Introduction
Juvenile polyps (JPs), which are considered to be a
type of hamartomatous polyp (1),
account for a small proportion of colorectal polyps. Solitary
sporadic JPs are often observed in childhood, with a prevalence of
1–2% (2,3). When JPs meet the following criteria,
juvenile polyposis syndrome (JPS), which is an autosomal dominant
disorder characterized by the presence of multiple JPs in the
gastrointestinal tract, is diagnosed: ⅰ) >3–5 JPs in the
colorectum; ⅱ) JPs throughout the intestinal tract; or ⅲ) any
number of JPs in combination with a positive family history of JPS
(4). JPs are typically considered to
be hamartomatous lesions with little malignant potential (5). JPS is reported as a risk factor for
colorectal cancer and it has been reported that patients with JPS
have a significantly elevated relative risk (34.0) of developing
colorectal cancer compared with the general population (6).
Although certain studies have reported an
association between their origin and genetic polymorphism, much
remains uncertain, including why JPs originate from the colorectal
mucosa, how they undergo malignant transformation and what the
typical endoscopic and chromoendoscopic findings are (7,8). In
clinical practice, JPs may present as childhood intussusceptions or
bloody stools, and they may also cause positive fecal occult blood
tests in adulthood. JPs are usually detected incidentally as a
result of colonoscopy for gastrointestinal tract complaints.
Following the identification of JPs on endoscopic examination,
lesions are resected endoscopically, and a definitive diagnosis is
usually confirmed by pathological examination (9,10).
Precise endoscopic diagnosis is important, however,
there are few reports describing the endoscopic features of JPs
(2). It is often difficult to
differentiate JPs from adenomas, inflammatory myoglandular polyps,
other hamartomatous polyps (such as solitary Peutz-Jeghers polyps),
inflammatory polyps and hyperplastic polyps with inflammatory
changes (10,11).
Therefore, the present study retrospectively
analyzed the endoscopic findings of JPs, particularly regarding the
magnifying chromoendoscopic findings. In addition, the current
study made the first attempt to evaluate JPs by endocytoscopy (EC).
The EC system allows observation of living gastrointestinal cells,
nuclei and vascularity in vivo under ultra-high
magnification, enabling real-time endoscopic assessment of
histological characteristics. The present study was approved by the
Ethics Committee of Showa University Northern Yokohama Hospital
(Yokohama, Japan; approval no. 1509-02) and the requirement for
consent was waived due to the retrospective nature of the
study.
Patients and methods
Patients
A total of 154 JPs from 128 patients treated
endoscopically at Showa University Northern Yokohama Hospital
between April 2001 and April 2014 were assessed in the present
study. Cases of JP diagnosed by colonoscopy were extracted from the
hospital database. Of those cases, the diagnosis of JP was
confirmed by histopathological diagnosis. In addition, endoscopic
features of all lesions were observed by retrospectively assessing
magnifying chromoendoscopy images captured prior to endoscopic
resection, and all were ultimately diagnosed pathologically as JPs.
Lesions that were pathologically diagnosed as hyperplastic polyp,
inflammatory polyp and adenoma were excluded. In addition, 20 of
these lesions from 16 patients were observed by EC following
magnifying chromoendoscopy. Characteristic information of patients
was obtained from patient history and interview data (Table I).
 | Table I.Relevant patient characteristics and
the gross appearance and endoscopic therapy of each juvenile
polypa (patients, n=128;
polyps, n=154). |
Table I.
Relevant patient characteristics and
the gross appearance and endoscopic therapy of each juvenile
polypa (patients, n=128;
polyps, n=154).
| Characteristic | MCa | MC+ECa | All patients |
|---|
| Age, years |
|
|
|
| Mean | 47.2 | 46.7 | 47.1 |
|
Median | 47.0 | 47.5 | 47.0 |
|
Range | 2-88 | 27-77 | 2-88 |
| Gender, n |
|
|
|
| Male | 91 | 5 | 96
(75.0%) |
|
Female | 17 | 15 | 32
(25.0%) |
| Gross appearance,
n |
|
|
|
|
Pedunculated | 53 | 9 | 62
(40.3%) |
|
Semi-pedunculated | 49 | 8 | 57
(37.0%) |
|
Non-pedunculated | 32 | 3 | 35
(22.7%) |
| Therapy, n |
|
|
|
| Hot
biopsy | 6 | 0 | 6
(3.9%) |
|
Polypectomy | 90 | 12 | 102 (66.2%) |
|
Endoscopic mucosal
resection | 36 | 7 | 43
(27.9%) |
|
Endoscopic piecemeal mucosal
resection | 0 | 1 | 1
(0.7%) |
|
Endoscopic submucosal
dissection | 2 | 0 | 2
(1.3%) |
Assessment
Magnified chromoendoscopic images were
retrospectively assessed to clarify the characteristic findings of
JPs. To identify the characteristic findings, the evaluation
focused on gross appearance, color, pit pattern and surface
inflammatory changes. Furthermore, the morphology of glandular
cavities, nuclei of glandular cells and interstitial features were
also evaluated in EC images, and the EC findings were compared with
findings on conventional pathological examination.
