Introduction
Prostate cancer is the most common malignancy in the
world, affecting 1 in 9 men >65 years of age. Currently, there
is no effective cure for advanced-stage prostate cancer and it is
the second leading cause of cancer-associated mortality in men
(1,2).
Identification of novel endogenous factors responsible for the
proliferation, migration and invasion of prostate cancer will
facilitate our understanding of the progression of prostate cancer,
and the development of novel approaches for its diagnosis and
therapy.
There are four mitogen-activated protein (MAP)
kinase pathways in mammalian cells: The extracellular
signal-regulated kinase (ERK)1/2, Janus kinase, p38 and big
mitogen-activated protein kinase 1 (BMK1) pathways (3–5). The BMK1
pathway was the last to be identified and the is least studied
mammalian MAP kinase cascade. BMK1 is most similar to ERK1/2, as
each contains the Thr-Glu-Tyr dual phosphorylation motif. However,
BMK1 has a unique activating loop structure and an unusually large
C-terminal non-kinase domain. The C-terminal half of BMK1 contains
a nuclear localization signal that is critical for the nuclear
localization of BMK1 (6). The ERK1/2
and BMK1 cascades are activated by mitogens and oncogenic signals,
and are strongly indicated to be involved in tumorigenesis
(3–5).
Moreover, deregulated BMK1 signaling has been associated with
properties of human malignancies, including the chemoresistance of
breast tumor cells (7), the
uncontrolled proliferation of erb-b2 receptor tyrosine kinase
2-overexpressing carcinomas (8), the
metastatic potential of prostate tumor cells (9) and tumor-associated angiogenesis
(10). Although the role of BMK1 has
been demonstrated to be required for growth factor-induced cell
proliferation and cell cycle regulation (11,12), its
biological significance for the development of prostate cancer
remains elusive.
The present study sought to examine the biological
functions of activated BMK1 in the cell proliferation and cell
cycle regulation of prostate cancer cells.
Materials and methods
Cell culture
The prostate cancer PC-3 cell line and the normal
prostate epithelial RWPE-2 cell line were obtained from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in a 5% CO2 humidified atmosphere at 37°C. The cells
were grown in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and
1:100 penicillin/streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.).
Antibodies and reagents
Rabbit monoclonal anti-ERK5 antibody (also known as
BMK1-antibody; 1:3,000; ab40809), rabbit polyclonal cyclin D1
antibody (1:3,000; ab7958), mouse monoclonal cyclin E antibody
(1:3,000; ab3927), mouse monoclonal cyclin A antibody (1:3,000;
ab38) and mouse monoclonal cyclin B antibody (1:3,000; ab72) were
obtained from Abcam (Cambridge, UK) and rabbit monoclonal
anti-phospho-ERK1/2 antibody (1:4,000; #4377) was purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Rabbit
polyclonal anti-EGF antibody (1:5,000; #SAB2104809) was purchased
from Sigma-Aldrich (St. Louis, MO, USA), while mouse monoclonal
anti-GAPDH antibody (1:5,000: #A01622-40) and mouse monoclonal
actin antibody (1:5,000; #A00702-100) were obtained from GenScript
(Piscataway, NJ, USA). Horseradish peroxidase-labeled goat
anti-mice immunoglobulin G (1:5,000; #115-035-062) or goat
anti-rabbit (1:3,000; #111-035-003) immunoglobulin G antibody was
purchased from Jackson ImmunoResearch Laboratories, Inc. (West
Grove, PA, USA). XMD8–92, a BMK1 inhibitor that specifically
inhibits the phosphorylation of BMK1, but not the phosphorylation
of ERK1/2, and PD184352, which is an ERK1/2 inhibitor that only
blocks ERK1/2 but not BMK1 activated by EGF treatment, were
purchased from Selleck Company (Houston, TX, USA). EGF was obtained
from Sino Biological Inc. (Beijing, China). The final
concentrations for the treatments of XMD8–92, PD184352, or EGF were
5 µM, 1 µM and 0.5 ng/ml, respectively.
Establishment of a stable
MEK5-expressing cell line
To establish the stable transfectant expressing the
MEK5 protein, the RWPE-2 cells were transfected with
pcDNA3.1(+)-MEK5 recombinant plasmid using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. After 48 h, the transfected cells were
selected for in DMEM supplemented with 400 µg/ml of G-418
antibiotic (Invitrogen; Thermo Fisher Scientific, Inc.) for 2
weeks, and then maintained in culture medium containing 200 µg/ml
G-418. The RWPE-2 cells were transfected with pcDNA3.1(+) and
selected for using G-418 treatment, as in the control cells.
