Introduction
Osteosarcoma (OS) is one of the most prevalent types
of malignant bone tumors, which predominantly occurs in adolescents
and young adults; in addition, OS has a morbidity rate of ~5 cases
per million (1). The conventional
treatment of OS consists of surgery and radiotherapy; however,
additional radiotherapy may not be used due to the risk of
radiation-induced necrosis of surrounding structures (2). In addition, there is a high risk of
relapse or metastasis for OS patients, even following curative
resection; therefore, a substantial portion of patients with OS
respond poorly to chemotherapy (3).
Thus, elucidating the mechanisms underlying the metastasis of OS
may lead to the development of novel therapeutic strategies for the
treatment of OS.
Twist is a member of a highly conserved basic
helix-loop-helix transcription factor family and is located on
human chromosome 7 (4). It has been
reported that Twist mediates cell migration and differentiation
under various physiological conditions (5). In addition, Twist has been demonstrated
to significantly enhance tumor malignancies, including breast
cancer (6), hepatocellular carcinoma
(HCC) (7) and prostate cancer
(8). It was reported that the role of
Twist in tumor cell invasion and metastasis may be associated with
the regulation of cancer-associated functions, such as angiogenesis
(9). Furthermore, Twist was found to
regulate apoptosis and angiogenesis under a variety of pathological
conditions (10). Therefore, it was
hypothesized that Twist was closely correlated with malignant
potential, progression and survival in patients with OS. However,
to the best of our knowledge, there is limited information
regarding the pathological roles of Twist expression in human OS
tissues.
Angiogenesis is a key factor that mediates tumor
metastasis; in addition, correlations between angiogenesis and
patient survival in OS have been previously reported (11). Microvessel density (MVD) was
demonstrated to be increased in histopathologically aggressive
cancers, including esophageal squamous cell carcinoma (12) and mammary carcinoma (13). High MVD was also reported to be
correlated with metastasis and poor survival in various types of
cancer, such as OS (14). Increasing
evidence has suggested that MVD may be considered an indirect
marker of neoangiogenesis, which is commonly labeled by CD31 or
CD34 (14).
Vascular endothelial growth factor (VEGF), the most
important mediator of vascular formation, is essential for the
initiation of immature vessel formation (15). VEGF has been reported to be directly
involved in the angiogenesis process, tumor growth and metastasis
(16). Neovascularization, promoted
by VEGF, is known to reflect the aggressiveness and the metastatic
potential of OS (17). However,
current understanding of the correlation of Twist, VEGF and MVD in
human OS tissues is limited.
The present study aimed to investigate Twist, VEGF
and CD34 expression levels in OS tissues, using immunohistochemical
(IHC) staining, in order to illustrate correlations among these
components in OS.
Materials and methods
Tissues
A total of 32 cases of different phase OS and 10
cases of osteochondroma (OC), a benign tumor of the bone, were
obtained from patients who underwent surgery between June 2011 and
March 2013 at the Department of Orthopedics, Xiangya Hospital of
Central South University (Changsha, China). Patients with OS
underwent amputation surgery, while patients with OC underwent
curettage. All OS tumor tissues were formalin-fixed and
paraffin-embedded following resection and then pathologically
diagnosed (18): Phase I OS, 3 cases;
phase II OS, 17 cases; and phase III OS, 12 cases. Ages of patients
range from 9 to 54 years old (mean, 23.21±8.73), including 18 males
and 14 females. In 20 cases OS was identified in the femur, in 8
cases the malignancy was located in the tibia and the remaining 4
cases were located in other bone regions. OC tissues were used as
the control. All tissues were collected prior to any
chemoradiotherapy. Written informed consent was obtained from the
patients. The study was approved by the Ethics Committee of the
Xiangya Hospital of Central South University.
