Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-associated mortality worldwide (1,2). The poor
prognosis of HCC is primarily due to the high rate of tumor
recurrence following surgery, or the occurrence of intrahepatic
metastasis (3,4). Accumulating evidence indicates that the
occurrence of liver disease may be strongly associated with bone
marrow cells (5–7). Bone marrow mesenchymal stem cells
(BM-MSCs) have an inhibitory role in HCC metastasis (8).
Angiogenesis is a process that leads to the
formation of novel blood vessels from preexisting vascular
networks. Angiogenesis is a necessary process for tumor growth,
invasion and metastasis (9).
Intratumor microvessel density (MVD) and vascular endothelial
growth factor (VEGF) are significant biomarkers for the assessment
of angiogenesis (10). Due to the
hypervascular nature of HCC tumors, angiogenesis has a significant
role in the progression of HCC (11).
VEGF and MVD have been demonstrated to be potential predictors for
clinical outcomes and HCC metastatic recurrence (12).
It has been reported that BM-MSCs may contribute to
tumor vascularization (13). BM-MSCs
are a progenitor for angioblasts, which subsequently differentiate
into cells that express endothelial markers, which are capable of
functioning in vitro as mature endothelial cells, as well as
contributing to neoangiogenesis in vivo (14). MSCs are additionally capable of
releasing various cytokines, including VEGF and transforming growth
factor (TGF)β1 (8,15). These cytokines are able to influence
angiogenesis in tumors (16–18). However, the potential role of MSCs in
the angiogenesis of HCC tumors remains to be elucidated.
In a previous study conducted by the authors of the
present study, it was identified that MSCs are able to home to the
sites of HCC tumors, and affect tumor growth and progression via
the action of TGFβ1 (8). However, the
role of MSCs in the angiogenesis of HCC tumors remains to be
elucidated. Therefore, in the present study, the aim was to
investigate the potential role of MSCs in the angiogenesis of HCC
tumors.
Materials and methods
Cell lines
Human BM-derived MSCs were purchased from Sciencell
Research Laboratories (Carlsbad, CA, USA). These cells were
isolated from human bone marrow, and characterized by
immunofluorescent methods, using cluster of differentiation (CD)44
and CD90 antibodies and lipid staining following differentiation.
Following 5 passages of subculture, the cells were re-evaluated by
immunocytochemical staining in order to assure that the normal
phenotype of MSCs had been retained. The 5th passage
MSCs did not express the surface marker CD34, expressed low levels
of fetal liver kinase (Flk)-1 and increased levels of CD29 and
CD105. Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) revealed that the MSCs expressed octamer-binding
transcription factor-4 and Flk-1, which was concordant with
previously published data concerning MSC cell surface markers
(19). Cells were cultured in
α-modified minimum essential medium (Gibco; Thermo Fisher
Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific) and 100 U/ml
penicillin/streptomycin solution (Sciencell Research Laboratories).
The medium was replaced when the cell density reached 5,000
cells/cm2 and the culture reached 50% confluence. Cells
were subcultured when 90% confluence was reached. Cells that had
undergone between 5 and 8 passages were utilized for the following
experiments.
MHCC97-H human HCC cell line was purchased from the
Liver Cancer Institute of Fudan University (Shanghai, China). These
cells exhibit a high metastatic potential, are positive for
α-fetoprotein, albumin and cytokeratin 8 expression, and negative
for hepatitis B (HBV) surface antigen. Fluorescence PCR has
revealed the occurrence of HBV DNA integration in the cellular
genome (20,21). The cells were cultured in high glucose
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific), supplemented with 10% FBS at 37°C in a humidified
incubator with an atmosphere of 5% CO2.
Animal models
In total, 8 female mice and 9 male mice were
randomly assigned into two groups, an experimental group and a
control group. All mice were fed under specific-pathogen free
conditions, food and water were treated by high pressure steam
sterilization, and the feeding temperature was 28°C. A total of
6×106 MHCC97-H cells were subcutaneously implanted into
nude mice. A total of 30 days subsequent to implantation,
subcutaneous tumor tissue was removed and cut into 1×1×1
mm3 tissue sections. These sections were subsequently
orthotopically implanted into the livers of 17 nude mice.
