-year-old female patient was admitted to Laiwu
Hospital in March 2014 with a 1.5-year history of worsening hearing
loss in the left ear, headache, dizziness, a four-month history of
left-sided facial numbness, coughing when consuming liquids,
dysphagia and an unsteady gait. Two months prior to admission, the
aforementioned symptoms had suddenly worsened, and the patient
experienced a severe headache and was unable to walk due to ataxia.
Physical examination revealed impaired sensation in the maxillary
and mandibular divisions of the left trigeminal nerve, ataxia and
dysmetria on the finger-nose test. The pure-tone average in the
left ear was determined to be 45 dB. The pure-tone thresholds were
determined for the left and right ears using the modified
Hughson-Westlake method at frequencies of 0.25, 0.50, 1.0, 2.0, 4.0
and 8.0 kHz (13). In addition to
assessing absolute hearing values, clinical function was
categorized into the following hearing loss (HL) ranges: <20 dB,
normal hearing; 20–40 dB, mild HL; 40–60 dB, moderate HL; 60–70 dB,
moderately severe HL; 70–90 dB, severe HL; and >90 dB, profound
HL.
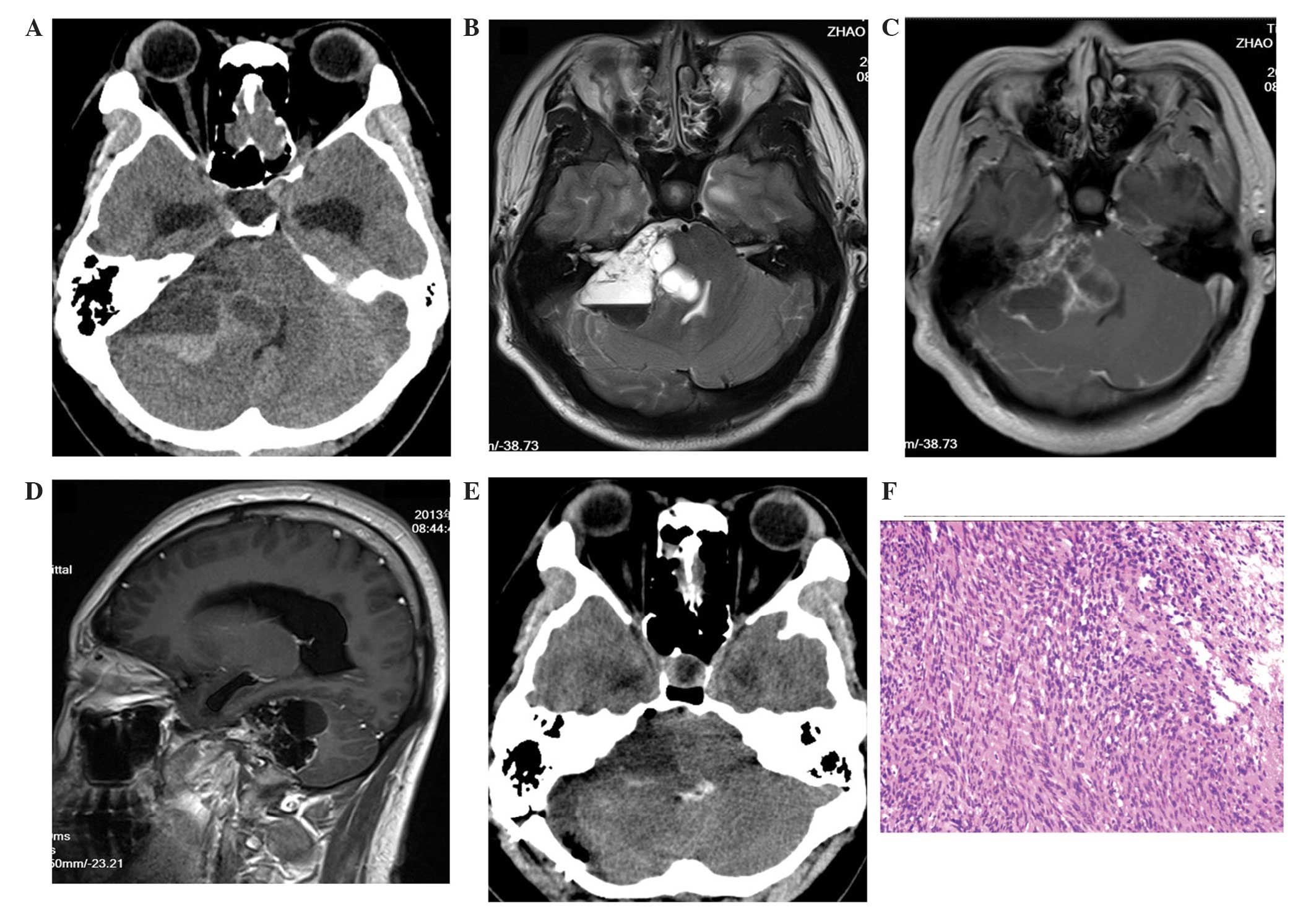
MRI scans revealed a multicystic lesion (Fig. 2) with two apparent fluid-fluid levels
in the left cerebellopontine area. The mass, which measured
4.6×3.4×3.4 cm, compressed the left cerebellum, the brainstem and
the fourth ventricle, forming a cerebellar tonsil hernia with no
hydrocephalus. Prior to surgery, the House-Brackmann grade was