Immunohistochemical staining
Immunohistochemical staining was performed using the
streptavidin-peroxidase (SP) method (SP kit; ZSGB-Bio, Beijing,
China), according to the manufacturer's instructions. Slides were
deparaffinized with xylene (BaiYi, Co. Ltd., Jining, China) twice
for 30 min each, dehydrated three times in a gradient series of
ethanol (100, 95, 90, 80 and 70%) and rinsed with
phosphate-buffered saline (PBS). Following 15 min of treatment with
3% H2O2, slides were blocked using normal
goat serum (Jackson ImmunoResearch, West Grove, PA, USA) for 20
min. Slides were incubated with the following primary antibodies
overnight at 4°C: Rabbit polyclonal anti-Twist (1:50; cat. no.
ab50581; Abcam, Cambridge, UK), mouse monoclonal anti-VEGF (1:150;
cat. no. ab1316; Abcam) or mouse monoclonal anti-CD34 (1:100; cat.
no. ab8536; Abcam). Slides were subsequently washed three times
with PBS for 15 min. Slides were treated with SP reagent for 20 min
and then incubated with a secondary antibody for 90 min at 37°C,
using an SP rabbit and mouse horseradish peroxidase kit (cat. no.
CW0120; CWBiotech Co. Ltd., Wuhan, China) according to the
manufacturer's instructions. Subsequently, the slides were washed
twice with PBS for 15 min per wash, and visualized using
3,3′-diaminobenzidine for 5 min and then counterstained with
hematoxylin (Solarbio Science ﹠ Technology Co., Ltd., Beijing,
China). The slides were mounted and dried. Images were captured
using an Olympus microscope (C-7070; Olympus Corporation, Tokyo,
Japan).
Evaluation of staining
Slides were evaluated by two investigators, who were
blinded to experiments, under a light microscope (BX43; Olympus
Corporation). Twist and VEGF staining intensity were scored as
follows: 0, negative; 1, weak; 2, medium; and 3, strong. Extent of
staining was scored as: 0, 0%; 1, 1–25%; 2, 26–50%; 3, 51–75%; and
4, 76–100%. The final staining score (0–7) was calculated as the
sum of the intensity score and extent score. Staining scores of 0–1
were considered to be negative, scores of 2–3 were considered as
low expression and score of >3 were considered as high
expression.
Measurement of MVD
Slides were examined at low-power magnification