The experiments were performed following the
approval of Xijing Medical Ethics Committee (Xi'an, China), and
were also in compliance with the Helsinki Declaration and Animal
Research: Reporting in vivo experiments guidelines (22).
MSC injection
In order to evaluate the potential effects of MSCs
on pulmonary metastasis of HCC, 8 of the mice were treated with
5×105 human MSCs via tail vein injection 3 times in one
week. The remaining 9 mice were treated with phosphate-buffered
saline (PBS; 0.2 ml), 3 times a week, as a control. A total of 35
days following injection, the mice were sacrificed and tumors were
removed, weighed and subsequently cryopreserved at −80°C, with a
1:4 dilution of optimal cutting temperature compound (Sakura
Finetek, Inc., Torrance, CA, USA) in PBS. Liver and lung tissues
were fixed in paraformalin (Sigma-Aldrich, St. Louis, MO, USA) and
embedded in paraffin.
Evaluation of lung metastasis of
HCC
The incidence and tumor foci number of the lung
metastases were evaluated using hematoxylin and eosin (Beyotime
Institute of Biotechnology, Inc., Shanghai, China) staining in 10
consecutive sections, with an interval of 50 µm, and were
additionally assessed by two independent pathologists.
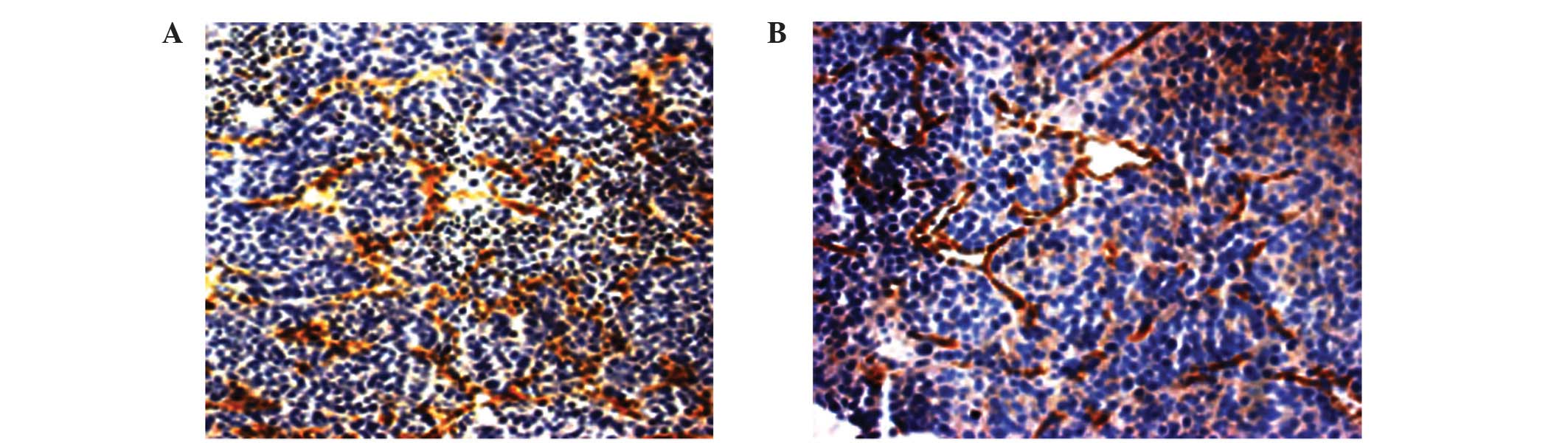
MVD counting
Fresh-frozen 8-µm sections of tumor were fixed in
acetone sections (Spectrum Chemical Manufacturing Corporation, New
Brunswick, NJ, USA), and stained using monoclonal mouse anti-CD31
(CBL1337; dilution, 1:200; EMD Millipore, Billerica, MA); and
subsequently donkey anti-mouse Immunoglobulin G-horseradish
peroxidase (AP189; dilution, 1:500; EMD Millipore, Billerica, MA)
was used as a secondary antibody. Following 3,3-diaminobenzidine
(ZSGB-BIO, Beijing, China) staining, the maximum density of
positive staining was selected as microscope field (magnification,
×20) and microvessels were counted.