determined to be I (normal).
The tumor was completely resected using a
retrosigmoid approach. The cystic fluid was yellow-green in color
with unclotted blood, and the solid section was yellow-white. The
tumor arose from the eighth cranial nerve and was compressing and
adherent to cranial nerves X, XI and XII, as well as the lower
cranial nerves, which were carefully dissected from the tumor under
intraoperative neurophysiological monitoring. Histological
examination indicated that the tumor was composed of spindle-shaped
cells with palisade arrangement (Antoni type A) and some nuclei
were enlarged and darkly stained; a diagnosis of VS was therefore
determined. The patient developed House-Brackmann grade III facial
nerve palsy and had no effective hearing on the left side during
the three-month follow-up period. No tumor recurrence was
observed.
Case three
A 27-year-old male patient presented to Beijing
Tiantan Hospital in March 2014 with right-sided tinnitus and
hearing loss that lasted for ~1.5 years, and a six-month history of
intermittent dizziness, nausea, vomiting and an unsteady gait.
During the month prior to admission, the aforementioned symptoms
suddenly worsened. In addition, right-sided facial numbness and
limb shaking developed, and the patient was unable to walk due to
ataxia. Physical examination revealed impaired sensation in the
maxillary division of the left trigeminal nerve, as well as ataxia.
The pure tone average in the left ear was determined to be 95
dB.
