Introduction
Fast-track surgery (FTS) was first proposed in a
study by Kehlet et al, which focused on using a variety of
measures to control a patient's perioperative pathophysiological
reaction, to reduce surgical stress and complications, to enhance
post-operative rehabilitation and to improve prognosis (1). FTS can be considered as multimodal
perioperative care. The main components include simplified bowl
preparation, skipping the routine mechanical enema, using epidural
anesthesia and analgesia, laparoscopic surgery, early removal of
the nasogastric and drainage tubes, and early post-operative
activities.
Randomized controlled trials and meta-analyses have
shown that the skipping of the routine mechanical enema, the use of
epidural anesthesia and analgesia, the early removal of the
nasogastric tube and the early post-operative daily activities,
which are components of FTS, do not increase the incidence of
post-operative colorectal surgery complications, such as
anastomotic leakage, post-operative intestinal obstruction and
abdominal infection (2–4). However, few physicians have adopted FTS
in clinical practice in China. One reason for this may be that
Chinese physicians are waiting for convincing evidence that FTS is
better than conventional laparoscopic surgery (5,6). The
aforementioned randomized controlled trials and meta-analyses are
mostly based on patients in Europe and America. Thus, the present
randomized controlled study was conducted on Chinese patients in
mainland China.
Subjects and methods
Subjects
A randomized blinded study was conducted (trial
number, NCT01969591). All clinical data was collected from 70 cases
of colorectal cancer patients who were admitted to the Department
of Gastrointestinal Surgery, First Hospital of Jilin University
(Changchun, Jilin, China) between January 2011 and July 2012, and
met the selection criteria listed in Table I. Subsequent to obtaining written
informed consent, the patients were randomly divided into two
groups. Post-operative observations and surgical outcomes were
compared. The efficacy of FTS applied in colorectal cancer was
evaluated. The study protocol was approved by the Ethics Committee
of the First Hospital of Jilin University.
 | Table I.Inclusion and exclusion criteria. |
Table I.
Inclusion and exclusion criteria.
| Inclusion
criteria | Exclusion
criteria |
|---|
| Age ≤75 years | Age >75 years |
| Good nutrition and no
systemic infection | Malnutrition or an
organ system infection |
| Elective laparoscopic
surgery | Associated with
obstruction, bleeding, emergency surgery or surgical
intervention |
|
| Tumor with extensive
metastasis |
|
| Prior to surgery,
patient was fasting, underwent gastrointestinal decompression and
received nutritional support |
|
| Previous history of
abdominal surgery |
|
| Patient had
previously undergone gastrostomy |
Methods
The 70 patients with colorectal cancer were divided
into two groups, the FTS group (31 cases) and the control group (39
cases), according to the perioperative treatment. The differences
in perioperative care between the two groups are listed in Table II. Patient age, gender, basic
disease(s), tumor-node-metastasis stage (7), surgical approach, duration of surgery
and blood loss volume are listed in Table III.
 | Table II.Perioperative treatment of the FTS and
control groups. |
Table II.
Perioperative treatment of the FTS and
control groups.