Each magnified chromoendoscopic image was obtained
using a CF-Q240Z or CF-H260AZI (Olympus Corporation, Tokyo, Japan)
instrument. The ultra-high magnification images were obtained with
an integrated type endocytoscope (CF-Y0020-I, prototype; Olympus
Corporation). The endocytoscope has a single lens on its tip with a
hand lever, enabling the magnifying power of conventional
endoscopic images to be increased to the ultra-high magnification
power of ×380, which covers a 700×600 µm area of tissue.
To obtain the magnified chromoendoscopic images,
indigo carmine (Nagase Medicals Co., Ltd., Hyogo, Japan) was
sprayed directly onto lesions through an endoscope instrument
channel or using a non-traumatic catheter (12) (Olympus Corporation). To obtain the EC
images, the non-traumatic catheter was subseqeuntly used to spray
0.05% crystal violet (Koso Chemical Co., Ltd., Gyoda, Japan) and
1.0% methylene blue dyes (Wako Pure Chemical Industries Ltd.,
Osaka, Japan) softly onto lesion to stain the cell cytoplasm and
cell nuclei, respectively.
To evaluate the lesions, the pit pattern
classification for magnifying chromoendoscopy (13) and the EC classification for EC
(14) were used. The pit pattern
classification relies on the features of the glandular duct lumen:
