Introduction
Extranodal natural killer/T-cell lymphoma (ENKTL),
an Epstein-Barr virus (EBV)-associated lymphoma, has a racial and
geographical predisposition, and accounts for 5–10% of all
malignant lymphomas in China (1). For
patients with stage IE ENKTL without any risk factors, radiotherapy
(RT) alone has been shown to achieve excellent outcomes (2). Such risk factors include an age of
>60 years, B symptoms, an Eastern Cooperative Oncology Group
performance status of ≥2, elevated lactate dehydrogenase levels,
regional node involvement, local tumor invasion (bone or skin),
high Ki-67 staining on histology, and an EBV DNA titer of
≥6.1×107 copies/ml (3).
However, for individuals with stage IE disease with risk factors or
those with stage IIE ENKTL, an increasing number of studies have
recommended concurrent (4) or
sequential (5) chemoradiation, in
order to reduce the relapse rate and improve the long-term
outcome.
Overexpression of multidrug-resistant (MDR) genes,
such as MDR-1, MRP, LRP and BCRP, leads to increased levels of
P-glycoprotein in NK/T-cell lymphoma cells. P-glycoprotein actively
exports doxorubicin and vincristine, which are the main components
of the CHOP chemotherapy regimen, subsequently leading to reduced
treatment efficacy and worse survival outcomes (6,7). Thus, due
to a high expression of MDR genes, ENKTL is resistant to
anthracycline-based chemotherapy (8).
L-asparaginase has been found to exhibit a specific anticancer
mechanism; it can hydrolyze and exhaust serum asparagines in
NK/T-cell lymphoma cells, which are unable to synthesize
L-asparagines, subsequently producing an anticancer effect
(9). Notably, asparaginase is not
affected by P-glycoprotein (10,11), as it
is not a substrate of P-glycoprotein and thus is not exported from
tumor cells (12). A number of
studies have incorporated asparaginase into existing chemotherapy
regimens and have achieved promising short-term results (5,9,13). However, to the best of our knowledge,
no long-term efficacy and safety data of asparaginase-based
treatment have been reported, due to relatively short follow-up
periods. As previously reported (5),
this group previously conducted a phase II clinical trial,
evaluating the efficacy and safety of a combination of gemcitabine,
oxaliplatin and asparaginase (GELOX), followed by RT in the
treatment of localized ENKTL, for which follow-up has been ongoing
since 2008. At the end of treatment, the overall response rate
(ORR) was 96.3%, with a complete remission (CR) rate of 74.1%, and
side effects were well tolerated. In the initial analysis, the
median follow-up time was 27 months, and 2-year overall survival
(OS) and progression-free survival (PFS) were each 86.0%. As of 6th
July 2014, the majority of patients had been followed up for >5
years. The current study therefore reports long-term outcomes of
GELOX-based treatment. Furthermore, patterns of failure, salvage
treatments and the involvement of EBV DNA in the early detection of
relapse are discussed.
Patients and methods
Patients
From January 2008 to July 2011, 27 patients, who
were newly diagnosed with stage IE or stage IIE ENKTL, were
enrolled. The primary site for all patients was the upper
aerodigestive tract, and the patients were enrolled regardless of
risk factors. Written informed consent was obtained from all
patients prior to enrollment, and the study was approved by the Sun
Yat-sen University Cancer Center Research Ethics Board. Inclusion
and exclusion criteria were as previously reported (5).
Treatment
Patients were treated with an initial GELOX regimen.
Following ≥2 cycles of GELOX, patients were referred for RT.
Subsequently, they received 2–4 cycles of GELOX within 1 week of
the completion of RT, resulting in a maximum total of 6 cycles of
GELOX. The dosage of the GELOX regimen and RT were as previously
reported (5).
Statistical analysis
PFS was calculated from the date of diagnosis to the
date of identification of disease progression, and was censored at
the date of the last follow-up visit. OS was calculated from the
date of diagnosis to the date of death from any cause, and was
censored at the date of the last follow-up visit. All statistical
analysis was performed using PASW Statistics 18.0 software (Apache
Software Foundation, Forest Hill, MD, USA). Survival outcomes were
assessed the Kaplan-Meier method and log-rank test. Differences
between the results of comparative tests were considered
significant if the two-sided P-value was <0.05.