(x40; microscope, BX43) to identify the areas with the highest
density of microvessels (labeled by CD34). In each case, the most
vascularized area was selected and the microvessels within a
high-power magnification (x200) field of vision were counted three
times. Macrovascular structures with smooth muscle cells were
excluded. The mean of the three highest counts per tumor was used
for analysis.
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used to perform statistical analysis. The ratio of high
expression of Twist and VEGF in different phases of OS was compared
using chi-squared tests. MVD in different phases of OS was compared
using Student's t-tests. Associations among Twist, VEGF and MVD
were assessed using the Spearman's rank correlation test. P<0.05
was considered to indicate a statistically significant difference
between values.
Results
Expression of Twist, VEGF and CD34 in
OS and OC tissues
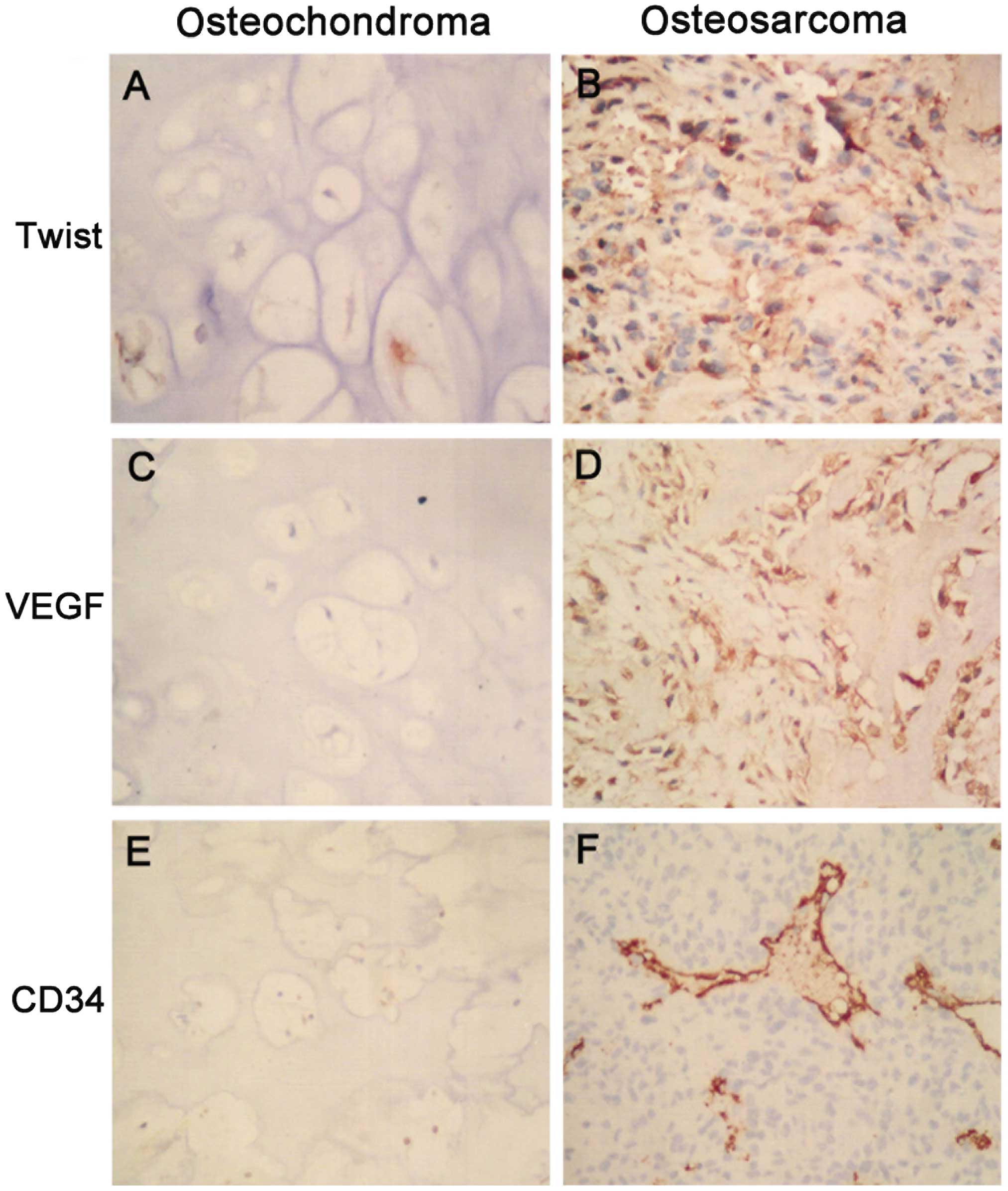
Immunohistochemical staining data revealed that
Twist was located in the nuclei and cytoplasm, VEGF was identified
in the cytoplasm and CD34 (for MVD) in the microvessels (Fig. 1A–F). The positive expression of Twist
was detected in 18 of the total 32 OS tissues, but in only 1 of the
10 OC tissues; therefore, the positive rate of Twist in OS tissues
was significantly increased compared with that in OC tissues
(χ2=6.579, P=0.01) (Table
I). In addition, the positive expression of VEGF was detected
in 23 out of 32 OS tissues and in only 2 of the 10 OC tissues,
demonstrating that the positive rate of VEGF in OS tissues was
significantly higher compared with that of OC tissues
(χ2 =8.510, P=0.004) (Table
II). Furthermore, MVD in OS tissues was significantly elevated
compared with that in OC tissues (t=17.086, P=0.008) (Table III).
 | Table I.Twist expression in osteochondroma and
osteosarcoma. |
Table I.
Twist expression in osteochondroma and
osteosarcoma.
| Tumor | Cases | Negative cases | Positive cases | Positive rate, % | χ2 | P-value |
|---|
| Osteochondroma | 10 | 9 | 1 | 10.00 | 6.579 | 0.01 |
| Osteosarcoma | 32 | 14 | 18 | 56.25 |
 | Table II.Vascular endothelial growth factor
expression in osteochondroma and osteosarcoma. |
Table II.