Cell proliferation assay using cell
counting kit (CCK)-8
The cells were seeded at 2×103 cells/well
in phenol red-free DMEM (Gibco; Thermo Fisher Scientific, Inc.)
with 10% fetal bovine serum (FBS; 100 µl/well) in a 96-well culture
plate. The growth rates of the cells were determined using the
CCK-8 assay (Beyotime Institute of Biotechnology, Haimen, China). A
total of 10 µl CCK-8 working solution was added to each well on
days 1–5, followed by incubation for 2 h at 37°C, and the
absorbance was finally measured at 450 nm using a model 3550
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Cell cycle analysis by flow
cytometry
Nuclear DNA content was measured by propidium iodide
(PI; Sigma-Aldrich) staining and fluorescence-activated cell
sorting analysis according to the manufacturer's protocols. In
brief, the cells were trypsinized and collected. Subsequent to
three washes with phosphate-buffered saline (PBS), the cells were
resuspended in 70% methanol and fixed in 4°C for 30 min. The cells
were then washed and resuspended in PBS containing 20 µg/ml RNase A
and 50 µg/ml PI, and incubated on ice for 30 min. Cell cycle
analysis was performed in a Coulter Epics XL flow cytometer using
the CellQuest program (Beckman Coulter, Inc., Brea, CA, USA) with
manually set regions for the G0/G1, S and
G2/M phases. Data from 10,000 cells were collected for
each data file.
Western blot analysis
To prepare the protein extracts, the cells were
washed with PBS and harvested in 1 ml PBS. Following
centrifugation, the cells were resuspended and extracted in lysis
buffer (Thermo Fisher Scientific, Inc.) for 30 min on ice. The
lysates were centrifuged at 15,000 × g for 10 min at 4°C. The
supernatants of the lysates were mixed with a 6X SDS sample buffer
and boiled for 10 min. The samples were separated in a 10% SDS
polyacrylamide gel and then transferred to a polyvinylidene
difluoride membrane (Millipore, Billerica, MA, USA). The membrane
was blocked with 5% (w/v) skimmed dry milk and then blotted with
the corresponding antibody in PBST buffer (0.1% Tween-20 in PBS)
with gentle shaking at room temperature for 2 h. Subsequent to
being washed with PBST four times, the membranes were incubated
with the indicated secondary antibody. The signals were detected
using a SuperSignal West Pico Substrate kit (Thermo Fisher
Scientific, Inc.). The signals were measured by fluorescence
intensity with ImageJ software (National Institutes of Health,
Bethesda, MD, USA).
Human EGF ELISA assay
A total of 1×105 cells were seeded in a
24-well plate, and maintained in 0.5 ml DMEM cell culture medium
with 0.5% FBS in each well. The tissue culture mediums were
collected after 48 h. The EGF concentrations were measured with a
Human EGF ELISA Assay kit according to the manufacturer's protocol
(Signosis Inc., Santa Clara, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol™
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was
performed using a SYBR PrimeScript RT-PCR kit (Takara Bio Inc.,
Otsu, Japan) on a Rotor-Gene 6000 Real-Time Genetic Analyzer