Results
Patient characteristics and initial
findings
27 patients were enrolled in the phase II clinical
trial (mean age, 47 years; range, 21–74 years). Of these, 66.7%
(18/27) had stage IE disease, and all patients had an International
Prognostic Index score of 0–1. As previously reported, the ORR was
96.3% at the completion of first-line treatment, with a CR of 74.1%
and a PR of 22.2%.
Updated survival data
The data used for this analysis were updated in July
2014, and detailed follow-up information was available for all 27
patients. The median follow-up time was 63.15 months (range,
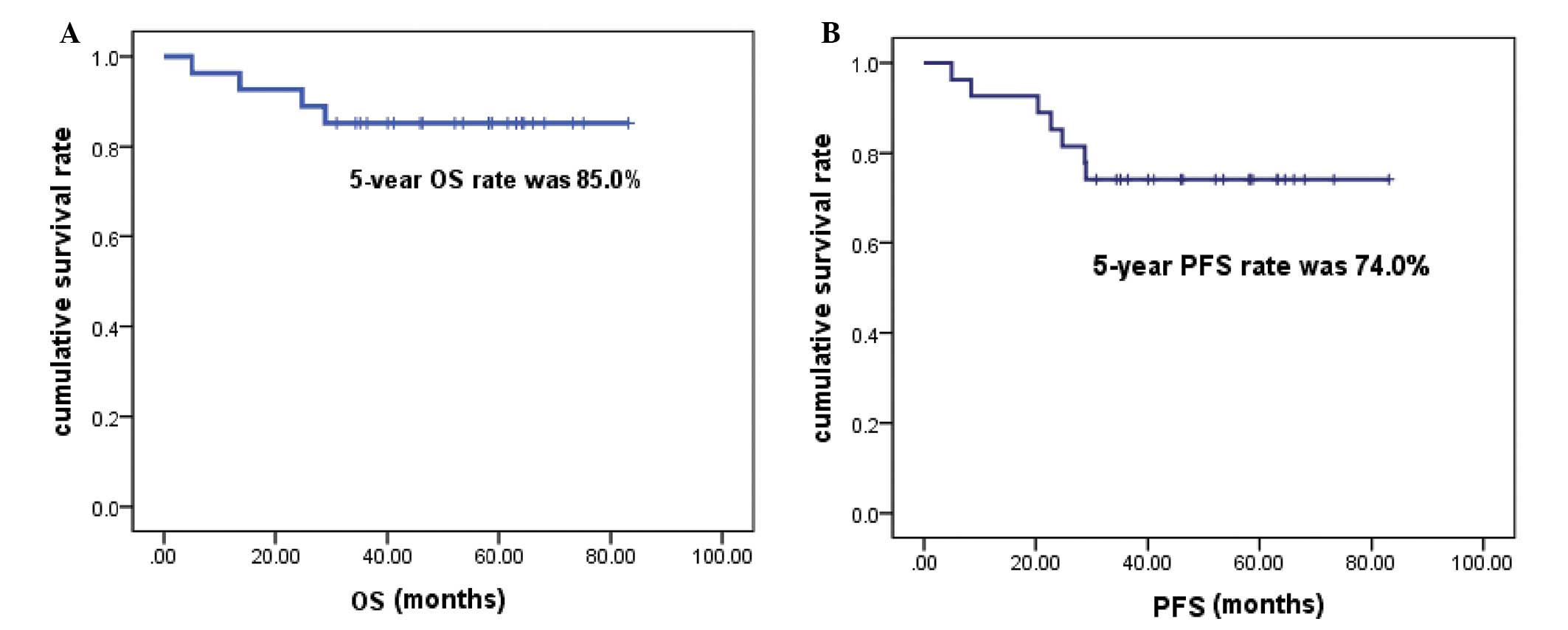
30.92–83.15 months). The 5-year OS rate was 85.0%, and the 5-year
PFS rate was 74.0% (Fig. 1). Four
patients were not alive at the end of the follow-up period, and no
further mortality occurred following the initial analysis. A total
of 7 patients exhibited disease progression, of whom 4 did not
survive, due to failure of salvage therapy. Recurrence within the
RT field was observed in 3 patients, and thus the planning
target-volume control rate at 5 years was 88.9% (24 out of 27
patients). Two patients with locoregional relapse responded to
subsequent GELOX and pegaspargase-based chemotherapy and achieved a
CR. One patient developed a lung recurrence following locoregional
relapse. This was a male patient, aged 41, who was diagnosed with
stage IE ENKTL in April 2009. He was treated with 6 cycles of GELOX
followed by RT (56 Gy) and subsequently achieved CR. However, he
developed locoregional relapse in September 2010. Seven cycles of a
combination of pegaspargase and IMVP-16 (ifosfamide, methotraxate
and etoposide) were administered and unconfirmed CR was attained in
the locoregional relapse site. However, a single small solid
nodule, 8×7 mm in size, was identified in the left lung during the
salvage chemotherapy, and a biopsy confirmed the diagnosis of
ENKTL. Due to the relapsed/refractory nature of this patient's