Type I, round pits, regular in size; Type II, larger in size than
normal, star-shaped or onion-like pits; Type IIIs, lesions with
compactly arranged pits, smaller in size than normal ones;
TypeIII-L, elongated pit; Type IV, branched pit; Type V, irregular
pit (13). We further divided Type IV
pit patterns into Type IVb (branch-like) and Type IVv
(villous-like) lesions. The EC classification, relies on the
features of glandular duct lumens and the shape of nuclei in the
superficial layers of tumors: EC1a, rounded lumens and fusiform
nuclei; EC1b, narrow, serrated lumens and small rounded nuclei;
EC2, slit-like, smooth lumens and uniform fusiform or rounded
nuclei; EC3a, irregular and rough lumens and a large number of
rounded nuclei; EC3b, unclear gland formation and agglomeration of
distorted nuclei (14).
Results
Patient characteristics
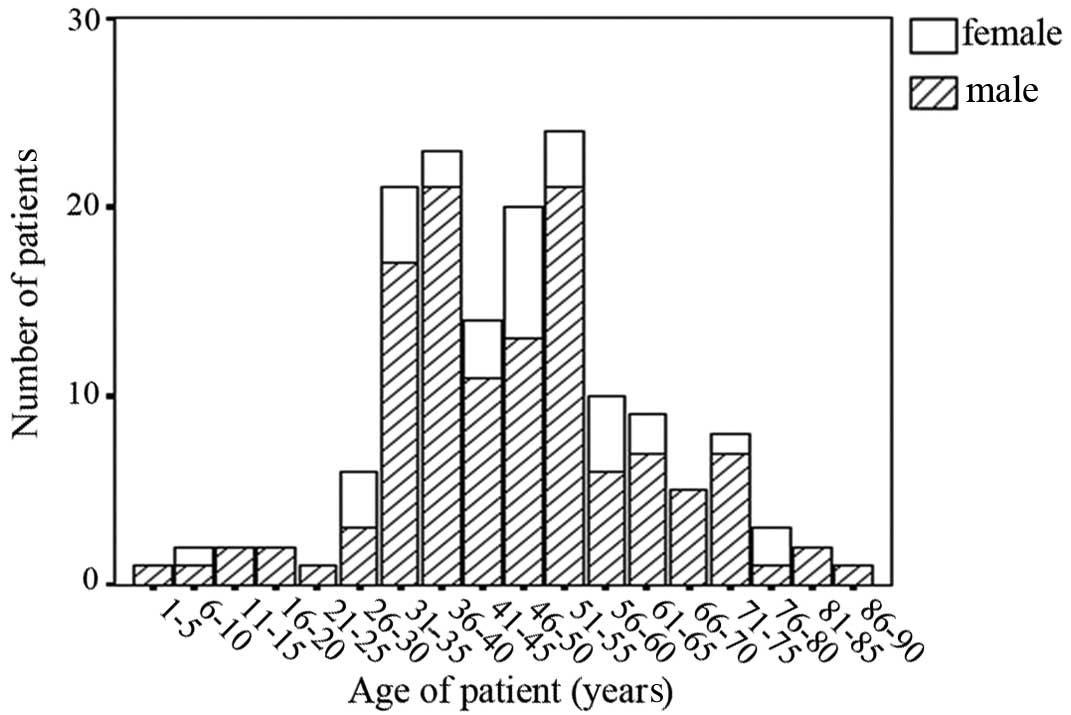
A total of 128 patients, comprising 96 males and 32
females with a mean age of 47.1 years, a median age of 47 years,
and an age range of 2–88 years, were included in the study
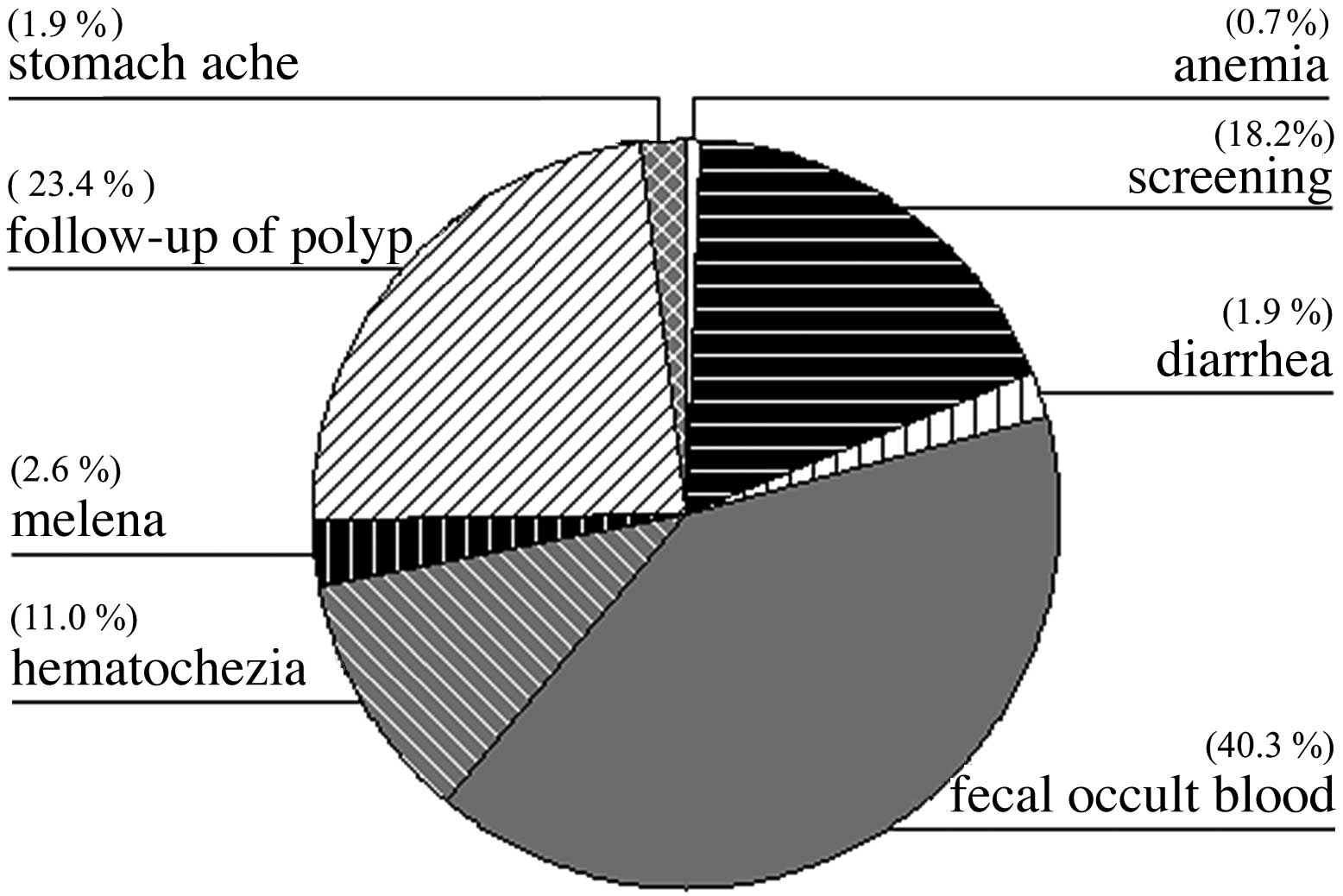
(Fig. 1; Table I). The most common symptomatic
presentations were associated with bleeding from the
gastrointestinal tract [total, 53.9%; melena (2.6%); hematochezia
(11.0%); detection of fecal occult blood (40.3%)] (Fig. 2).