RNA extraction and RT-qPCR
Total RNA of tumor tissues was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Waltham, MA,
USA) according to the manufacturer's protocols. SYBR Green Realtime
PCR Master Mix (Toyobo Co., Ltd., Osaka, Japan) was used to perform
RT-qPCR analysis, in order to identify the expression levels of
TGFβ1, Smad2 and Smad7. The primers were designed using Primer
Premier software version 5.0 (PREMIER Biosoft, Palo Alto, CA, USA),
and the sequences used are listed in Table I. Amplification conditions were as
follows: 95°C for 9 min, followed by 45 cycles at 95°C for 30 sec,
57°C for 30 sec and 72°C for 15 sec, followed by a final extension
step at 72°C for 5 min. β-actin served as a control for detection
of the presence of amplifiable complementary DNA. The mRNA
expression levels were quantified using the 2−∆∆Cq
method. In brief, the Cq value for the target gene was subtracted
from the Cq value of β-actin in order to give a ∆Cq value. The
average ∆Cq value was calculated for the control group, and this
value was subsequently subtracted from the ∆Cq value of all other
samples (including the control group). This resulted in the
production of a ∆∆Cq value for all samples, which was subsequently
used in order to calculate the fold-induction of mRNA expression of
the target genes using the formula 2−∆∆Cq, as
recommended by the manufacturer's protocols (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). In the present study, MHCC97-H model
samples served as the control group.
 | Table I.Primer sequences used for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-polymerase chain reaction.
| Gene | Primer | Sequence 5′→3′ |
|---|
| TGFβ1 | Sense |
5′-GGCGATACCTCAGCAACCG-3′ |
|
| Antisense |
5′-CTAAGGCGAAAGCCCTCAAT-3′ |
| Smad2 | Sense |
5′-TACTACTCTTTCCCCTGT-3′ |
|
| Antisense |
5′-TTCTTGTCATTTCTACCG-3′ |
| Smad7 | Sense |
5′-CAACCGCAGCAGTTACCC-3′ |
|
| Antisense |
5′-CGAAAGCCTTGATGGAGA-3′ |
| β-actin | Sense |
5′-TCGTGCGTGACATTAAGGAG-3′ |
|
| Antisense |
5′-ATGCCAGGGTACATGGTAAT-3′ |
Protein extraction and ELISA
Tumor samples were lysed in radioimmunoprecipitation
assay buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 0.5% sodium
deoxycholate; 1% NP-40; 0.1% sodium dodecyl sulfate) plus serine
protease inhibitors. Protein was extracted by microcentrifugation
for 30 min at 13,000 × g. Protein concentration was determined
using Bradford Reagent (Beyotime Institute of Biotechnology, Inc.).
The VEGF and TGFβ1 concentration in the protein extracts was
determined using the Human VEGF Quantikine ELISA assay kit (DVE00;
R&D Systems, Inc., Minneapolis, MN, USA) and Human TGF-beta 1
Quantikine ELISA kit (R&D Systems, Inc.)according to the
manufacturer's protocol. The optical density values were measured
using a Spectramax M5 microplate reader (Molecular Devices, LLC.,
Sunnyvale, CA, USA) at 450 nm wavelength.
Statistical analysis
Data was analyzed using SPSS version 11.5 (SPSS,
Inc., Chicago, IL, USA). Student's t test was utilized for analysis
of the significance of differences between tumor weight and volume,
and mRNA expression levels of target genes for independent samples.
Fishers' exact test was performed for the comparison of ratio
involved. All statistical tests performed were two-sided, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
MSC treatment reduces the rate of
pulmonary metastasis of HCC
No difference in tumor weight was observed between
the group treated with MSCs and the untreated control group; the
ratios of tumor to body weight were 0.14±0.05 vs. 0.13±0.04,
respectively (P=0.75). Compared with the control group, the
pulmonary metastasis rate of the MSC-treated mice (100 vs. 50%, for
the control and MSC-treated mice, respectively) was statistically
reduced (100%; P=0.029; Table
II).
 | Table II.Inhibitory effects of MSC on the
in vivo pulmonary metastasis of hepatocellular
carcinoma. |
Table II.