(Corbett Life Science; Qiagen, Inc., Valencia, CA, USA) according
to the manufacturer's protocols. The primer sequences of cyclin A,
cyclin B1, cyclin D1, cyclin E and GAPDH are shown in Table I. The PCR protocol included a
denaturation program (95°C for 2 min), followed by 40 cycles of an
amplification and quantification program (95°C for 5 sec and
55–57°C for 30 sec) and a melting curve program (55–95°C, with a
0.5°C increment each cycle). Each sample was replicated three
times.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction assay. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction assay.
| Name | Forward sequence | Reverse sequence |
|---|
| Cyclin A |
5′-atgagaccggctttcccgca-3′ |
5′-cccctggccacaggtcctcc-3′ |
| Cyclin B1 | 5′
atggcgctccgagtcaccag-3′ |
5′-ctctggcactggctcagaca-3′ |
| Cyclin D1 | 5′
atggaacaccagctcctgtg-3′ |
5′-ctgcaggcggctctttttca-3′ |
| Cyclin E |
5′-atgccgagggagcgcaggga-3′ |
5′-ggatggtgcaataatccgag-3′ |
| GAPDH |
5′-atggggaaggtgaaggtcgg-3′ |
5′-gccagtggactccacgacgt-3′ |
Statistical analysis
All results were analysis by SPSS statistical
software, version 10.0 (SPSS, Inc., Chicago, IL, USA) and presented
as the arithmetic mean ± standard error of the mean. Student's
t-test was performed for statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Activation of the ERK/MEK5/BMK1
pathway induces prostate cell proliferation
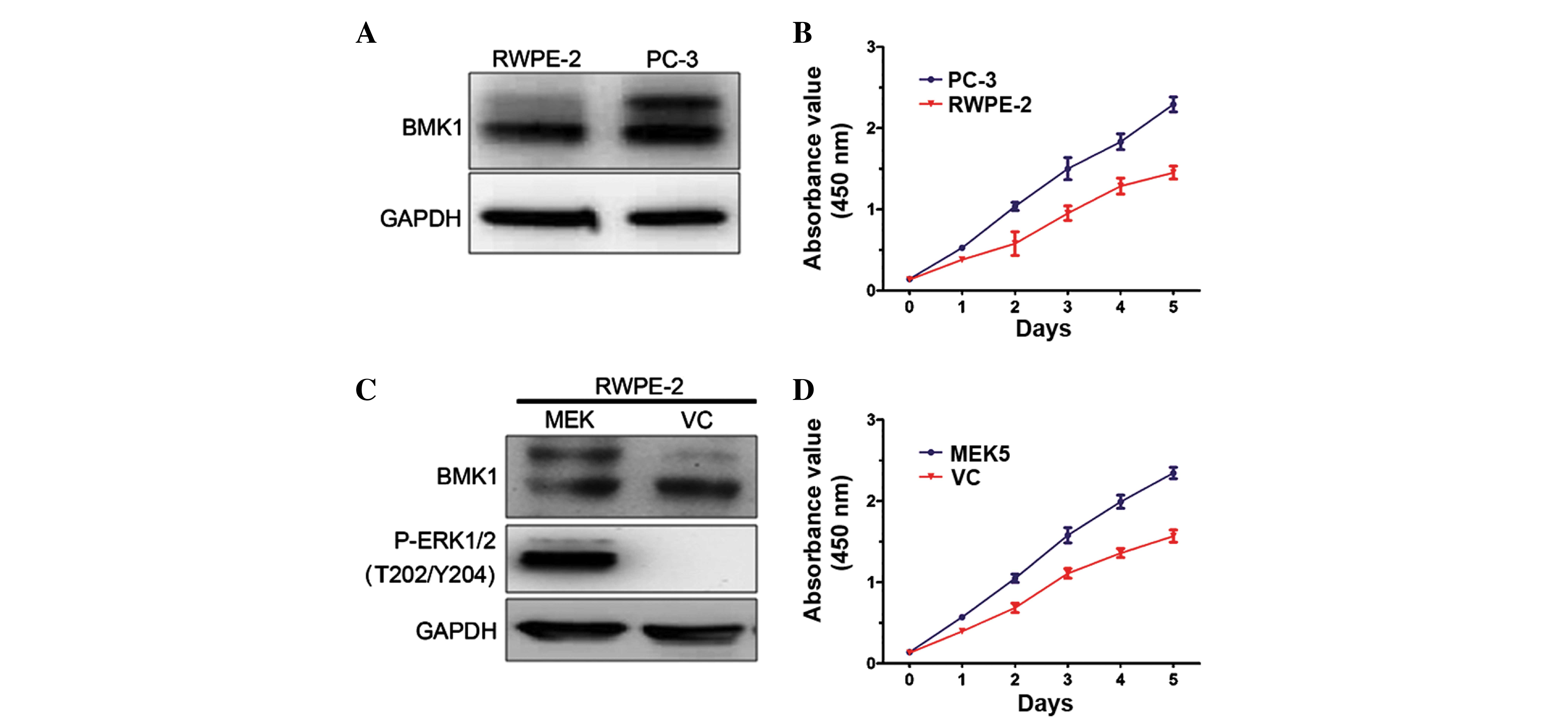
To determine the biological functions of BMK1 in
prostate cancer, the expression levels of BMK1 were determined in
prostate cancer PC-3 cells and normal prostate epithelial RWPE-2
cells. It was found that these two cell lines each express BMK1
protein. However, the expression level of phosphorylated BMK1 in
the PC-3 cells was much higher than that in the RWPE-2 control
cells, suggesting that the expression of phosphorylated BMK1 may
play a role in prostate cancer (Fig.
1A). Next, the proliferation of the PC-3 and RWPE-2 cells was
measured. Notably, the proliferation rate of the PC-3 cells was
significantly higher than that of the RWPE-2 cells (Fig. 1B). These results encouraged the
further investigation of the biological functions of the activation
of BMK1 in prostate cancer.
To investigate the association between
phosphorylated BMK1 and proliferation, the expression level of
phosphorylated BMK1 was upregulated in the RWPE-2 cells, and then
the proliferation of the cells with or without the activation of
BMK1 was measured. It is known that the overexpression of MEK5 can
activate the ERK/MEK/BMK1 pathway in vitro (13). Therefore, phosphorylated BMK1 or
ERK1/2 was found in the stable MEK5-overexpressing RWPE-2 cells
(Fig. 1C). Moreover, it was found
that the proliferation increased by >50% as measured by CCK-8
assay in a culture time ranging from 1 to 5 days. This was
accompanied by the activation of ERK/MEK5/BMK1, which was induced
by the MEK5 overexpression in the RWPE-2 cells, suggesting that
ERK/MEK5/BMK1 activation may promote cell proliferation (Fig. 1D).