Lesion characteristics
Of the 154 polyps assessed, the median size of the
lesions was 11.6 mm. With regard to location, 3 lesions were
detected in the cecum, 25 in the ascending colon, 19 in the
transverse colon, 8 in the descending colon, 59 in the sigmoid
colon and 40 in the rectum. In terms of gross appearance, 62 of the
lesions were pedunculated polyps, 57 semi-pedunculated polyps and
35 non-pedunculated polyps. Sporadic JPs accounted for 126 of the
cases. There were only 2 cases of JPS with multiple polypoid polyps
in the colorectum. The polyps were treated by hot biopsy in 6
cases, polypectomy in 102 cases, endoscopic mucosal resection in 43
cases, endoscopic piecemeal mucosal resection in 1 case and
endoscopic submucosal dissection in 2 cases (Table I). No adenomatous or malignant changes
were detected in any of the JPs.
Endoscopic findings
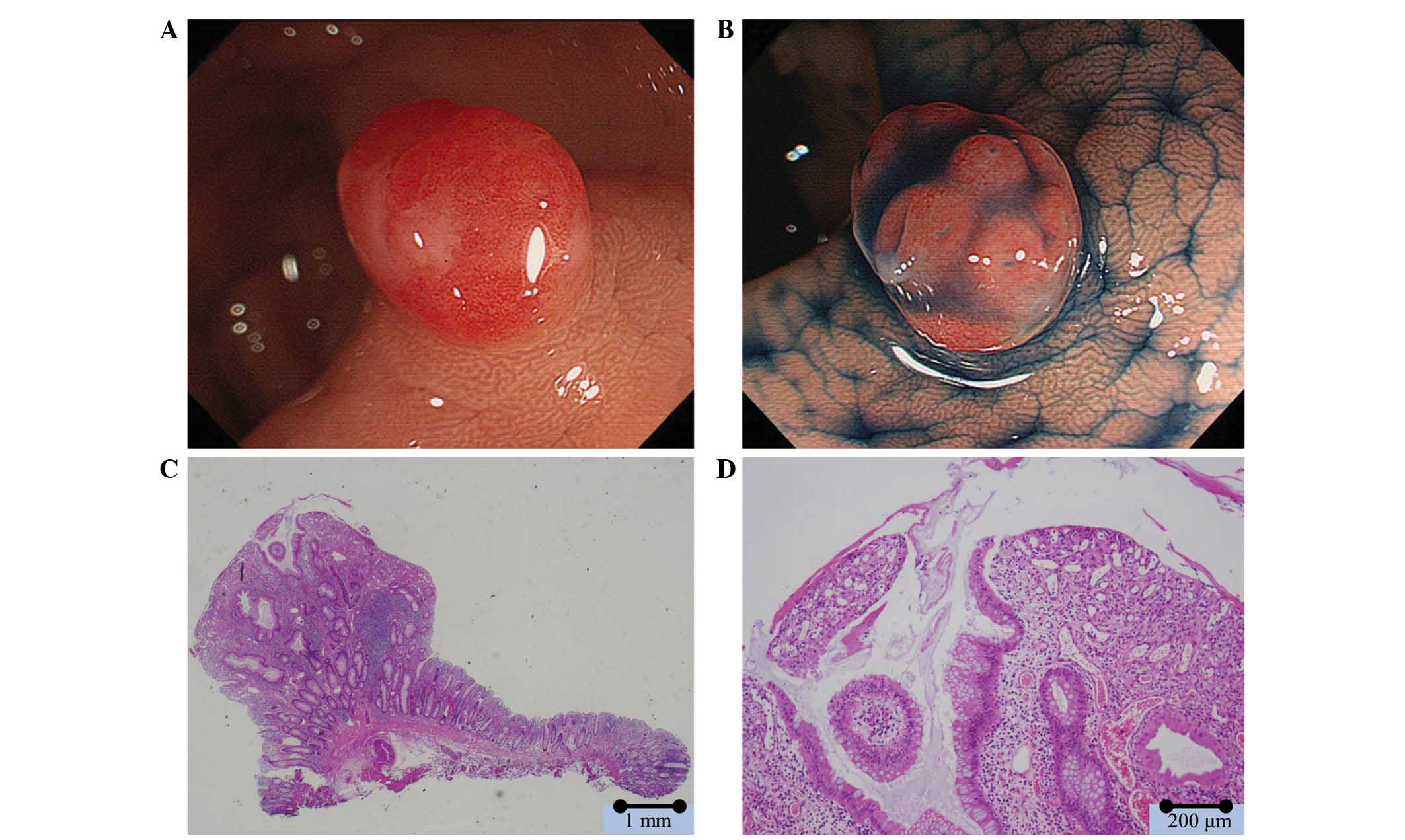
In white light, 151 polyps were observed to have
reddish surfaces. Caps of white mucus adhering to the surfaces of
the polyps were observed in 45 lesions (Fig. 3) and reddish areas, reflecting an
increase in vascularity surrounding the pits were present in 148
lesions. Type II pit patterns were observed in 140 lesions, type
III L pit patterns in 11 lesions, and a type IVb pit pattern in 2
lesions. There were frequent, relatively small, round pits in the
majority of the polyps with the type II pit pattern. Open pits were