| Treatment | FTS group | Control group |
|---|
| Pre-operative |
|
|
| Bowel
preparation | No mechanical bowel
preparation | Mechanical bowel
preparation |
| Diet
control | Pre-operative fasting
for 2 h for liquids and for 6 h for solid food | Pre-operative fasting
for 24 h prior to surgery |
|
Nutritional support | Enteral nutrition 24
h prior to surgery and 500 ml of 10% glucose solution 3 h prior to
surgery | Semi-liquid diet
initiated 72 h prior to surgery, and fasting prescribed on the
morning of surgery |
|
Antimicrobial prophylaxis | Intravenous
antibiotics 30 min prior to surgery | Orally administered
metronidazole and amikacin 72 h prior to surgery, and intravenous
antibiotics 30 min prior to surgery |
| Intraoperative |
|
|
|
Restricted fluid
replacement | Colloidal fluid
consumption limited to 500 ml and crystalloid fluid consumption
limited to 150ml; vasoactive drugs may be used when necessary | Sufficient fluid
administered according to urine volume |
| Post-operative |
|
|
| Standard
anesthetic protocol | Continuous epidural
analgesia (up to 48 h post-surgery) | Intermittent
injection of meperidine |
|
Post-operative nutrition | At 6 h post-surgery,
the patient can consume a liquid diet, with restoration of a solid
diet at 24 h post-surgery | Fluid diet fed after
the passage of first flatus, at 3–4 days post-surgery |
|
Nasogastric tube | No nasogastric tube
used, and if used, removed at the end of the surgery | Removed after 3–4
days |
| Drainage
tube | No drainage tube | Removed at 3–5
days |
| Urinary
catheter | Removed on the first
post-operative day | Removed at 3–4
days |
|
Post-operative activity | Ambulation started on
the first post-operative day | Ambulation started at
3–4 days post-surgery; ambulation cannot start until full recovery
of physical strength |
 | Table III.General information comparison of
patients in the FTS and control groups. |
Table III.
General information comparison of
patients in the FTS and control groups.
| Parameter | FTS group (n=31) | Control group
(n=39) | P-values |
|---|
| Age, years (mean ±
SD) | 58.5±8.4 | 57.4±10.1 | 0.629 |
| Gender, n
(male/female) | 22/9 | 20/19 | 0.095 |
| Underlying diseases,
n |
|
| 0.546 |
|
Diabetes | 2 | 4 | 0.690 |
|
Hypertension | 4 | 9 | 0.357 |
| Heart
disease | 0 | 0 | 1.000 |
|
Anemia | 4 | 6 | 0.987 |
| TNM
staginga, n |
|
| 0.834 |
| Stage
I | 1 | 1 |
|
| Stage
II | 16 | 20 |
|
| Stage
III | 14 | 18 |
|
| Surgical approach,
n |
|
| 0.984 |
| Right
colectomy | 8 | 11 |
|
|
Transverse colectomy | 1 | 2 |
|
| Left
colectomy | 2 | 3 |
|
| Sigmoid
colectomy | 8 | 8 |
|
| Anterior
resection of the rectum | 12 | 15 |
|
| Surgical duration,
min (mean ± SD) | 83±18 | 88±15 | 0.208 |
| Blood loss volume, ml
(mean ± SD) | 14.5±9.5 | 16.4±8.9 | 0.394 |
Statistical analysis
The SPSS 16.0 statistical software package (SPSS,
Inc., Chicago, IL, USA) was used to process the data, recorded as
mean ± standard deviation, and processed using a t-test for
two independent samples. Count data was compared using the
χ2 test. P<0.05 was used to indicate a statistically
significant difference.