disease, autologous stem cell transplantation (ASCT) was performed
on 7th May 2011 (day 0), using BEAM as the conditioning regimen
(carmustine 300 mg/m2, day −6; cytarabine 200
mg/m2, twice daily, days −5 to −2; VP-16 200
mg/m2, days −5 to −2; and melphalan 140
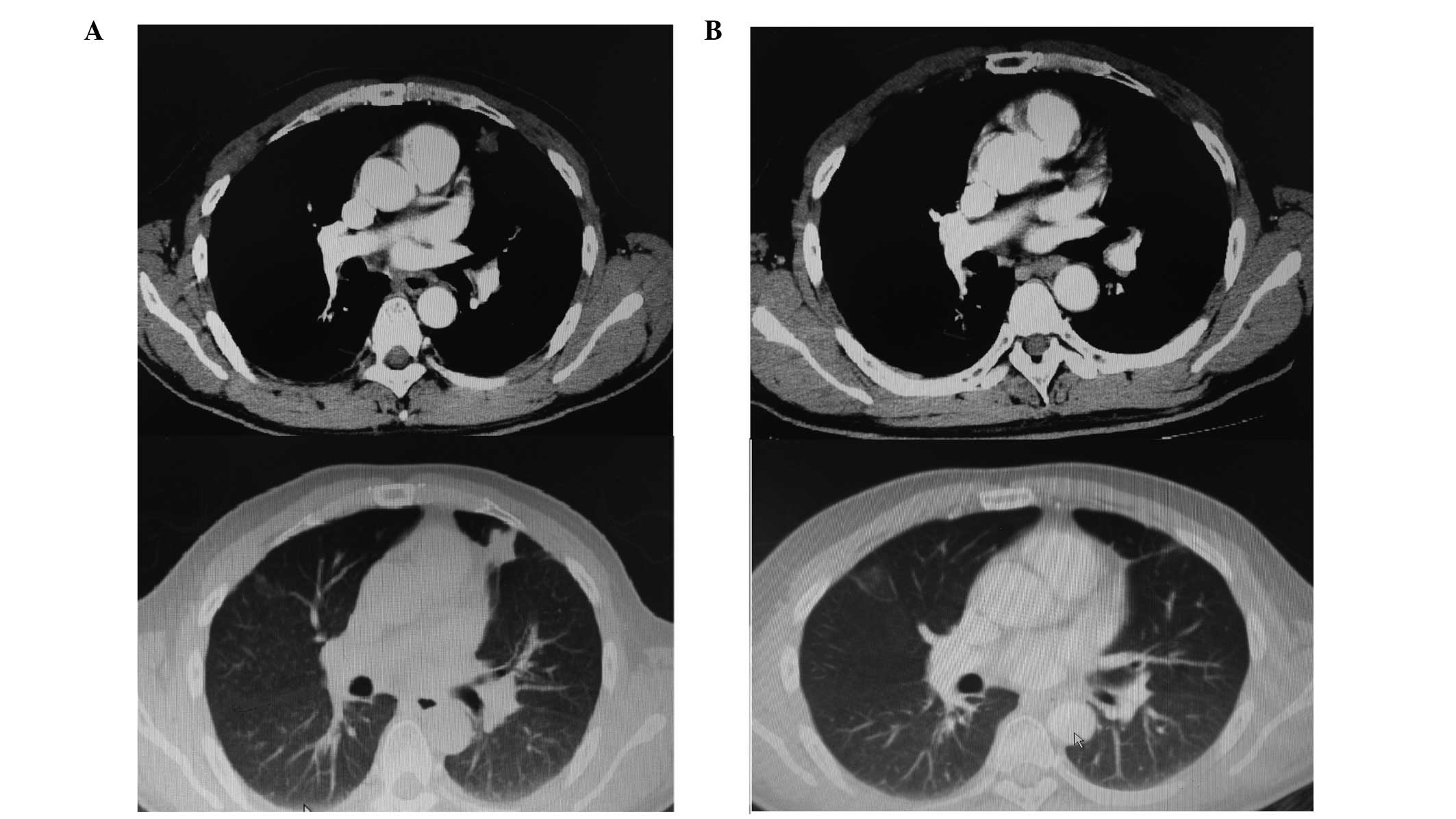
mg/m2, day −1). As shown in Fig. 2A, at 6 months post-ASCT, a 25×21 mm
solid nodule was observed in the left lung, which was significantly
larger than the nodule had been pre-ASCT. At this time, the EBV DNA
load was 4.01×103copies/mL, which indicated persistence
of ENKTL. Lenalidomide was then administered as salvage therapy, at
a dosage of 15 mg/d, from days 1 to 21, followed by a rest for 1
week. Treatment was well tolerated. Four months after lenalidomide
treatment, the CT scan demonstrated no discernible lesion in the
left lung (Fig. 2B) and the EBV DNA
load was undetectable. Lenalidomide was administered as maintenance
therapy for a total of 2 years, and regular follow-up CT scans
demonstrated no evidence of relapse. The patient was alive without
disease at the last follow-up visit.
Toxicity
No clinically significant late-onset toxicities were
observed during follow-up visits. As reported previously, <grade
3 mucositis and dermatitis occurred frequently during RT, and
resolved in all patients without compromising quality of life
[assessed using the European Organization for Research and
Treatment of Cancer Quality of Life Questionnaire, QLQ-C30
(14)]. No treatment-related
mortality occurred. However, a 52 year-old old female patient
developed left breast intraductal papilloma with atypical
hyperplasia 4 years after the completion of treatment for ENKTL. No
causality was found and this finding may be unrelated to the
treatment she received.
Discussion
It has been reported that ENKTL is resistant to
anthracycline-base chemotherapy regimens due to high expression of
P-glycoprotein (8).
L-asparaginase-based regimens, such as combined dexamethasone,