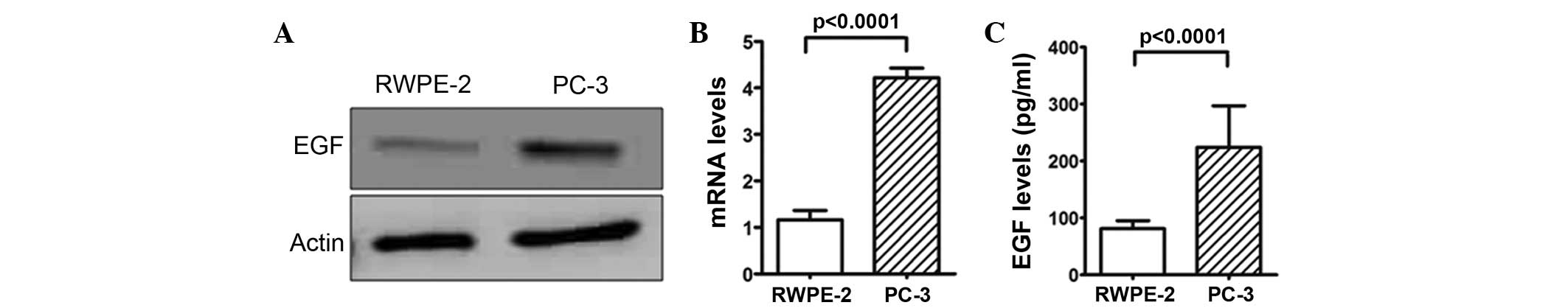
EGF-mediated activation of BMK1
induces proliferation in prostate cancer cells
Since EGF has also been described as an activator of
the ERK/MEK5/BMK1 pathway (11), the
present study next tested whether EGF expression was involved in
the cell proliferation of the prostate cancer cells. The analysis
of EGF protein expression level in the PC-3 and RWPE-2 cells found
a higher expression level in the PC-3 cells (Fig. 2A). RT-qPCR analysis and ELISA assays
confirmed this result for mRNA and protein expression, respectively
(P<0.0001; Fig. 2B and C),
suggesting that the higher expression level of EGF protein in the
prostate cancer cells may be associated with cell proliferation.
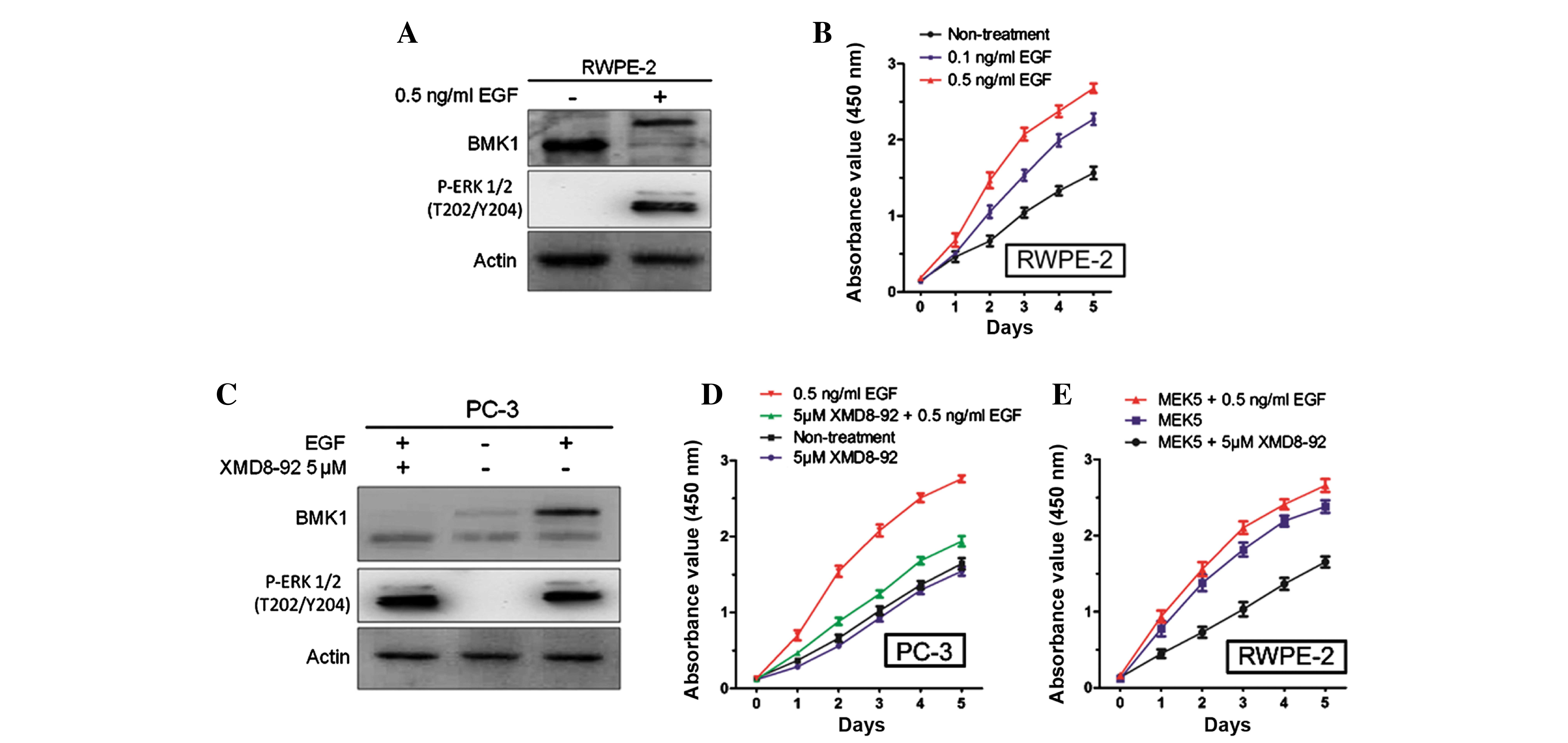
Furthermore, it was also found that the stimulation of 0.5 ng/ml
EGF (Sino Biological, Inc., Beijing, China) could significantly
activate the phosphorylation of BMK1 and ERK1/2 in the RWPE-2 cells
(Fig. 3A), which is consistent with a
previous study in HeLa cells (14).