Results
Hospital stay duration
The average length of total hospital stay for the
FTS and control groups was 5.9±0.8 and 10.9±1.3 days, respectively
(P<0.05), and the length of post-operative hospital stay was
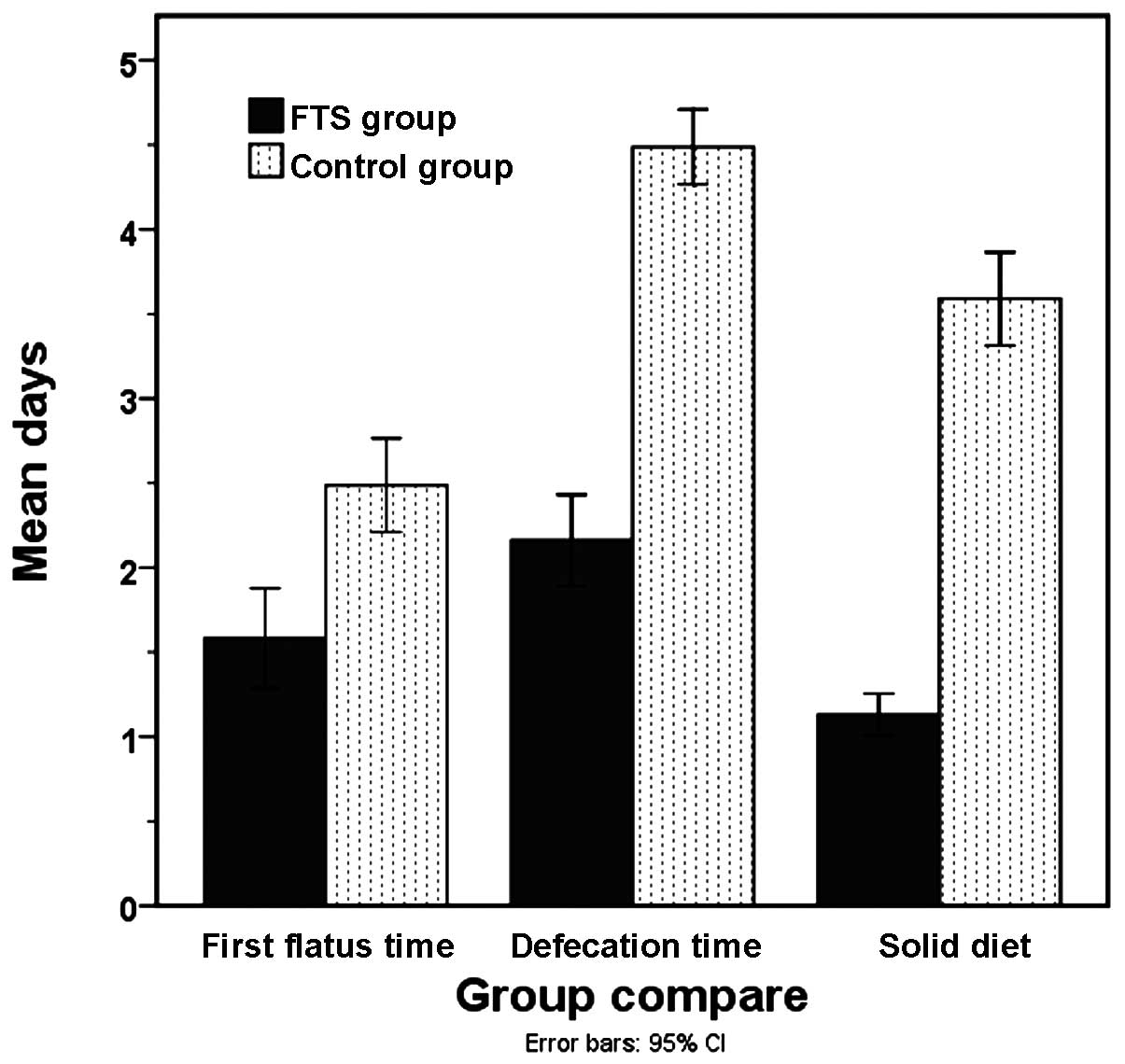
4.3±0.8 and 8.0±1.1 days, respectively (P<0.05). First flatus
time for the FTS and control groups was 1.6±0.8 and 2.5±0.9 days,
respectively (P<0.05), while defecation time was 2.2±0.7 and
4.5±0.7 days, respectively (P<0.05). The time to restoration of
a solid diet also exhibited a significant difference between the
FTS and control groups (1.1±0.3 vs. 3.6±0.9 days; P<0.05)
(Fig. 1).
Post-operative analgesic
adminstration
In the first 3 post-operative days, the results for
the FTS group show that 18 patients (58.1%) did not use analgesics,
whereas 5 patients (16.1%) used analgesics once, 6 patients (19.4%)
used them twice and 2 patients (6.5%) used them 3 times. In the
first 3 post-operative days for the control group, 21 patients
(53.9%) did not use analgesics, whereas 9 patients (23.1%) used
analgesics once, 7 patients (18.0%) used them twice and 2 patients
(5.1%) used them 3 times. The use of analgesic between the two
groups showed no significant difference (P>0.05).