methotrexate, ifosfamide, L-asparaginase and etoposide (SMILE)
(9), and combined asparaginase,
methotrexate and dexamethasone (AspaMetDex) (13), have produced promising survival
outcomes. Since January 2008, this group has been conducting a
phase II clinical trial, evaluating the efficacy of GELOX in the
treatment of early stage ENKTL (5).
As previously reported, the short-term outcomes were significantly
better than those of CHOP-based regimens (15). In this updated analysis, with a median
follow-up time of 63.15 months, the 5-year PFS and OS were 74.0 and
85.0%, respectively, which were significantly better than those of
previous CHOP-based results, in which 5-year OS rate was 48.0%
(15). The Japan Clinical Oncology
Group Study (JCOG0211) (16) updated
the long-term outcomes of concomitant chemoradiotherapy using the
DeVIC regimen (dexamethasone, etoposide, ifosfamide and
carboplatin) for localized ENKTL, and the 5-year PFS and OS were
63% and 70%, respectively, which appear to be inferior to the
results of the present study. In addition, in comparison with the
safety profiles of the current study (5), a greater number of grade 3/4
hematological events and RT-related adverse events were documented
in the JCOG0211 study (4).
Furthermore, no overt late toxicities were observed in the present
group, while in the JCOG0211 group, one patient developed a grade 4
late RT adverse event (perforation of the nasal skin, which
required plastic surgery) and 11 patients developed grade 1 or 2
late RT adverse effects of the eye (16). Therefore, GELOX-based sequential
chemoradiotherapy appears to be highly efficacious and safe in the
treatment of localized ENKTL. Currently, this groups is undertaking
a phase II clinical trial, evaluating the efficacy and safety of
concomitant chemoradiotherapy using the GELOX regimen, which aims
to investigate whether this strategy may further reduce the relapse
rate, while maintaining acceptable toxicity profiles (NCT02080234;
http://clinicaltrials.gov).