Inhibitory effects of MSC on the
in vivo pulmonary metastasis of hepatocellular
carcinoma.
| Variable | MSC injection | No MSC
injection | P-value |
|---|
| No. of animals | 8 | 9 | N/A |
| Body weight, g |
21.99±2.90a |
22.08±1.69a | 0.918 |
| Tumor weight,
g |
2.90±0.80a |
2.86±0.72a | 0.906 |
| Tumor weight/body
weight, g |
0.14±0.05a |
0.13±0.04a | 0.754 |
| Lung metastasis,
% | 50% | 100% | 0.029 |
| No. of lung
metastases |
1.37±1.60a |
1.77±0.97a | 0.263 |
| Cellular no. of
metastases |
50.37±60.19a |
74.44±84.22a | 0.292 |
MSC treatment exerts differential
effects on the expression of mRNA of TGFβ1 and Smads in HCC
tumors
Levels of TGFβ1 mRNA in the MSC-treated group were
2.15-fold higher compared with the untreated controls (1.27±0.61
vs. 0.59±0.39; P=0.033), however, the levels of Smad7 mRNA were
downregulated compared with untreated controls (0.76±0.29 vs.
1.41±0.50; P=0.006; Table III).
 | Table III.Expression of TGFβ1, Smads and VEGF
were influenced by MSC injection in mouse models. |
Table III.
Expression of TGFβ1, Smads and VEGF
were influenced by MSC injection in mouse models.
| Variable | MSC, mean ± SD | No MSC, mean ±
SD | Fold change | P-value |
|---|
| TGFβ1 mRNA |
1.27±0.61 |
0.59±0.39 | +2.15 | 0.033 |
| Smad2 mRNA |
1.01±0.14 |
1.25±0.38 | −1.25 | 0.114 |
| Smad7 mRNA |
0.76±0.29 |
1.41±0.50 | −1.86 | 0.006 |
| TGFβ1 protein |
0.02±0.01 |
0.03±0.01 | −1.50 | 0.267 |
| VEGF protein |
0.29±0.05 |
0.31±0.13 | −1.07 | 0.631 |
| Microvessel
density | 28.00±9.19 | 18.11±3.30 | +1.55 | 0.006 |
MSC treatment exerts differential
effects on MVD and VEGF expression in HCC
The expression of VEGF in the MSC-treated group did
not significantly differ compared with the untreated control group
(0.29±0.05 vs. 0.31±0.13; P=0.631). However, MVD was significantly