C-reactive protein levels
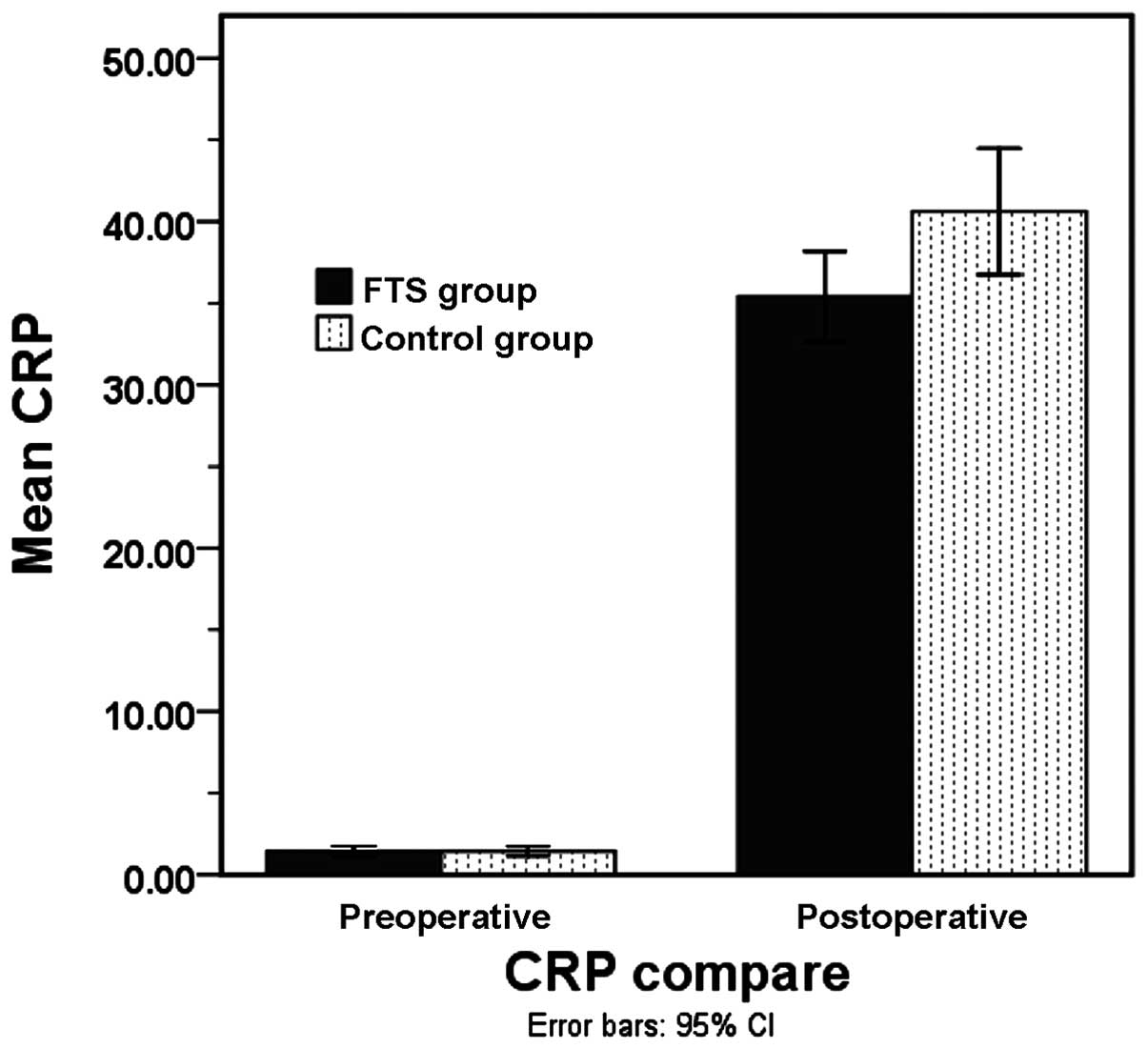
The pre-operative C-reactive protein (CRP) levels
between the FTS (1.4±0.9 mg/l) and control (1.4±0.9 mg/l) groups
exhibited no significant difference (P>0.05). On post-operative
day 1, the CRP level of the FTS group was high, but lower than the
CRP level in the control group (35.4±7.5 mg/l vs. 40.6±11.9 mg/l;
P<0.05) (Fig. 2).