Vascular endothelial growth factor
expression in osteochondroma and osteosarcoma.
| Tumor | Cases | Negative cases | Positive cases | Positive rate, % | χ2 | P-value |
|---|
| Osteochondroma | 10 | 8 | 2 | 20.00 | 8.510 | 0.004 |
| Osteosarcoma | 32 | 9 | 23 | 71.88 |
 | Table III.MVD values in osteochondroma and
osteosarcoma. |
Table III.
MVD values in osteochondroma and
osteosarcoma.
| Tumor | Cases | MVDa | χ2 | P-value |
|---|
| Osteochondroma | 10 |
7.97±3.67 | 17.086 | 0.008 |
| Osteosarcoma | 32 | 37.38±7.20 |
Expression of Twist, VEGF and CD34 in
different phases of OS
The positive expression of Twist was detected in 10
of the total 12 phase III OS tissues and in 8 of the 20 phase I/II
OS tissues; therefore, the positive rate of Twist in phase III OS
was significantly higher compared with that in phase I/II OS
(χ2=5.732, P=0.018) (Table
IV). The positive expression of VEGF was detected in all 12
phase III OS tissues and in 11 out of 20 phase I/II OS tissues;
thus, the positive rate of VEGF in phase III OS was significantly
increased compared with that in phase I/II OS (χ2=7.513,
P=0.006) (Table V). The MVD in phase
III OS tissues (41.2±4.17 per field) was significantly increased
compared with that of phase I/II OS tissues (31.08±3.36 per field)
(t=7.536, P<0.001) (Table
VI).
 | Table IV.Twist expression in different phase of
osteosarcoma. |
Table IV.
Twist expression in different phase of
osteosarcoma.
| Phase | Cases | Negative cases | Positive cases | Positive rate, % | χ2 | P-value |
|---|
| I | 3 | 3 | 0 | 0.00 |
|
|
| II | 17 | 9 | 8 | 47.06 | 5.732a | 0.018a |
| III | 12 | 2 | 10 | 83.33 |
|
|
 | Table V.Vascular endothelial growth factor
expression in different phases of osteosarcoma. |
Table V.
Vascular endothelial growth factor
expression in different phases of osteosarcoma.
| Phase | Cases | Negative cases | Positive cases | Positive rate,
% | χ2 | P-value |
|---|
| I | 3 | 3 | 0 | 0.00 |
|
|
| II | 17 | 6 | 11 | 64.71 | 7.513a | 0.006a |
| III | 12 | 0 | 12 | 100.00 |
|
|
 | Table VI.MVD values in different phase of
osteosarcoma. |
Table VI.
MVD values in different phase of
osteosarcoma.
| Phase | Cases | MVDa | t-value | P-value |
|---|
| I | 3 | 23.33±4.36 |
|
|
| II | 17 | 37.15±3.74 |
|
|
| I and II | 20 | 31.08±3.36 | 7.536b |
<0.0001b |
| III | 12 |
41.20±4.17 |
|
|
Associations among Twist, VEGF and MVD
expression in correlation analysis
Spearman's rank correlation analysis revealed that
Twist expression was positively associated with VEGF expression
(r=0.371, P=0.002) and with MVD (r=0.393, P=0.001) in OS; in
addition, VEGF expression was demonstrated to have a positive
correlation with MVD (r=0.469, P=0.001).
Discussion
OS is an aggressive type of cancer that affects the
skeletal system. Although investigated by numerous previous
studies, the molecular mechanisms of the etiology and pathogenesis
underlying OS remain to be elucidated (19,20).
Therefore developing effective therapeutic strategies for the
treatment of OS is challenging. The present study demonstrated that
Twist expression was positively correlated with tumor phase and
metastasis in patients with OS. It has previously been reported
that Twist expression was significantly higher in cancer cells of
sarcomatoid renal cell carcinoma compared with those at the edge of
the tumors; in addition, Twist was associated with tumor
aggressiveness and poor prognosis in patients with renal cell
carcinoma (21). Oncogenic activation
of Twist was reported to be essential for the
epithelial-mesenchymal transition (EMT) that initializes invasion
and metastasis (22). Twist has been
demonstrated to couple aberrant signals from EMT to senescence and
was found to be an important candidate biomarker for cervical
cancer prognosis (23). In addition,
the downregulation of Twist expression was suggested to facilitate
apoptosis and recover the sensitivity of chemoresistance induced by
cisplatin in ovarian cancer (24).