observed in 139 lesions. There were 22 lobulated polyps. Low pit
density was observed in 139 lesions. Erosion was observed in
epithelial cells between glandular cavities in 142 lesions
(Fig. 3; Table II).
 | Table II.Endoscopic findings of juvenile polyps
(n=154 from 128 patients). |
Table II.
Endoscopic findings of juvenile polyps
(n=154 from 128 patients).
| Finding | MCa, n | MC+ECa, n | All patients, n
(%) |
|---|
| Color |
|
|
|
|
Reddish | 131 | 20 | 151 (98.1) |
|
Non-reddish | 3 | 0 | 3
(1.9) |
| Pit pattern |
|
|
|
| Type
I | 0 | 1 | 1
(0.7) |
| Type
II | 122 | 18 | 140 (90.9) |
| Type
III-L | 11 | 0 | 11
(7.1) |
| Type
IVb | 1 | 1 | 2
(1.3) |
| Open pit |
|
|
|
|
Present | 119 | 20 | 139 (90.3) |
|
Absent | 15 | 0 | 15 (9.7) |
| Mucus cap |
|
|
|
|
Present | 37 | 8 | 45
(29.2) |
|
Absent | 97 | 12 | 109 (70.8) |
| Lobular
appearance |
|
|
|
|
Present | 17 | 5 | 22
(14.3) |
|
Absent | 117 | 15 | 132 (85.7) |
| Decreased pit
density |
|
|
|
|
Present | 119 | 20 | 139 (90.3) |
|
Absent | 15 | 0 | 15 (9.7) |
| Surface
erosion |
|
|
|
|
Present | 122 | 20 | 142 (92.2) |
|
Absent | 12 | 0 | 12 (7.8) |
| Increase of
vascularity |
|
|
|
|
Present | 128 | 20 | 148 (96.1) |
|
Absent | 6 | 0 | 6
(3.9) |
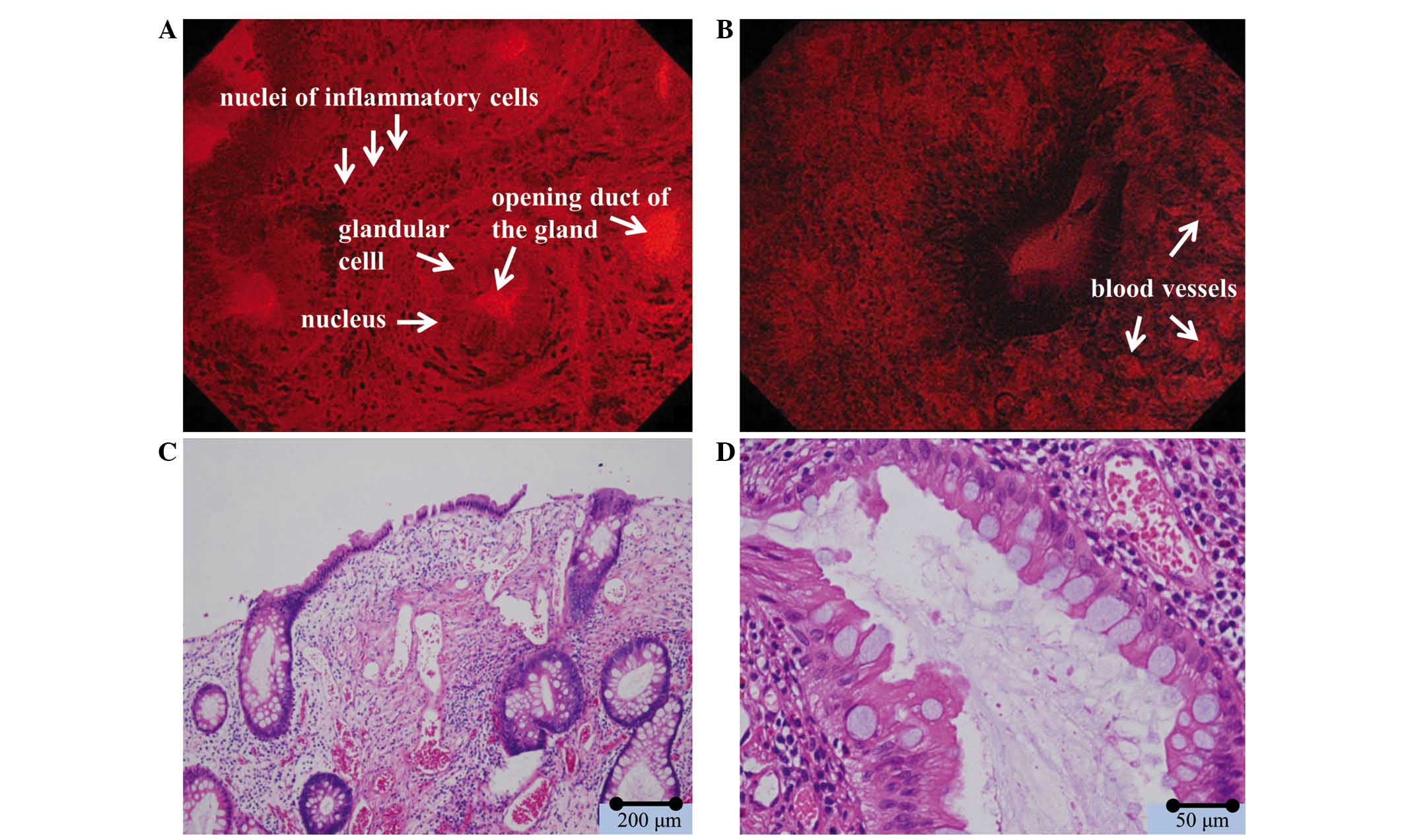
EC findings
Of the 20 polyps observed by EC, 1 polyp was
classified as EC1a and 19 polyps as EC1b according to the EC
classification criteria. No atypical changes were observed in the
nuclei of the glandular cells of any polyps. A number of the