increased in the MSC-treated group compared with the untreated
control group (28.00±9.19 vs. 18.11±3.30; P=0.006; Fig. 1; Table
III).
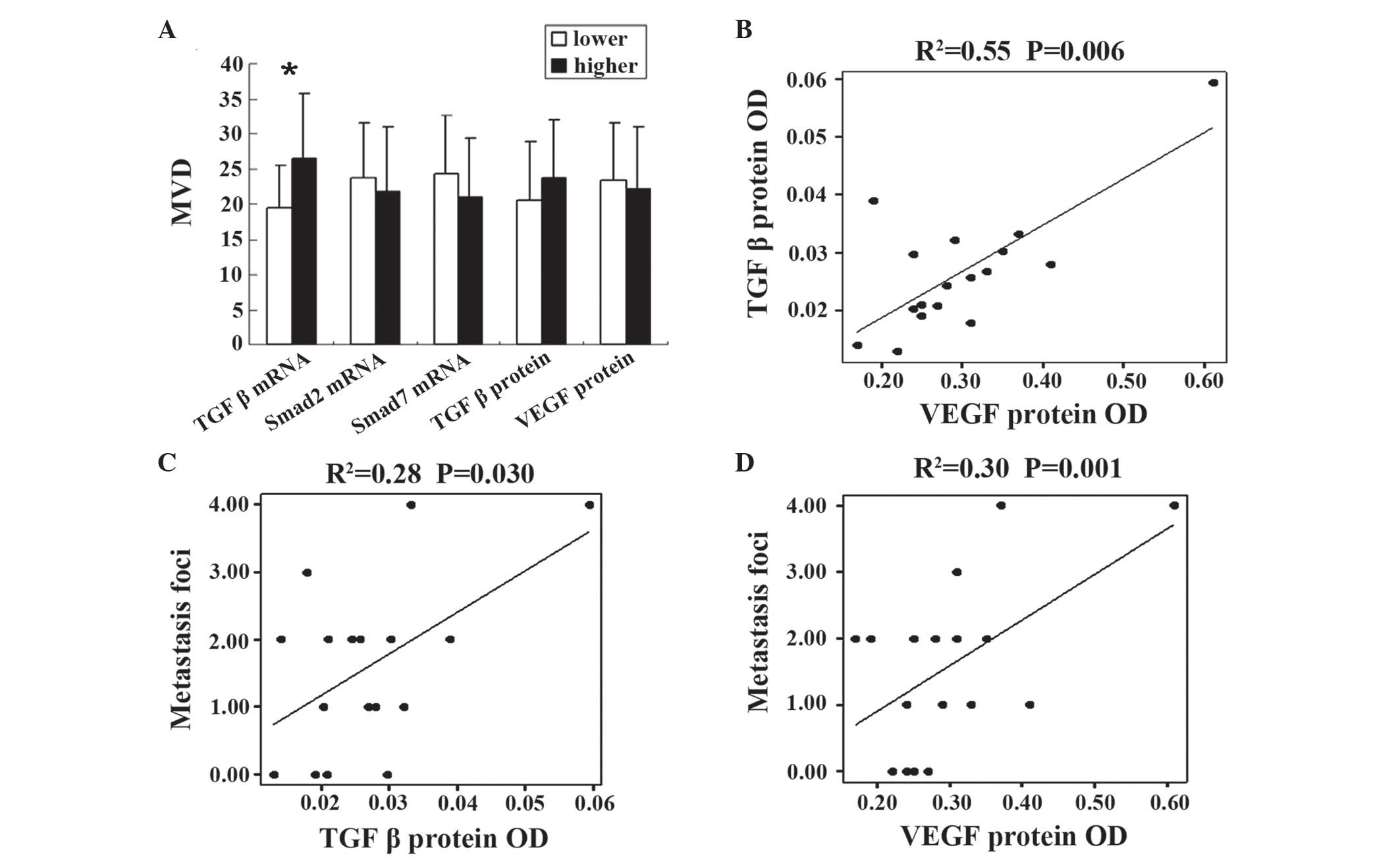
Levels of TGFβ1 mRNA are associated
with MVD
According to the median levels of TGFβ1 mRNA, Smad2
mRNA, Smad7 mRNA, TGFβ1 protein and VEGF protein, expression levels
were divided into two groups, high-level and low-level. MVD was
increased in the TGFβ1 mRNA high-level group compared with the
low-level group (26.50±9.11 vs. 19.44±6.14; P=0.038), however, the
levels of Smad2 mRNA (23.67±7.84 vs. 21.75±9.14; P=0.648), Smad7
mRNA (24.33±8.26 vs. 21.00±8.46; P=0.424), VEGF protein levels
(22.12±8.82 vs. 23.33±8.23; P=0.375) and TGFβ1 protein levels
(23.66±8.23 vs. 20.63±8.29; P=0.375) demonstrated no significant
difference between the two groups (Fig.
2A).
TGFβ1 and VEGF protein levels are
associated with metastasis
In the present study, it was identified that TGF
protein levels were closely correlated with VEGF protein levels
using linear correlation analysis (R2=0.55; P=0.006;
Fig. 2B). In addition, TGFβ1 and VEGF
protein levels were observed to be associated with the tumor foci
number of metastasis (R2=0.28 and P=0.030; and
R2=0.30 and P=0.001, respectively; Fig. 2C and D).
MVD was not observed to be associated
with tumor growth and metastasis of HCC
According to the median levels of MVD, samples were
divided into two groups, dependent on high or low MVD. No
statistically significant differences were identified between the
two groups, including for tumor weight, pulmonary metastasis rate,
tumor foci number of metastasis and cellular number of metastases
(Table IV).
 | Table IV.MVD and tumor progression of
hepatocellular carcinoma. |
Table IV.