Next, the proliferation of the RWPE-2 cells with or without the
treatment using different concentrations of EGF protein (0.1 and
0.5 ng/ml), was measured. It was found that the proliferation of
the RWPE-2 cells treated with EGF was much higher than that of the
non-treated cells. Also, the proliferation was increased in a
dose-dependent manner according to the EGF concentration (Fig. 3B).
Since either the ERK or the BMK1 pathway was
activated by EGF treatment, further studies were performed to
confirm which was involved in the proliferation of the prostate
cancer cells. Following the treatment with EGF and/or 5 µM XMD8–92,
(Fig. 3C), it was found that the
proliferation of the PC-3 cells was increased by 0.5 ng/ml EGF and
suppressed by 5 µM XMD8–92. This suggested that EGF-mediated BMK1
activation induced the proliferation of the PC-3 cells (Fig. 3D). Similarly, the treatment with 5 µM
XMD8–92 significantly suppressed the proliferation in the MEK5
overexpressed RWPE-2 cells (Fig. 3E).
All these results indicated that BMK1 activation induced by either
MEK5 overexpression or EGF stimulation is essential for the
proliferation of prostate cancer cells.
EGF-mediated BMK1 activation promotes
entry into the S phase in association with the upregulation of
cyclin expression
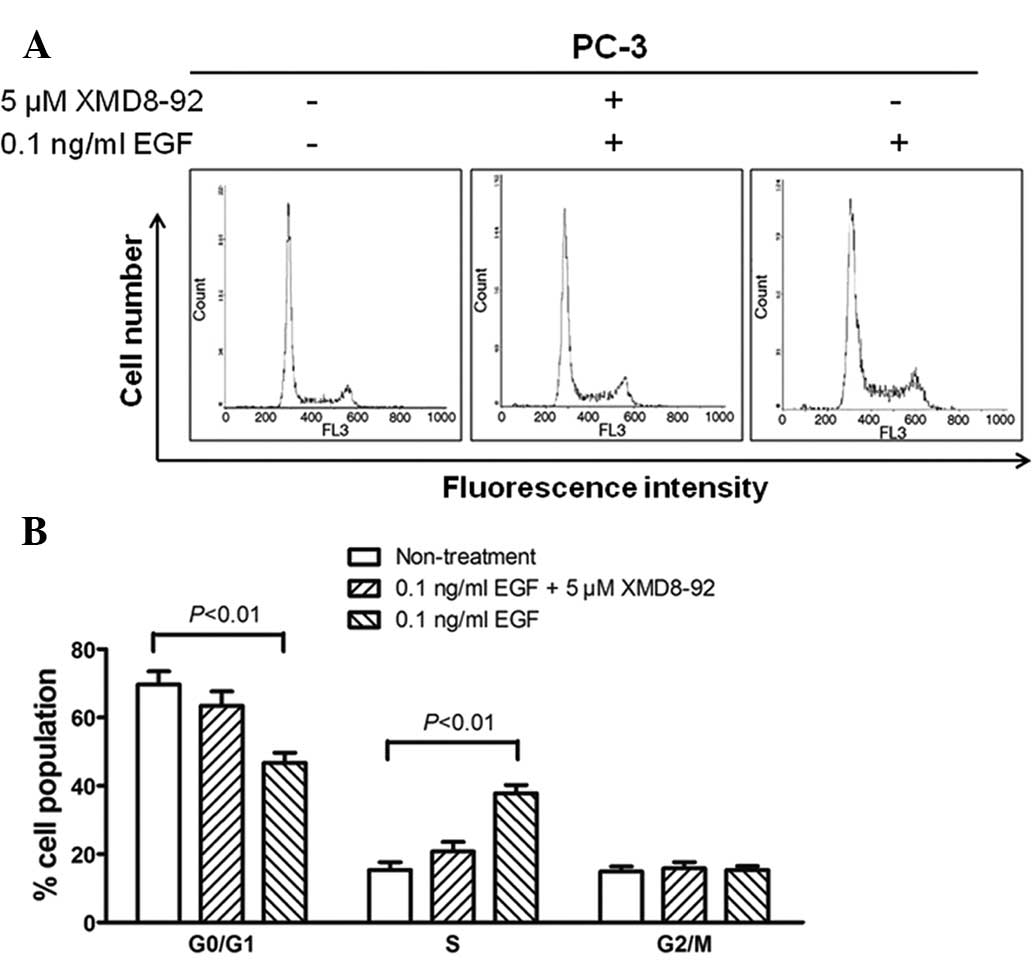
To determine whether the proliferation of
BMK1-activated cells was due to cell cycle regulation at certain
phase(s), flow cytometry analysis was performed based on DNA
content in nuclei stained with PI. The proportions of cells in the
G0/G1, S and G2/M phases for the
0.5 ng/ml EGF-stimulated PC-3 cells were 46.8, 37.8 and 15.4%,
respectively, whereas those for the non-treated cells were 69.7,
15.4 and 14.9%, respectively (P<0.01). Moreover, the percentages