glandular cavities were dilated by abundant mucus (Fig. 4). In addition, numerous compact
nuclei, which were considered to be nuclei of inflammatory cells,
were observed in the intercellular substance between glandular
cells (Fig. 4; Table III).
 | Table III.Endocytoscopic findings of juvenile
polyps (n=20). |
Table III.
Endocytoscopic findings of juvenile
polyps (n=20).
| Finding | n (%) |
|---|
| Endocytoscopy
classification |
|
| 1a | 1 (5.0) |
| 1b | 19 (95.0) |
| Atypical cells |
|
|
Present | 0 (0.0) |
|
Absent | 20 (100.0) |
| Dilated opening
duct |
|
|
Present | 20 (100.0) |
|
Absent | 0 (0.0) |
| Elongated distance
between gland basal layers |
|
|
Present | 20 (100.0) |
|
Absent | 0 (0.0) |
| Interstitial
infiltration of inflammatory cells |
|
|
Present | 20 (100.0) |
|
Absent | 0 (0.0) |
Discussion
The pathological characteristics of JPs are erosive
changes in the surface epithelium, inflammatory cell infiltration,
proliferation of blood vessels and cystic dilated glandular ducts
(4). These characteristics are
readily identifiable by optical microscopy, in which desquamated
epithelium consequent to inflammatory changes in the surfaces of
the polyps, serrated and cystic dilated glandular ducts, and
infiltration by inflammatory cells, such as lymphocytes, are
clearly visible (4). However, we
hypothesize that it is often difficult to visualize these
pathological characteristics endoscopically.