MVD and tumor progression of
hepatocellular carcinoma.
| Variable | Low MVD | High MVD | P-value |
|---|
| No. of animals | 8 | 9 | N/A |
| Body weight, g |
20.62±1.66a |
21.58±2.08a | 0.314 |
| Tumor weight,
g |
2.87±0.82a |
2.91±0.91a | 0.926 |
| Tumor weight/body
weight, g |
0.14±0.05a |
0.14±0.05a | 0.904 |
| Lung metastasis,
% | 75.0% | 44.4% | 0.335 |
| No. of lung
metastases |
1.75±1.16a |
1.22±1.56a | 0.447 |
| Cellular no. of
metastases |
32.38±35.55a |
75.33±97.11a | 0.257 |
Discussion
MVD is a direct reflection of tumor angiogenesis,
and may be visualized using immunohistochemical staining with
antibodies of anti-CD31, anti-CD34, factor VIII and α-smooth muscle
actin (23). In the present study, it
was identified that MSC is capable of enhancing the MVD of HCC
tumors, as well as inhibiting pulmonary metastasis. The results of
the present study provided evidence and indicated that MSC may
promote tumor angiogenesis and influence tumor progression.
In HCC, MVD is closely associated with tumor size,
recurrence and disease-free survival (24). However, the present study did not
identify an association between MVD and metastasis and tumor size.
Although intratumor MVD is a marker for angiogenesis, it is not
able to provide any functional information concerning the
underlying molecular pathways involved in the angiogenic activity
of a specific tumor (25).
Multivariate analysis has indicated that MVD is not an effective
prognostic factor when tumor size is >5 cm (9). In addition, three types of intratumor
microvessels may be identified in HCC, including capillary-like,
sinusoid-like and mixed-type, which make the prognostic value of
MVD uncertain (24).
Cytokines have a significant role in tumor
metastasis and angiogenesis (26,27). TGFβ1
may influence the invasiveness and metastasis of carcinoma
(28–30), and has been observed to particularly
promote angiogenesis (31–34). The present study identified that MSCs
were able to significantly enhance the expression of TGFβ1 mRNA,
however, inhibited the expression of Smad7 mRNA. Additionally,
TGFβ1 mRNA expression levels correlated with MVD. The results of
the present study suggested that MSCs may promote angiogenesis via
the TGFβ1/Smad signaling pathway, and may have revealed a novel
mechanism for the role of MSCs in tumor progression. The present
study detected TGFβ1 mRNA and protein levels and demonstrated that
MVD was correlated with the levels of TGFβ1 mRNA, however, was not
correlated with the levels of TGFβ1 protein. This divergence may be
due to the complicated post-transcriptional mechanisms involved in
translation of mRNA into proteins (35).
No association was identified between MSC injection
and VEGF expression, and VEGF was not observed to enhance MVD in
the present study. However, a close association was observed
between TGFβ1 and VEGF, and these cytokines were demonstrated to be
associated with tumor metastasis, which may indirectly reflect the
role of VEGF in tumor angiogenesis. It has been reported that VEGF
and TGFβ1 interact with each other during the process of
angiogenesis. This interaction complicates their role in
angiogenesis, and in some cases may induce an inhibitory effect on
cancer proliferation (36–39).
In conclusion, the results of the present study
suggested that MSCs may enhance the angiogenesis of HCC through the
action of TGFβ1. The TGFβ1/Smad signaling pathway and its
interaction with VEGF may partly explain the intricate role of MSCs
in tumor progression. Whether the increase in angiogenesis is due
to the differentiation of MSCs or caused by alternative vascular
regulators secreted by MSCs requires investigation in future
studies.
Acknowledgements
The authors would like to thank Dr Qiong Xue, Dr
Dongmei Gao and Dr Jun Chen in Liver Cancer Institute of Fudan
University (Shanghai, China) for their assistance with the animal
experiments, Dr Ruixia Sun and Dr Jie Chen in Zhongshan hospital of
Fudan University for their suggestions for cell culture, and Dr
Haiying Zeng and Dr Tengfang Zhu in the Pathological Department of
Zhongshan Hospital of Fudan University, for their assistance with
pathological experiments.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He J, Gu D, Wu X, Reynolds K, Duan X, Yao
C, Wang J, Chen CS, Chen J, Wildman RP, et al: Major causes of
death among men and women in China. N Engl J Med. 353:1124–1134.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye QH, Qin LX, Forgues M, He P, Kim JW,
Peng AC, Simon R, Li Y, Robles AI, Chen Y, et al: Predicting
hepatitis B virus-positive metastatic hepatocellular carcinomas
using gene expression profiling and supervised machine learning.