Post-operative complications
Two cases of intestinal obstruction (5.1%) were
observed in the control group, but there were no cases of
anastomotic leakage or wound infection. One case in the FTS group
experienced wound infection (3.3%). The incidence of complications
between the FTS and control groups showed no significant difference
(P>0.05) (Table IV).
 | Table IV.Comparison of FTS and control groups
with regard to length of hospital stay and other post-operative
parameters. |
Table IV.
Comparison of FTS and control groups
with regard to length of hospital stay and other post-operative
parameters.
| Parameter | FTS group (n=31) | Control group
(n=39) | P-values |
|---|
| Length of hospital
stay, daysa | 5.9±0.8 | 10.9±1.3 | <0.001 |
| Post-operative
daysa | 4.3±0.8 | 8.0±1.1 | <0.001 |
| Time until first
flatus, daysa | 1.6±0.8 | 2.5±0.9 | <0.001 |
| Time until first
defecation, daysa | 2.2±0.7 | 4.5±0.7 | <0.001 |
| Time until
resumption of solid diet, daysa | 1.1±0.3 | 3.6±0.9 | <0.001 |
| Analgesic
administration, n (%) |
|
| 0.891 |
|
None | 18 (58.1) | 21 (53.9) |
|
|
Once | 5 (16.1) | 9 (23.1) |
|
|
Twice | 6 (19.4) | 7 (17.9) |
|
| 3
times | 2 (6.5) | 2 (5.1) |
|
| CRP,
mg/la |
|
|
|
|
Pre-operative | 1.4±0.9 | 1.4±0.90 | 0.956 |
|
Post-operative day 1 | 35.4±7.5 | 40.6±11.9 | 0.029 |
| Complications,
n |
|
|
|
|
Anastomotic leakage | 0 | 0 | 1.000 |
|
Intestinal obstruction | 0 | 2 | 0.502 |
| Wound
infection | 1 | 0 | 0.438 |
Discussion
A number of studies documenting the efficacy of
laparoscopic surgery have been performed since its advent in the
early 1990′s. Minimally invasive laparoscopic surgery aids in
reducing post-operative pain, the incidence of hemorrhage, wound
infections and inflammation. Fast-track laparoscopic surgery for
colorectal resection can be defined as a coordinated pre-operative
approach aimed at reducing surgical stress and facilitating
post-operative recovery (8–10).
Stress, as a response to an unpleasant situation,
acts as a negative factor and may lead to adverse outcomes in a
number of diseases. In contrast to anxiety, stress may provoke
certain psychological and physiological changes in the body that
may result in decreasing activity, increasing prevalence of disease
and occasionally, mortality (11). In
the present study, CRP level was used as the stress indicator. On
post-operative day 1, the CRP level was significantly higher in the
control group compared with that of the FTS group (P<0.05).
Despite the routine use of mechanical bowel
preparation (MBP) in clinic, there is a degree of concern with
regard to post-operative complications. For instance, Pineda et
al (12) and Bucher et al
(13) reported that the application
of MBP in colorectal cancer patients was linked with anastomotic
leakage and post-operative infectious complications, thus the
present study excluded the use of MBP in patients receiving
FTS.
Studies suggest that the consumption of
carbohydrate-rich drinks prior to surgery facilitates early
recovery (14–16). In the present study, the patients in
the FTS group underwent a pre-operative fasting period of 2 h for
liquids and 6 h for solids, while the control group underwent a
fasting period of 24 h prior to surgery. Oral consumption of 500 ml
of 10% glucose solution 3 h prior to surgery was undertaken by the
FTS group, whereas a semi-liquid diet was initiated and fasting was
prescribed on the morning of surgery for the control group.