Furthermore, the enhanced production of Twist resulted in VEGF
secretion that promotes tumor angiogenesis in breast cancer cells,
which in turn enhances cancer invasion and metastasis (9).
VEGF, as a prime mediator of angiogenesis, has been
implicated in carcinogenesis and metastasis in human and murine OS
cells (25). It was reported that the
survival and proliferation of highly aggressive OS cells was
dependent on autocrine VEGF/VEGF receptor 1 signaling in
vitro (17). Numerous studies
have suggested that VEGF expression may act as a biomarker of
prognosis in OS patients (26–28). A
previous study reported that patients with high VEGF expression had
significantly reduced disease-free survival and overall survival
rates compared with OS patients with low or negative VEGF
expression (28). In line with
previous studies, the present study determined that the positive
rate of VEGF in OS tissues was significantly increased compared
with that in OC tissues with increased grade and metastasis,
suggesting that VEGF expression may be an efficient biomarker for
predicting the prognosis of OS patients. However, further
large-scale prospective trials are required in order to confirm the
prognostic value of VEGF for survival in OS patients.
The present study determined that Twist expression
was positively correlated with VEGF expression in OS. Niu et
al (7) reported that increased
Twist messenger RNA and protein expression levels were positively
associated with the upregulation of VEGF expression in HCC patients
with a poor prognosis, suggesting that Twist may have a critical
role in the angiogenesis and metastasis of HCC (7). In cases of supraglottic carcinoma, Twist
and VEGF expression levels in lymph node metastasis patients were
significantly increased compared with those in patients without
metastasis; in addition, the levels of VEGF were reported to be
positively correlated with those of Twist (29). One study reported that stable
overexpression of Twist in the MCF-7 breast cancer cell line
resulted in increased VEGF synthesis in vitro and xenograft
experiments with MCF-7 cells overexpressing Twist produced tumors
with a higher vascular volume and vascular permeability in
vivo (6). Overall, these previous
studies indicated that Twist overexpression may enhance cancer
invasion and metastasis through increasing VEGF expression,
resulting in the induction of angiogenesis, which is pivotal for
the transformation into an aggressive phenotype.
In the present study, the staining of endothelial
cells for CD34 was used to evaluate the MVD in OS tissues. The
results indicated that the MVD in OS tissues was significantly
increased compared with that of OC tissues; in addition, this
increase was correlated with OS phase. Spearman's rank correlation
analysis revealed that Twist expression was positively correlated
with MVD, while VEGF expression also exhibited a positive
correlation with MVD. Positive Twist expression in HCC specimens
was previously demonstrated to have an elevated MVD compared with
specimens with negative Twist expression (7). Another study demonstrated that the MVD
in paraffin sections from 97 patients with HCC was correlated with
the upregulation of Twist expression (30). In addition, Twist expression was
reported to be positively associated with MVD in cancer cells of
sarcomatoid renal cell carcinoma (21). It has been demonstrated that VEGF
regulated the development of microvessels and was significantly
correlated with MVD in Ewing's sarcoma family of tumors (31). Serum VEGF levels were identified to be
significantly elevated in OS patients with pulmonary metastasis
compared with patients without detectable disease relapse; in
addition, these VEGF levels were positively correlated with the
MVD, suggesting that the pre-therapeutic serum VEGF levels
reflected the angiogenic property of OS (32,33).
However, Ek et al (14)
demonstrated that the degree of MVD and VEGF expression did not
provide prognostic information for OS, as determined through a
small-scale (25 cases) investigation. Thus, the association between
VEGF expression and MVD in OS requires further large-scale
investigations, in addition to further studies regarding Twist and
VEGF expression.