Nat Med. 9:416–423. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Portolani N, Coniglio A, Ghidoni S,
Giovanelli M, Benetti A, Tiberio GA and Giulini SM: Early and late
recurrence after liver resection for hepatocellular carcinoma,
Prognostic and therapeutic implications. Ann Surg. 243:229–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petersen BE, Bowen WC, Patrene KD, Mars
WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS and Goff JP:
Bone marrow as a potential source of hepatic oval cells. Science.
284:1168–1170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang YY, Collector MI, Baylin SB, Diehl AM
and Sharkis SJ: Hematopoietic stem cells convert into liver cells
within days without fusion. Nat Cell Biol. 6:532–539. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alison MR and Lovell MJ: Liver cancer: The
role of stem cells. Cell Prolif. 38:407–421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li GC, Ye QH, Xue YH, Sun HJ, Zhou HJ, Ren
N, Jia HL, Shi J, Wu JC, Dai C, et al: Human mesenchymal stem cells
inhibit metastasis of a hepatocellular carcinoma model using the
MHCC97-H cell line. Cancer Sci. 101:2546–2553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rakesh KJ, Dan GD, Christopher GW,
Dushyant VS, Andrew XZ, Jay SL and Tracy TB: Biomarkers of response
and resistance to antiangiogenic therapy. Nat Rev Clin Oncol.
6:327–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poon RT, Ng IO, Lau C, Yu WC, Yang ZF, Fan
ST and Wong J: Tumor microvessel density as a predictor of
recurrence after resection of hepatocellular carcinoma, A
prospective study. J Clin Oncol. 20:1775–1785. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin LX and Tang ZY: Recent progress in
predictive biomarkers for metastatic recurrence of human
hepatocellular carcinoma: A review of the literature. J Cancer Res
Clin Oncol. 130:497–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lyden D, Hattori K, Dias S, Costa C,
Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et
al: Impaired recruitment of bone-marrow-derived endothelial and
hematopoietic precursor cells blocks tumour angiogenesis and
growth. Nat Med. 7:1194–1201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reyes M, Dudek A, Jahagirdar B, Koodie L,
Marker PH and Verfaillie CM: Origin of endothelial progenitors in
human postnatal bone marrow. J Clin Invest. 109:337–346. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kinnaird T, Stabile E, Burnett MS, Shou M,
Lee CW, Barr S, Fuchs S and Epstein SE: Local delivery of
marrow-derived stromal cells augments collateral perfusion through
paracrine mechanisms. Circulation. 109:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bellone G, Gramigni C, Vizio B, Mauri FA,
Prati A, Solerio D, Dughera L, Ruffini E, Gasparri G and Camandona
M: Abnormal expression of Endoglin and its receptor complex (TGF-β1
and TGF-β receptor II) as early angiogenic switch indicator in
premalignant lesions of the colon mucosa. Int J Oncol.
37:1153–1165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo SW, Ke FC, Chang GD, Lee MT and Hwang
JJ: Potential role of follicle-stimulating hormone (FSH) and
transforming growth factor (TGFβ1) in the regulation of ovarian
angiogenesis. J Cell Physiol. 226:1608–1619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mazzocca A, Fransvea E, Lavezzari G,
Antonaci S and Giannelli G: Inhibition of transforming growth
factor beta receptor I kinase blocks hepatocellular carcinoma
growth through neo-angiogenesis regulation. Hepatology.
50:1140–1151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Y, Jahagirdar BN, Reinhardt RL,
Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund
T, Blackstad M, et al: Pluripotency of mesenchymal stem cells
derived from adult marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue
Q, Chen J, Gao DM and Bao WH: Establishment of cell clones with
different metastatic potential from the metastatic hepatocellular
carcinoma cell line MHCC97. World J Gastroenterol. 7:630–636.