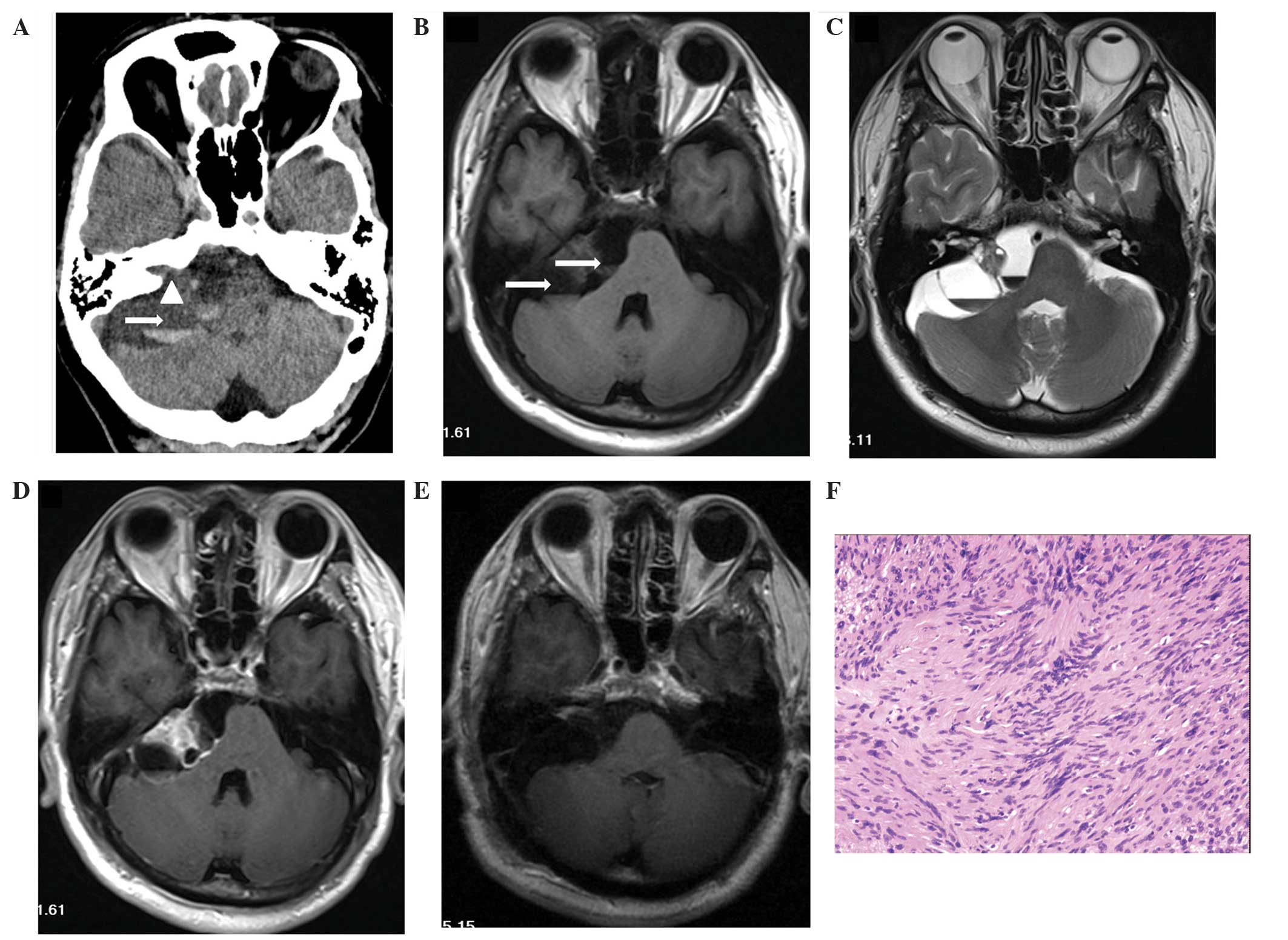
CT and MRI scans revealed a multicystic tumor with
one fluid-fluid level in the left CPA area (Fig. 3). The tumor measured 6.3×4.2×5.2 cm,
compressed the right cerebellum, the brainstem and the fourth
ventricle, and hydrocephalus was observed. The axial and sagittal
images identified a high-density matter in the inferior layer.
The patient underwent surgery using the retrosigmoid
approach to completely resect the tumor. The tumor was
predominantly composed of cysts in which xanthochromic fluid and
unclotted blood were observed. Subsequent histological analysis
indicated features characteristic of a schwannoma; most of the area
was focal cellular (Antoni A), and part was hypocellular with
vacuolar degeneration (Antoni B). Following surgery, the patient
developed House-Brackmann grade VI facial nerve palsy and had no
effective hearing on the right side during the three-month
follow-up period. Tumor recurrence was not observed.