However, in a randomized control study, Mathur et al
(17) showed that pre-operative
carbohydrate loading did not have any benefits for patients
undergoing major abdominal surgery. Therefore, pre-operative
carbohydrate loading for major surgeries requires further study. It
is unclear whether carbohydrate loading played a role in the
improved recovery of the FTS group in the present study.
The dietary protocol in the control group undergoing
conventional laparoscopic surgery was not easily adapted. The
restoration of diet was implemented at 24 h post-surgery, however,
the transition from liquid to solid required 3–4 days. Conversely,
in the FTS group, the dietary protocol was well tolerated.
Furthermore, the early restoration of diet following surgery is
also associated with a lower mortality rate (18).
The use of analgesia and the number of complications
in the two groups did not differ significantly (P>0.05).
However, the length of hospital stay did differ significantly
between the two groups (P<0.05). This is in agreement with
various studies reporting that the implementation of FTS shortens
the hospital stay of patients (19,20).
Meta-analyses of control trials and cohort studies
have suggested that FTS for colorectal cancer is effective in terms
of reducing hospital stay and post-operative pain, and enhancing
early recovery following surgery (21–25).
Similarly, the present study showed a reduced length of hospital
stay and a lower number of post-operative days in the FTS group
(6.7±1.4 and 4.1±0.8 days, respectively) compared with the control
group (9.2±2.3 and 6.4±1.8 days, respectively).
In conclusion, FTS has a better outcome compared
with conventional surgery for colorectal cancer.
References
|
1
|
Kehlet H: Fast-track colorectal surgery.
Lancet. 371:791–793. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holte K and Kehlet H: Epidural anaesthesia
and analgesia - effects on surgical stress responses and
implications for postoperative nutirion. Clin Nutr. 21:199–206.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raue W, Haase O, Junghans T, et al:
‘Fast-track’ multimodal rehabilitation program improves outcome
after laparoscopic sigmoidectomy: A controlled prospective
evaluation. Surg Endosc. 18:1463–1468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scatizzi M, Kröning KC, Boddi V, et al:
Fast-track surgery after laparoscopic colorectal surgery: Is it
feasible in a general surgery unit? Surgery. 147:219–226. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang G, Jiang ZW, Xu J, Gong JF, Bao Y,
Xie LF and Li JS: Fast-track rehabilitation program vs conventional
care after colorectal resection: A randomized clinical trial. World
J Gastroenterol. 17:671–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Q, Suo J, Jiang J, Wang C, Zhao YQ
and Cao X: Effectiveness of fast-track rehabilitation vs
conventional care in laparoscopic colorectal resection for elderly
patients: A randomized trial. Colorectal Dis. 14:1009–1013. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edge S, Byrd DR, Compton CC, et al: AJCC
Cancer Staging Manual. 7th. Springer; New York, NY: 2010
|
|
8
|
Kelley SR, Wolff BG, Lovely JK and Larson
DW: Fast-track pathway for minimally invasive colorectal surgery
with and without alvimopan (Entereg)™: Which is more
cost-effective? Am Surg. 79:630–633. 2013.PubMed/NCBI
|
|
9
|
Baek SJ, Kim SH, Kim SY, Shin JW, Kwak JM
and Kim J: The safety of a ‘fast-track’ program after laparoscopic
colorectal surgery is comparable in older patients as in younger
patients. Surg Endosc. 27:1225–1232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sommer T, Larsen JF and Raundahl U:
Eliminating learning curve-related morbidity in fast track
laparoscopic Roux-en-Y gastric bypass. J Laparoendosc Adv Surg Tech
A. 21:307–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