According to the current findings, the
characteristic endoscopic features of JPs are reddish surfaces,
surface erosion, caps of white mucus on the surfaces, open pits
surrounded by inflammatory changes, and low pit density. When the
endoscopic and pathological findings were compared, they were found
to correspond. The inflammatory changes lead to epithelial erosion
and account for the colonoscopic findings of reddish surfaces and
caps of white mucus adhering to the polyps (4). The open pits visible by colonoscopy
correspond with the cystic dilated glandular ducts filled with
mucus observed pathologically. The low pit density is caused by the
increased interstitial volume and cystic dilated glandular ducts
stretching the polyp surfaces (11).
Thus, the colonoscopic findings accurately reflect the pathological
findings. It may therefore be concluded that the following
tetralogy of endoscopic findings, which is found in most JPs, is
characteristic of these polyps: i) reddish surfaces; ii) surface
erosion; iii) open pits; and iv) low pit density.
The observations provided by EC assessment of the
JPs were also consistent with the pathological findings. On EC, the
areas that had appeared inflamed and reddish on magnifying
chromoendoscopy were observed to contain increased blood vessels,
particularly surrounding the pits. These findings suggest that the
following triad of EC findings is characteristic of JPs: i) dilated
ductal openings surrounded by normal glandular cells; ii) greater
distances between gland basal layers; and iii) interstitial
infiltration by inflammatory cells.
Patients with JPS are known to be at increased risk
of colorectal cancer (6,15). Adenomatous or malignant changes have
been observed in subjects with JPS and also in those with sporadic
JPs (16,17). In the present study, no malignant
changes were detected; nonetheless, EC is able to detect malignant
changes as it enables the assessment of nuclei in real time. A
number of studies have reported the usefulness of EC in the
diagnosis of epithelial neoplastic lesions (14,18,19). The
current study supports previously reported endoscopic findings
(6). However, to the best of our
knowledge, no previous studies have described the use of EC with
regard to JPs. Upon comparison of current EC results with previous
pathological findings, EC data was found to accurately reflect the
pathological findings (4,11). Considering that the current findings
indicate that EC images accurately reflect the pathological
findings of JPs, EC may be of value, not only for diagnosing
neoplastic lesions, but also for diagnosing non-neoplastic lesions
including JPs. JP has similar endoscopic features to other polyps,
such as inflammatory and hyperplastic polyps; therefore, clarifying
the features of magnifying endoscopic and EC findings will aid in
differentiating between these disease entities (11).
Although JPs are relatively rare, they are
occasionally discovered in daily medical practice, particularly by
colonoscopic examination (20). It is
important to make a precise diagnosis to enable optimal treatment
(5). The risk of bleeding after
colonoscopic polypectomy was reported as 0.3–6.1% (21). Precise endoscopic diagnosis will make
it possible to avoid endoscopic biopsy or resection for confirming
pathological histology, and, thus, enable the risk of bleeding
caused to be reduced, particularly in patients that have been
administered anticoagulant treatments. Furthermore, although the
malignant potential of JPs not particularly high, their diagnosis
of JPs on the basis of endoscopic features is important, as it
allows clinicians to consider whether immediate treatment is
required (5).
Precise endoscopic diagnosis prevents unnecessary
biopsy or resection, and reduces the risk of bleeding caused by
treatment. Therefore, the present findings are of great
significance in the endoscopic diagnosis and management of JPs.
Acknowledgements
The authors wish to thank Edanz Group Ltd. (Fukuoka,
Japan) for assistance with correcting English in this paper.
References
|
1
|
Morson BC: Precancerous and early
malignant lesions of the large intestine. Br J Surg. 55:725–731.