In the present cohort, three patients who had
relapsed, responded to subsequent asparaginase-based chemotherapy,
indicating that asparaginase may be used as salvage treatment,
regardless of prior exposure. Recently, a number of studies have
demonstrated that the tumor microenviroment may be important in
tumorigenesis and tumor progression (17). Lin et al (18) showed that high expression of
tumor-associated macrophages predicted a poor prognosis in patients
with ENKTL, indicating that therapies targeting the tumor
microenviroment may be effective in ENKTL. As shown in Fig. 2, the lung lesion in a patient with
relapsed disease, was refractory to ASCT, while it responded well
to lenalidomide monotherapy, indicating the potential efficacy of
immunomodulatory drugs in the treatment of ENKTL. This group is
also currently undertaking a phase III trial, comparing the
efficacy of a pegaspargase-Gemox regimen followed by thalidomide,
with that of the AspaMetDex regimen in patients with de novo
or relapsed ENKTL, with the aim of investigating the role of
thalidomide as maintenance therapy in ENKTL (NCT02085655;
http://clinicaltrials.gov).
A number of studies have indicated that the plasma
EBV DNA load at presentation may predict survival outcomes
(19,20). Unfortunately, measurement of the EBV
DNA load was initially not routinely conducted. Therefore, an
analysis of EBV DNA load was not included in the first analysis of
the current clinical trial. Over the past 2 years, this strategy
was modified to test the EBV DNA load in all patients with ENKTL at
each follow-up visit. In the patient with relapsed disease, who was
treated with ASCT and lenalidomide, the EBV DNA load was 0 copy/ml
on 28th September 2011 (4 months post-ASCT). It increased to
4.01×103 copy/ml on 26th December 2011 (6 months
post-ASCT), at which time the CT scan revealed progression of the
existing lung lesion. The EBV DNA load had returned to 0 copy/ml on
17th December 2012 (10 months after lenalidomide treatment), and
the CT scan at that time demonstrated no detectable lesions in the
lung, thereby confirming CR. Thus, the EBV DNA load may be used as
an surrogate biomarker for early detection of relapsed disease, in
addition to a reflection of treatment response. The two ongoing
clinical trials, have implemented required EBV DNA load testing,
and may validate the role of EBV DNA load measurement in monitoring
patient with ENKTL.
In conclusion, the present updated analysis
confirmed the long-term benefit of GELOX regimen followed by RT,
and demonstrated good safety profiles with this approach. This
strategy may currently be one of the most suitable options for
treatment of early stage ENKTL.
Acknowledgements
The authors would like to thank the doctors of
Sun-Yat Sen University Cancer Center, for recruiting the patients
involved in this study, and the pathologists of Sun-Yat Sen
University Cancer Center for their assistance. This study received
financial support from the National Natural Science Foundation of
China (grant no. 81400159), the Young Teachers' Cultivation Project
of Sun Yat-sen University (grant no. 12ykpy54) and the Outstanding
Young Talents Project of Sun Yat-sen University Cancer Center
(grant no. 04190101).