British Medical Association Health Policy
and Economic Research Unit, . Work related stress among senior
doctors - review of research. London: BMA; 2000
|
|
12
|
Pineda CE, Shelton AA, Hernandez-Boussard
T, Morton JM and Welton ML: Mechanical bowel preparation in
intestinal surgery: a meta-analysis and review of the literature. J
Gastrointest Surg. 12:2037–2044. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bucher P, Gervaz P, Soravia C, Mermillod
B, Erne M and Morel P: Randomized clinical trial of mechanical
bowel preparation versus no preparation before elective left-sided
colorectal surgery. Br J Surg. 92:409–414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nygren J, Thorell A and Ljungqvist O:
Preoperative oral carbohydrate nutrition: An update. Curr Opin Clin
Nutr Metab Care. 4:255–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ljungqvist O, Nygren J and Thorell A:
Modulation of post-operative insulin resistance by pre-operative
carbohydrate loading. In: Proc Nutr Soc. 61. pp. 329–336. 2002;
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brady M, Kinn S and Stuart P: Preoperative
fasting for adults to prevent perioperative complications. Cochrane
Database Syst Rev. CD0044232003.PubMed/NCBI
|
|
17
|
Mathur S, Plank LD, McCall JL, Shapkov P,
McIlroy K, Gillanders LK, Merrie AE, Torrie JJ, Pugh F, Koea JB,
Bissett IP and Parry BR: Randomized controlled trial of
preoperative oral carbohydrate treatment in major abdominal
surgery. Br J Surg. 97:485–494. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andersen HK, Lewis SJ and Thomas S: Early
enteral nutrition within 24 h of colorectal surgery versus later
commencement of feeding for postoperative complications. Cochrane
Database Syst Rev. CD0040802006.PubMed/NCBI
|
|
19
|
Morończyk DA and Krasnodębski IW:
Implementation of the fast track surgery in patients undergoing the
colonic resection: Own experience. Pol Przegl Chir. 83:482–487.
2011.PubMed/NCBI
|
|
20
|
Gouvas N, Gogos-Pappas G, Tsimogiannis K,
Tsimoyiannis E, Dervenis C and Xynos E: Implementation of
fast-track protocols in open and laparoscopic sphincter-preserving
rectal cancer surgery: A multicenter, comparative, prospective,
non-randomized study. Dig Surg. 29:301–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gouvas N, Tan E, Windsor A, Xynos E and
Tekkis PP: Fast-track vs standard care in colorectal surgery: a
meta-analysis update. Int J Colorectal Dis. 24:1119–1131. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wind J, Polle SW, Fung Kon, Jin PH, Dejong
CH, von Meyenfeldt MF, Ubbink DT, Gouma DJ and Bemelman WA:
Laparoscopy and/or Fast Track Multimodal Management Versus Standard
Care (LAFA) Study Group; Enhanced Recovery After Surgery (ERAS)
Group: Systematic review of enhanced recovery programmes in colonic
surgery. Br J Surg. 93:800–809. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gatt M, Khan S and MacFie J: In response
to: Varadhan KK Neal KR Dejong CH Fearon KC Ljungqvist O Lobo DN
The enhanced recovery after surgery (ERAS) pathway for patients
undergoing major elective open colorectal surgery: a meta-analysis
of randomized controlled trials. Clin Nutr. 29:2010.434–440
Clin Nutr. 29:689–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feroci F, Lenzi E, Baraghini M, Garzi A,
Vannucchi A, Cantafio S and Scatizzi M: Fast-track surgery in real
life: how patient factors influence outcomes and compliance with an
enhanced recovery clinical pathway after colorectal surgery. Surg
Laparosc Endosc Percutan Tech. 23:259–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feroci F, Baraghini M, Lenzi E, Garzi A,
Vannucchi A, Cantafio S and Scatizzi M: Laparoscopic surgery
improves postoperative outcomes in high-risk patients with
colorectal cancer. Surg Endosc. 27:1130–1137. 2013. View Article : Google Scholar : PubMed/NCBI
|