of cell populations in the EGF/XMD8–92 treated PC-3 cells were
similar to those in non-treated cells (Fig. 4A and B). These results indicated that
the proportion of cells in the S phase was significantly increased,
accompanied by a decrease in the cell proportion in the
G0/G1 phase, when compared with the
non-treated cells. This suggested that the cell proliferation
induced by activated BMK1 may be involved in the promotion of the
entry into the S phase.
It is known that cell cycle progression is regulated
using a complex network of positive or negative cell cycle
regulatory molecules (14). Cyclin D
and cyclin E are essential for the control of the cell cycle at the
G1/S transition, while cyclin A is associated with the
control of the cell cycle at the G1/S and
G2/M transitions, and cyclin B is involved with the
G2/M transition only. Therefore, the expression levels
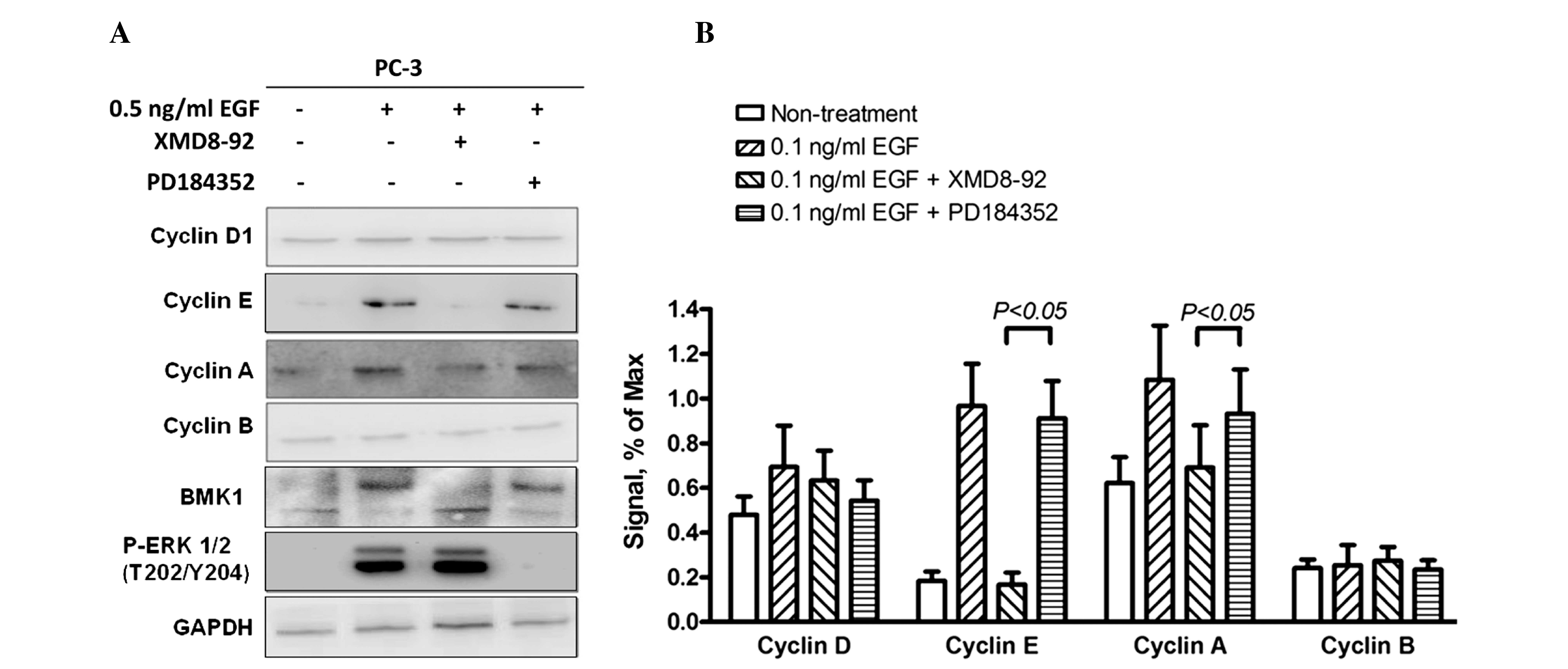
of these cyclin proteins were examined by western blot analysis to
investigate the molecular mechanisms involved in cell proliferation
and cell cycle regulation induced by phosphorylated BMK1 in PC-3
cells. As expected, the expression levels of cyclin A and cyclin E
(associated with the G1/S transition) were significantly
increased, and the quantitative analysis indicated that expression
levels were 2- and 10-fold higher, respectively, in the EGF-treated
cells. This suggested that EGF-mediated BMK1 activation can promote
the G1/S transition through upregulation of the
expression levels of cyclins A and E. Furthermore, the EGF cells
stimulated with XMD8–92 or PD184352, an ERK1/2 inhibitor, only
blocked ERK1/2 but not BMK1 activation by EGF treatment, were also
determined to reveal which pathway was involved in this process
(Fig. 5A and B).