References
|
1
|
Li YX, Liu QF, Fang H, Qi SN, Wang H, et
al: Variable clinical presentations of nasal and Waldeyer ring
natural killer/T-cell lymphoma. Clin Cancer Res. 15:2905–2912.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li YX, Wang H, Jin J, Wang WH, Liu QF, et
al: Radiotherapy alone with curative intent in patients with stage
I extranodal nasal-type NK/T-cell lymphoma. Int J Radiat Oncol Biol
Phys. 82:1809–1815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kohrt H, Lee M and Advani R: Risk
stratification in extranodal natural killer/T-cell lymphoma. Expert
Rev Anticancer Ther. 10:1395–1405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamaguchi M, Tobinai K, Oguchi M, Ishizuka
N, Kobayashi Y, Isobe Y, et al: Phase I/II study of concurrent
chemoradiotherapy for localized nasal natural killer/T-cell
lymphoma: Japan Clinical Oncology Group study JCOG0211. J Clin
Oncol. 27:5594–5600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Wang ZH, Chen XQ, Li YJ, Wang KF,
et al: First-line combination of gemcitabine, oxaliplatin, and
L-asparaginase (GELOX) followed by involved-field radiation therapy
for patients with stage IE/IIE extranodal natural killer/T-cell
lymphoma. Cancer. 119:348–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baekelandt M, Lehne G, Tropé CG, Szántó I,
Pfeiffer P, Gustavssson B and Kristensen GB: Phase I/II trial of
the multidrug-resistance modulator valspodar combined with
cisplatin and doxorubicin in refractory ovarian cancer. J Clin
Oncol. 19:2983–2993. 2001.PubMed/NCBI
|
|
7
|
Shiraki N, Hamada A, Ohmura T, Tokunaga J,
Oyama N and Nakano M: Increase in doxorubicin cytotoxicity by
inhibition of P-glycoprotein activity with lomerizine. Biol Pharm
Bull. 24:555–557. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang B, Li XQ, Ma X, Hong X, Lu H and Guo
Y: Immunohistochemical expression and clinical significance of
P-glycoprotein in previously untreated extranodal NK/T-cell
lymphoma, nasal type. Am J Hematol. 83:795–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamaguchi M, Kwong YL, Kim WS, Maeda Y,
Hashimoto C, Suh C, Izutsu K, Ishida F, Isobe Y, Sueoka E, et al:
Phase II study of SMILE chemotherapy for newly diagnosed stage IV,
relapsed, or refractory extranodal natural killer (NK)/T-cell
lymphoma, nasal type: The NK-Cell Tumor Study Group study. J Clin
Oncol. 29:4410–4416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin JK, Sun W, Moraga-A D, Schuster SM
and Wylie DE: An investigation into the mechanism of L-asparaginase
resistance in L5178Y murine leukemia cells. Amino Acids. 5:51–69.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tse E and Kwong YL: How I treat NK/T-cell
lymphomas. Blood. 121:4997–5005. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van den Berg H: Asparaginase revisited.
Leuk Lymphoma. 52:168–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jaccard A, Gachard N, Marin B, Rogez S,
Audrain M, et al: Efficacy of L-asparaginase with methotrexate and
dexamethasone (AspaMetDex regimen) in patients with refractory or
relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood.
117:1834–1839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chie WC, Yang CH, Hsu C and Yang PC:
Quality of life of lung cancer patients: Validation of the Taiwan
Chinese version of the EORTC QLQ-C30 and QLQ-LC13. Qual Life Res.
13:257–262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Xia ZJ, Huang HQ, et al:
Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)
in the treatment of stage IE/IIE extranodal natural killer/T cell
lymphoma, nasal type: 13-year follow-up in 135 patients. Int J
Hematol. 96:617–623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaguchi M, Tobinai K, Oguchi M, Ishizuka
N, Kobayashi Y, Isobe Y, et al: Concurrent chemoradiotherapy for
localized nasal natural killer/T-cell lymphoma: An updated analysis
of the Japan clinical oncology group study JCOG0211. J Clin Oncol.
30:4044–4046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosenquist R, Davi F and Ghia P: The
microenvironment in lymphomas - dissecting the complex crosstalk
between tumor cells and ‘by-stander’ cells. Semin Cancer Biol.
24:1–2. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin ZX, Bai B, Cai QC, et al: High numbers
of tumor-associated macrophages correlate with poor prognosis in
patients with mature T- and natural killer cell lymphomas. Med
Oncol. 29:3522–3528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HS, Kim KH, Kim KH, Chang MH, Ji SH,
et al: Whole blood Epstein-Barr virus DNA load as a diagnostic and
prognostic surrogate: Extranodal natural killer/T-cell lymphoma.
Leuk Lymphoma. 50:757–763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki R, Yamaguchi M, Izutsu K, Yamamoto
G, Takada K, Harabuchi Y, et al: Prospective measurement of
Epstein-Barr virus-DNA in plasma and peripheral blood mononuclear
cells of extranodal NK/T-cell lymphoma, nasal type. Blood.
118:6018–6022. 2011. View Article : Google Scholar : PubMed/NCBI
|