1968. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brosens LA, Langeveld D, van Hattem WA,
Giardiello FM and Offerhaus GJ: Juvenile polyposis syndrome. World
J Gastroenterol. 17:4839–4844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cichy W, Klincewicz B and Plawski A:
Juvenile polyposis syndrome. Arch Med Sci. 10:570–577. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Hattem WA, Langeveld D, de Leng WW,
Morsink FH, van Diest PJ, Iacobuzio-Donahue CA, Giardiello FM,
Offerhaus GJ and Brosens LA: Histologic variations in juvenile
polyp phenotype correlate with genetic defect underlying juvenile
polyposis. Am J Surg Pathol. 35:530–536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giardiello FM, Hamilton SR, Kern SE, et
al: Colorectal neoplasia in juvenile polyposis or juvenile polyps.
Arch Dis Child. 66:971–975. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brosens LA, van Hattem A, Hylind LM,
Iacobuzio-Donahue C, Romans KE, Axilbund J, Cruz-Correa M,
Tersmette AC, Offerhaus GJ and Giardiello FM: Risk of colorectal
cancer in juvenile polyposis. Gut. 56:965–967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barros R, Mendes N, Howe JR, Reis CA, de
Bolos C, Carneiro F, David L and Almeida R: Juvenile polyps have
gastric differentiation with MUC5AC expression and downregulation
of CDX2 and SMAD4. Histochem Cell Biol. 131:765–772. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marsh Durban V, Jansen M, Davies EJ,
Morsink FH, Offerhaus GJ and Clarke AR: Epithelial-specific loss of
PTEN results in colorectal juvenile polyp formation and invasive
cancer. Am J Pathol. 184:86–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stojcev Z, Borun P, Hermann J, Krokowicz
P, Cichy W, Kubaszewski L, Banasiewicz T and Plawski A:
Hamrtomatous polyposis syndromes. Hered Cancer Clin Pract.
11:42013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shussman N and Wexner SD: Colorectal
polyps and polyposis syndromes. Gastroenterol Rep (Oxf). 2:1–15.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ricci MT, Salemme M, Villanacci V and
Varesco L: The genetics of inherited predispositions to colorectal
polyps: A quick guide for clinicians. Colorectal Dis. 17(Suppl 1):
S3–S9. 2015. View Article : Google Scholar
|
|
12
|
Fujii T, Hasegawa RT, Saitoh Y, et al:
Chromoscopy during colonoscopy. Endoscopy. 33:1036–1041.
2001.PubMed/NCBI
|
|
13
|
Kudo S, Rubio CA, Teixeira CR, Kashida H
and Kogure E: Pit pattern in colorectal neoplasia: Endoscopic
magnifying view. Endoscopy. 33:367–373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kudo S, Wakamura K, Ikehara N, Mori Y,
Inoue H and Hamatani S: Diagnosis of colorectal lesions with a
novel endocytoscopic classification-a pilot study. Endoscopy.
43:869–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Howe JR, Mitros FA and Summers RW: The
risk of gastrointestinal carcinoma in familial juvenile polyposis.
Ann Surg Oncol. 5:751–756. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedman CJ and Fechner RE: A solitaly
juvenile polyp with hyperplastic and adenomatous glands. Dig Dis
Sci. 27:946–948. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang SH, Chung WS, Hyun CL, Moon HS, Lee
ES, Kim SH, Sung JK, Lee BS and Jeong HY: A rare case of a signet
ring cell carcinoma of the colon mimicking a juvenile polyp. Gut
Liver. 6:129–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inoue H, Kudo SE and Shiokawa A:
Technology insight: Laser-scanning confocal microscopy and
endocytoscopy for cellular observation of the gastrointestinal
tract. Nat clin Pract Gastroenterol Hepatol. 2:31–37. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mori Y, Kudo S, Ikehara N, Wakamura K,
Wada Y, Kutsukawa M, Misawa M, Kudo T, Kobayashi Y, Miyachi H, et
al: Comprehensive diagnostic ability of endocytoscopy compared with
biopsy for colorectal neoplasms: A prospective randomized
noninferiority trial. Endoscopy. 45:98–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Howe JR, Sayed MG, Ahmed AF, Ringold J,
Larsen-Haidle J, Merg A, Mitros FA, Vaccaro CA, Petersen GM,
Giardiello FM, et al: The prevalence of MADH4 and BMPR1A mutations
in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1
mutations. J Med Genet. 41:484–491. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosen L, Bub DS, Reed JF 3rd and Nastasee
SA: Hemorrhage following colonoscopic polypectomy. Dis Colon
Rectum. 36:1126–1131. 1993. View Article : Google Scholar : PubMed/NCBI
|