In conclusion, the present study investigated the
expression of Twist, VEGF and CD34 (MVD) in 32 cases of OS at
different phases and analyzed the associations among Twist, VEGF
and MVD. The results revealed that OS tissues exhibited elevated
expression levels of Twist and VEGF as well as high MVD values. In
addition, it was indicated that Twist overexpression in OS may
enhance cancer invasion and metastasis in OS through increasing
VEGF expression, which in turn may result in increased MVD.
Therefore, inhibition of Twist expression may have potential
therapeutic use for the treatment of OS.
Acknowledgements
This study was supported by grants from the 2014
Hunan Provincial Innovation Foundation For Postgraduates (no.
CX2014) and the Open-End Fund for the Valuable and Precision
Instruments of Central South University (no. CSUZC2014046).
References
|
1
|
Yin K, Liao Q, He H and Zhong D:
Prognostic value of Twist and E-cadherin in patients with
osteosarcoma. Med Oncol. 29:3449–3455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mullen JT, Hornicek FJ, Harmon DC, et al:
Prognostic significance of treatment-induced pathologic necrosis in
extremity and truncal soft tissue sarcoma after neoadjuvant
chemoradiotherapy. Cancer. 120:3676–3682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Zang X, Huang Z and Zhang C: TWIST
interacts with endothelin-1/endothelin A receptor signaling in
osteosarcoma cell survival against cisplatin. Oncol Lett.
5:857–861. 2013.PubMed/NCBI
|
|
4
|
Levens D and Larson DR: A new twist on
transcriptional bursting. Cell. 158:241–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khan MA, Chen HC, Zhang D and Fu J: Twist:
A molecular target in cancer therapeutics. Tumour Biol.
34:2497–2506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mironchik Y, Winnard PT Jr, Vesuna F, et
al: Twist overexpression induces in vivo angiogenesis and
correlates with chromosomal instability in breast cancer. Cancer
Res. 65:10801–10809. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niu RF, Zhang L, Xi GM, et al:
Up-regulation of Twist induces angiogenesis and correlates with
metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res.
26:385–394. 2007.PubMed/NCBI
|
|
8
|
Wallerand H, Robert G, Pasticier G, et al:
The epithelial-mesenchymal transition-inducing factor TWIST is an
attractive target in advanced and/or metastatic bladder and
prostate cancers. Urol Oncol. 28:473–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sossey-Alaoui K, Pluskota E, Davuluri G,
et al: Kindlin-3 enhances breast cancer progression and metastasis
by activating Twist-mediated angiogenesis. FASEB J. 28:2260–2271.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Low-Marchelli JM, Ardi VC, Vizcarra EA,
van Rooijen N, Quigley JP and Yang J: Twist1 induces CCL2 and
recruits macrophages to promote angiogenesis. Cancer Res.
73:662–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka T, Yui Y, Naka N, et al: Dynamic
analysis of lung metastasis by mouse osteosarcoma LM8: VEGF is a
candidate for anti-metastasis therapy. Clin Exp Metastasis.
30:369–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakurai T, Okumura H, Matsumoto M, et al:
Endoglin (CD105) is a useful marker for evaluating microvessel
density and predicting prognosis in esophageal squamous cell
carcinoma. Anticancer Res. 34:3431–3438. 2014.PubMed/NCBI
|
|
13
|
Wang WQ, Liu L, Xu HX, et al: The
combination of HTATIP2 expression and microvessel density predicts
converse survival of hepatocellular carcinoma with or without
sorafenib. Oncotarget. 5:3895–3906. 2014.PubMed/NCBI
|
|
14
|
Ek ET, Ojaimi J, Kitagawa Y and Choong PF:
Does the degree of intratumoural microvessel density and VEGF
expression have prognostic significance in osteosarcoma. Oncol Rep.
16:17–23. 2006.PubMed/NCBI
|
|
15
|
He S, Xiao Z, Chen L and Xiong S: Comment
on, Xu XW, et al: Prognostic significance of VEGF expression
in osteosarcoma: A meta-analysis. Tumour Biol. 35:6193–6194. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Becker RG, Galia CR, Morini S and Viana
CR: Immunohistochemical expression of vegf and her-2 proteins in
osteosarcoma biopsies. Acta Ortop Bras. 21:233–238. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohba T, Cates JM, Cole HA, et al:
Autocrine VEGF/VEGFR1 signaling in a subpopulation of cells
associates with aggressive osteosarcoma. Mol Cancer Res.