Discussion
A fluid-fluid level is considered to be an uncommon
and non-specific phenomenon in tumors (4). To date, there is no consensus regarding
the mechanism underlying the formation of these levels; however,
two mechanisms have been proposed in the literature (2,4,6–8,10,14). The
first refers to tumor necrosis causing liquefaction and exudation
of the tumor tissue. The fluid formed initially is more
proteinaceous compared with the interstitial fluid formed later;
thus, fluid separation occurs based on viscosity and protein
content (7,10). The second proposed mechanism is
hemorrhage, typically of unclotted blood. The red blood cells or
the products of red blood cell breakdown constitute the inferior
fluid layer, while serous blood constitutes the superior fluid
layer based upon sedimentation (2,4,6,8,14). The present authors consider the second
mechanism to be more accurate, since unclotted blood was observed
in all the cases reported in the current study. Furthermore, the
growing tumor is hypothesized to compress and erode internal blood
vessels, resulting in occlusion, thrombus, ischemia and the
elastolytic function of enzymes. Thus, tumor growth may result in
the destruction and degeneration of blood vessels, including the
formation of pseudoaneurysms and subsequent bleeding (2–4). Due to
the different densities of blood cells or blood cell breakdown
products and serum, the fluid-fluid levels are proposed to form
through the sedimentation effect. In addition, inflammatory
exudation, induced by blood breakdown products, possibly occurred
during the formation of the layers. Acute hemorrhage within the
tumor was considered to be the cause of the sudden worsening of
symptoms in cases two and three of the current study (9). By contrast, in case one, which presented
with chronic evolution of the condition, microhemorrhages may have
occurred.
The presence of fluid-fluid levels can be clarified
by performing CT or MRI scans. On CT scans, the superior fluid
layer had a lower density, whereas the inferior fluid layer had a
higher density, compared with brain tissue (Figs. 1A and 3A). On T1-weighted MRI scans, the superior
fluid layer was hypointense and the inferior fluid layer was
isointense (Figs. 1B and 2A). Furthermore, on T2-weighted MRI scans,
the superior fluid layer presented marked hyperintensity, while the
inferior fluid layer was hypointense (Figs. 1C, 2B
and 3B).
A number of studies have investigated the
significance of fluid-fluid levels in tumors. For instance, Sommer
et al (5) proposed that
fluid-fluid levels in hepatic metastases are a characteristic
indication of metastases of neuroendocrine origin. In addition, Xia
et al (3) determined that
fluid-fluid levels in VSs were a predictor of peritumoral adhesion
and are associated with a less favorable surgical outcome. In
agreement with this, VS with fluid-fluid levels adhered to the
facial nerve, other cranial nerves or the brain stem were observed
during the surgical procedures performed in the current cases.
However, determination of the fundamental causes and implications
of fluid-fluid levels using biochemistry may be required.
VSs with fluid-fluid levels are not appropriate for
‘watch and wait’ treatment approaches due to the relatively high
probability of sudden tumor enlargement due to hemorrhage (3,5,15). Furthermore, radiosurgery is not
recommended, as the expansion of the cystic components and possible
hemorrhage following radiosurgery may result in sudden
deterioration (2,16,17).
Therefore, surgery is the optimal treatment strategy for such
patients. Although cystic VSs with fluid-fluid levels indicate
greater adhesiveness, it is essential that total resection is
performed during the initial surgical procedure due to the tendency
for accelerated regrowth of residual cystic schwannoma (8,18).
In conclusion, the current study reported three rare
cases of multicystic VS with fluid-fluid levels. Fluid-fluid levels
in VSs are predominantly identified on CT or MRI scans. Hemorrhage
in multicystic VSs may be the major mechanism of fluid-fluid level
formation, with acute hemorrhage resulting in sudden deterioration
of the patient's clinical condition and microhemorrhages resulting
in chronic evolution of the patient's condition. Furthermore,
fluid-fluid levels in VSs indicate greater adhesiveness and a
poorer prognosis. Therefore, ‘watch and wait’ approaches or
radiosurgery are not appropriate; instead, surgery is recommended
as the optimal treatment strategy.