Discussion
Prostatic adenocarcinoma is the most frequently
diagnosed type of malignancy in American men, accounting for
>35% of male cancers (15), with
~20% of patients eventually succumbing to the disease. BMK1
promotes tumor development not only by inhibiting the tumor
suppressor (16), but also by
supporting tumor angiogenesis (10,17,18), tumor
metastasis (19–21) and the chemoresistance of tumor cells
(7). Furthermore, the knockout of
BMK1 in various tissues of mice has been shown to have no marked
effect on the development, behavior, reproduction and aging of the
animals (10), suggesting that BMK1
should be an attractive target for pharmaceutical intervention in
cancer therapy. The present study demonstrated that EGF-mediated
BMK1 activation in prostate cancer is associated with cellular
proliferation by promoting entry into the S phase of the cell
cycle. Furthermore, it was also found that the expression levels of
cyclin A and cyclin E are regulated by the activation of BMK1 in
this process.
In the present study, the level of phosphorylation
of BMK1 in the PC-3 cells was higher than that in the RWPE-2 cells,
suggesting that the higher proliferation of the PC-3 cells may be
associated with the phosphorylated BMK1 (Fig. 1A and B). Following the activation of
BMK1 induced by the overexpression of MEK5 in the RWPE-2 cells
(Fig. 1C), it was found that this
proliferation phenotype was also induced in MEK5-overexpressing
cells. This can promote the activation of the ERK/MEK5/BMK1 pathway
(13), suggesting that the activation
of BMK1 or ERK1/2 may play a role in the proliferation of the
prostate cancer cells (Fig. 1D).
Different expression levels of EGF protein were also noted in the
RWPE-2 and PC-3 cells (Fig. 2). The
EGF treatment was found to be an ERK/MEK5/BMK1 pathway activator in
the RWPE-2 cells (Fig. 3A).
Furthermore, this proliferation can be inhibited by XMD8–92,
suggesting that the activation of BMK1, not ERK1/2, induces the
proliferation in the prostate cancer cells (Fig. 3D and E). Taken together, these results
suggest that the high expression of EGF may result in this
proliferation through the induction of activated BMK1 in the
prostate cancer cells.
Since cell proliferation is usually associated with
cell cycle regulation, a flow cytometry analysis was performed to
measure the proportion of cells in the various cycle phases. A
higher proportion of S-phase cells indicated that the activated
BMK1 increased G1/S transition in the cell cycle
progression, which lead to the cell proliferation in the prostate
cancer cells (Fig. 4A and B). As is
known, cyclins A, B, D and E are all involved in the
G1/M transition (14).
Therefore, western blot analysis revealed that the expression
levels of cyclins A and E were increased significantly. By
contrast, there was no apparent change in the levels of cyclins B
and D (Fig. 5). Moreover, the total
RNA of EGF-stimulated cells and non-treated cells was extracted,
and further RT-qPCR analysis demonstrated that the levels of
cyclins A and E mRNA were also markedly increased (data not shown).
In previous studies, BMK1/MEK5 has been found to induce
AP-1-mediated transcription (9), and
BMK1 can suppress the functions of promyelocytic leukemia protein,
which is associated with the transcription factors through
phosphorylation (16,22). This suggests that the effects of BMK1
activation on prostate cell proliferation and cell cycle
progression may be a transcriptional event.