12:1100–1111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roberts CC, Daffner RH, Weissman BN, et
al: ACR appropriateness criteria on metastatic bone disease. J Am
Coll Radiol. 7:400–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou W, Hao M, Du X, Chen K, Wang G and
Yang J: Advances in targeted therapy for osteosarcoma. Discov Med.
17:301–307. 2014.PubMed/NCBI
|
|
20
|
Rettew AN, Getty PJ and Greenfield EM:
Receptor tyrosine kinases in osteosarcoma: not just the usual
suspects. Adv Exp Med Biol. 804:47–66. 2014.PubMed/NCBI
|
|
21
|
Ohba K, Miyata Y, Matsuo T, et al: High
expression of Twist is associated with tumor aggressiveness and
poor prognosis in patients with renal cell carcinoma. Int J Clin
Exp Pathol. 7:3158–3165. 2014.PubMed/NCBI
|
|
22
|
Galvan JA, Helbling M, Koelzer VH, et al:
TWIST1 and TWIST2 promoter methylation and protein expression in
tumor stroma influence the epithelial-mesenchymal transition-like
tumor budding phenotype in colorectal cancer. Oncotarget.
6:874–885. 2015.PubMed/NCBI
|
|
23
|
Wang T, Li Y, Wang W, et al: Twist2, the
key Twist isoform related to prognosis, promotes invasion of
cervical cancer by inducing epithelial-mesenchymal transition and
blocking senescence. Hum Pathol. 45:1839–1846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang T, Li Y, Tuerhanjiang A, et al:
Twist2 contributes to cisplatin-resistance of ovarian cancer
through the AKT/GSK-3beta signaling pathway. Oncol Lett.
7:1102–1108. 2014.PubMed/NCBI
|
|
25
|
Wang SW, Liu SC, Sun HL, et al: CCL5/CCR5
axis induces vascular endothelial growth factor-mediated tumor
angiogenesis in human osteosarcoma microenvironment.
Carcinogenesis. 36:104–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu XW, Wu TY, Yi X, et al: Prognostic
significance of VEGF expression in osteosarcoma: A meta-analysis.
Tumour Biol. 35:155–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Q, Zhu Y, Deng Z, Long H, Zhang S and
Chen X: VEGF and EMMPRIN expression correlates with survival of
patients with osteosarcoma. Surg Oncol. 20:13–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen D, Zhang YJ, Zhu KW and Wang WC: A
systematic review of vascular endothelial growth factor expression
as a biomarker of prognosis in patients with osteosarcoma. Tumour
Biol. 34:1895–1899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu SM, Yu L, Tian JJ, et al: Twist
modulates lymphangiogenesis and correlates with lymph node
metastasis in supraglottic carcinoma. Chin Med J (Engl).
124:1483–1487. 2011.PubMed/NCBI
|
|
30
|
Che N, Zhao XL, Sun T, et al: The role of
Twist1 in hepatocellular carcinoma angiogenesis: A clinical study.
Hum Pathol. 42:840–847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dalal S, Berry AM, Cullinane CJ, et al:
Vascular endothelial growth factor: A therapeutic target for tumors
of the Ewing's sarcoma family. Clin Cancer Res. 11:2364–2378. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaya M, Wada T, Kawaguchi S, et al:
Increased pre-therapeutic serum vascular endothelial growth factor
in patients with early clinical relapse of osteosarcoma. Br J
Cancer. 86:864–869. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oda Y, Yamamoto H, Tamiya S, et al: CXCR4
and VEGF expression in the primary site and the metastatic site of
human osteosarcoma: Analysis within a group of patients, all of
whom developed lung metastasis. Mod Pathol. 19:738–745. 2006.
View Article : Google Scholar : PubMed/NCBI
|