2001.PubMed/NCBI
|
|
21
|
Li Y, Tang Y, Ye L, Liu B, Liu K, Chen J
and Xue Q: Establishment of a hepatocellular carcinoma cell line
with unique metastatic characteristics through in vivo
selection and screening for metastasis-related genes through cDNA
microarray. J Cancer Res Clin Oncol. 129:43–51. 2003.PubMed/NCBI
|
|
22
|
Ferdowsian H: Human and animal research
guidelines: A ligning ethical constructs with new scientific
developments. Bioethics. 25:472–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen ZY, Wei W, Guo ZX, Lin JR, Shi M and
Guo RP: Morphologic classification of microvessels in
hepatocellular carcinoma is associated with the prognosis after
resection. J Gastroenterol Hepatol. 26:866–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun HC, Tang ZY, Li XM, Zhou YN, Sun BR
and Ma ZC: Microvessel density of hepatocellular carcinoma, Its
relationship with prognosis. J Cancer Res Clin Oncol. 125:419–426.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gasparini G: Prognostic value of vascular
endothelial growth factor in breast cancer. Oncologist. 5(Suppl 1):
37–44. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Littlepage LE, Egeblad M and Werb Z:
Coevolution of cancer and stromal cellular responses. Cancer Cell.
7:499–500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leek RD, Harris AL and Lewis CE: Cytokine
networks in solid human tumors, Regulation of angiogenesis. J
Leukoc Biol. 56:423–435. 1994.PubMed/NCBI
|
|
28
|
Oft M, Heider KH and Beug H: TGFbeta
signaling is necessary for carcinoma cell invasiveness and
metastasis. Curr Biol. 8:1243–1252. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iyer S, Wang ZG, Akhtari M, Zhao W and
Seth P: Targeting TGFbeta signaling for cancer therapy. Cancer Biol
Ther. 4:261–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Welm AL: TGFβ primes breast tumor cells
for metastasis. Cell. 133:27–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu Q and Stamenkovic I: Cell
surface-localized matrix metalloproteinase-9 proteolytically
activates TGF-beta and promotes tumor invasion and angiogenesis.
Genes Dev. 14:163–176. 2000.PubMed/NCBI
|
|
32
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Salcedo X, Medina J, Sanz-Cameno P,
García-Buey L, Martín-Vilchez S and Moreno-Otero R: Review article
Angiogenesis soluble factors as liver disease markers. Aliment
Pharmacol Ther. 22:23–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brogi E, Wu T, Namiki A and Isner JM:
Indirect angiogenic cytokines upregulate VEGF and bFGF gene
expression in vascular smooth muscle cells, whereas hypoxia
upregulates VEGF expression only. Circulation. 90:649–652. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Greenbaum D, Colangelo C, Williams K and
Gerstein M: Comparing protein abundance and mRNA expression levels
on a genomic scale. Genome Biol. 4:1172003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferrari G, Pintucci G, Seghezzi G, Hyman
K, Galloway AC and Mignatti P: VEGF, a prosurvival factor, acts in
concert with TGF-beta1 to induce endothelial cell apoptosis. Proc
Natl Acad Sci USA. 103:17260–17265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeon SH, Chae BC, Kim HA, Seo GY, Seo DW,
Chun GT, Kim NS, Yie SW, Byeon WH, Eom SH, et al: Mechanisms
underlying TGF-beta1-induced expression of VEGF and Flk-1 in mouse
macrophages and their implications for angiogenesis. J Leukoc Biol.
81:557–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee KS, Park SJ, Kim SR, Min KH, Lee KY,
Choe YH, Hong SH, Lee YR, Kim JS, Hong SJ and Lee YC: Inhibition of
VEGF blocks TGF-beta1 production through a PI3K/Akt signalling
pathway. Eur Respir J. 31:523–531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ferrari G, Cook BD, Terushkin V, Pintucci
G and Mignatti P: Transforming growth factor-beta 1 (TGF-beta1)
induces angiogenesis through vascular endothelial growth factor
(VEGF)-mediated apoptosis. J Cell Physiol. 219:449–458. 2009.
View Article : Google Scholar : PubMed/NCBI
|