In summary, the present data provided evidence to
indicate the functions of BMK1 in the cell proliferation and cell
cycle regulation of human prostate cancer cells. The association
between EGF and BMK1 activation in proliferation and cell cycle
regulation were also clarified, suggesting the significance of a
high expression level of EGF in human prostate cancer. However,
further investigation in required into the novel mechanisms
underlying proliferation and cell cycle regulation, further factors
that may be involved in the EGF/BMK1 pathway, and the effects of
BMK1 on tumor angiogenesis, differentiation and tumor metastasis in
prostate cancer cells. Ongoing studies on BMK1 function may lead to
the identification of an effective approach for treating human
prostate cancer.
Acknowledgements
This study was supported by the startup fund from
the First Affiliated Hospital of Yangtze University (Jingzhou,
China).
References
|
1
|
Debes JD and Tindall DJ: Mechanisms of
androgen-refractory prostate cancer. N Engl J Med. 351:1488–1490.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schröder FH: Progress in understanding
androgen-independent prostate cancer (AIPC): A review of potential
endocrine-mediated mechanisms. Eur Urol. 53:1129–1137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JD, Ulevitch RJ and Han J: Primary
structure of BMK1: A new mammalian map kinase. Biochem Biophys Res
Commun. 213:715–724. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weldon CB, Scandurro AB, Rolfe KW, Clayton
JL, Elliott S, Butler NN, Melnik LI, Alam J, McLachlan JA and Jaffe
BM: Identification of mitogen-activated protein kinase kinase as a
chemoresistant pathway in MCF-7 cells by using gene expression
microarray. Surgery. 132:293–301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Esparis-Ogando A, Díaz-Rodriguez E,
Montero JC, Yuste L, Crespo P and Pandiella A: Erk5 participates in
neuregulin signal transduction and is constitutively active in
breast cancer cells overexpressing ErbB2. Mol Cell Biol.
22:270–285. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehta PB, Jenkins BL, McCarthy L, Thilak
L, Robson CN, Neal DE and Leung HY: MEK5 overexpression is
associated with metastatic prostate cancer and stimulates
proliferation, MMP-9 expression and invasion. Oncogene.
22:1381–1389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayashi M and Lee JD: Role of the
BMK1/ERK5 signaling pathway: Lessons from knockout mice. J Mol Med
(Berl). 82:800–808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato Y, Tapping RI, Huang S, Watson MH,
Ulevitch RJ and Lee JD: Bmk1/Erk5 is required for cell
proliferation induced by epidermal growth factor. Nature.
395:713–716. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hayashi M, Tapping RI, Chao TH, Lo JF,
King CC, Yang Y and Lee JD: BMK1 mediates growth factor-induced
cell proliferation through direct cellular activation of serum and
glucocorticoid-inducible kinase. J Biol Chem. 276:8631–8634. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cameron SJ, Abe J, Malik S, Che W and Yang
J: Differential role of MEK5alpha and MEK5beta in BMK1/ERK5
activation. J Biol Chem. 279:1506–1512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
John PC, Mews M and Moore R: Cyclin/Cdk
complexes: Their involvement in cell cycle progression and mitotic
division. Protoplasma. 216:119–142. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Q, Deng X, Lu B, Cameron M, Fearns C,
Patricelli MP, Yates JR III, Gray NS and Lee JD: Pharmacological
inhibition of BMK1 suppresses tumor growth through promyelocytic
leukemia protein. Cancer Cell. 18:258–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayashi M, Fearns C, Eliceiri B, Yang Y
and Lee JD: Big mitogen-activated protein kinase 1/extracellular
signal-regulated kinase 5 signaling pathway is essential for
tumor-associated angiogenesis. Cancer Res. 65:7699–7706.
2005.PubMed/NCBI
|
|
18
|
Pi X, Garin G, Xie L, Zheng Q, Wei H, Abe
J, Yan C and Berk BC: BMK1/ERK5 is a novel regulator of
angiogenesis by destabilizing hypoxia inducible factor 1alpha. Circ
Res. 96:1145–1151. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sawhney RS, Liu W and Brattain MG: A novel
role of ERK5 in integrin-mediated cell adhesion and motility in
cancer cells via Fak signaling. J Cell Physiol. 219:152–161. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sticht C, Freier K, Knöpfle K,
Flechtenmacher C, Pungs S, Hofele C, Hahn M, Joos S and Lichter P:
Activation of MAP kinase signaling through ERK5 but not ERK1
expression is associated with lymph node metastases in oral
squamous cell carcinoma (OSCC). Neoplasia. 10:462–470. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou C, Nitschke AM, Xiong W, Zhang Q,
Tang Y, Bloch M, Elliott S, Zhu Y, Bazzone L and Yu D: Proteomic
analysis of tumor necrosis factor-alpha resistant human breast
cancer cells reveals a MEK5/Erk5-mediated epithelial-mesenchymal
transition phenotype. Breast Cancer Res. 10:R1052008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chatterjee A, Chatterjee U and Ghosh MK:
Activation of protein kinase CK2 attenuates FOXO3a functioning in a
PML-dependent manner: Implications in human prostate cancer. Cell
Death Dis. 4:e5432013. View Article : Google Scholar : PubMed/NCBI
|