References
|
1
|
Arthurs BJ, Lamoreaux WT, Giddings NA,
Fairbanks RK, Mackay AR, Demakas JJ, Cooke BS and Lee CM: Gamma
Knife radiosurgery for vestibular schwannoma: case report and
review of the literature. World J Surg Oncol. 7:1002009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park CK, Kim DC, Park SH, Kim JE, Paek SH,
et al: Microhemorrhage, a possible mechanism for cyst formation in
vestibular schwannomas. J Neurosurg. 105:576–580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xia L, Zhang H, Yu C, Zhang M, Ren M, Qu
Y, et al: Fluid-fluid level in cystic vestibular schwannoma: A
predictor of peritumoral adhesion. J Neurosurg. 120:197–206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu ZH and Wu M: Unusual features in an
adult pancreatic hemangioma: CT and MRI demonstration. Korean J
Radiol. 14:781–785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sommer WH, Zech CJ, Bamberg F, Auernhammer
CJ, Helck A, Paprottka PM, et al: Fluid-fluid level in hepatic
metastases: A characteristic sign of metastases of neuroendocrine
origin. Eur J Radiol. 81:2127–2132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu H, Hao SY, Jia GJ, Zhang JT and Zhang
LW: A cystic vestibular schwannoma with a fluid-fluid level. Chin
Med J (Engl). 125:39202012.PubMed/NCBI
|
|
7
|
Catalano P, Fang-Hui E and Som PM:
Fluid-fluid levels in benign neurogenic tumors. AJNR Am J
Neuroradiol. 18:385–387. 1997.PubMed/NCBI
|
|
8
|
Li WC, Hong XY, Wang LP, Ge PF, Fu SL and
Luo YN: Large cystic hypoglossal schwannoma with fluid-fluid level:
A case report. Skull Base. 20:193–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gagliardo C, Martines F, Bencivinni F, La
Tona G, Lo Casto A and Midiri M: Intratumoral haemorrhage causing
an unusual clinical presentation of a vestibular schwannoma.
Neuroradiol J. 26:30–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chin KF, Babar J, Tzifa K, Chavda SV and
Irving RM: Vestibular schwannomas with fluid-fluid level. J
Laryngol Otol. 121:902–906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
House JW and Brackmann DE: Facial nerve
grading system. Otolaryngol Head Neck Surg. 93:146–147.
1985.PubMed/NCBI
|
|
12
|
Yamakami I, Uchino Y, Kobayashi E, Yamaura
A and Oka N: Removal of large acoustic neurinomas (vestibular
schwannomas) by the retrosigmoid approach with no mortality and
minimal morbidity. J Neurol Neurosurg Psychiatry. 75:453–458. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carhart R and Jerger J: Preferred method
for clinical determination of pure-tone thresholds. J Speech Hear
Disord. 24:330–345. 1959. View Article : Google Scholar
|
|
14
|
Chang WC, Huang GS, Lee HS, Lee CH and Hsu
YC: Fluid-fluid level in peripheral nerve schwannoma: Report of a
case with histological correlation. Clin Imaging. 33:248–251. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sinha S and Sharma BS: Cystic acoustic
neuromas: Surgical outcome in a series of 58 patients. J Clin
Neurosci. 15:511–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Ipolyi AR, Yang I, Buckley A, Barbaro
NM, Cheung SW and Parsa AT: Fluctuating response of a cystic
vestibular schwannoma to radiosurgery: Case report. Neurosurgery.
62:E1164–E1165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ganslandt O, Fahrig A and Strauss C:
Hemorrhage into cystic vestibular schwannoma following stereotactic
radiation therapy. Zentralbl Neurochir. 69:204–206. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kameyama S, Tanaka R, Honda Y, Hasegawa A,
Yamazaki H and Kawaguchi T: The long-term growth rate of residual
acoustic neurinomas. Acta Neurochir (Wien). 129:127–130. 1994.
View Article : Google Scholar : PubMed/NCBI
|