Introduction
Breast cancer accounts for almost 1/4 of all cancers
diagnosed in women (1). Among the
molecular subtypes of breast cancer, estrogen receptor
(ER)-positive subtypes respond to anti-estrogen therapy (2), but have been observed to develop
resistance (3). Triple-negative
breast cancer, which does not express ER, progesterone receptor or
human epidermal growth factor receptor 2 (HER2), is more aggressive
and has a reduced number of treatment options (4). Anti-estrogens and trastuzumab are not
effective for the treatment of triple-negative cancer, as cells do
not express ER or HER2 (5); therefore
chemotherapy is the only effective treatment option (6). Besides being expensive, radiotherapy and
chemotherapy may cause serious side effects (7). Therefore, it is necessary to discover
novel anticancer compounds that cause fewer adverse effects. Plants
and other natural sources have provided ~60% of anti-cancer agents
currently in use (8); however, there
are a number of traditionally used plants that remain to be
scientifically validated.
Mangifera zeylanica (family, Anacardiaceae)
is a plant endemic to Sri Lanka, and is typically found in the
intermediate and wet zone forests (9). It is commonly known as ‘Etamba’, and
grows as a wild species that bears edible fruit. M.
zeylanica has been used traditionally for cancer therapy in Sri
Lanka. However, these claims have not been scientifically
validated. Mangiferin is the only reported compound isolated from
M. zeylanica (10). Therefore,
the present study was conducted to evaluate the potential cytotoxic
and apoptotic effects of M. zeylanica on breast and ovarian
cancer cells and to identify phytochemical constituents in active
fractions obtained from bioactivity-guided fractionation.
Materials and methods
Plant material, chemicals, cell lines
and cell culture reagents
Approval was obtained from the Department of
Wildlife Conservation, Government of Sri Lanka (Columbo, Sri Lanka)
for collecting M. zeylanica bark for research. The bark (2.5 kg)
was collected from Imaduwa (Galle, Sri Lanka) and the plant was
identified by a botanist at Bandaranayke Memorial Ayurvedic
Research Institute (BMARI; Nawinna, Maharagama, Sri Lanka). The
voucher specimen (#1221 A) was deposited at BMARI. All chemicals
were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless
otherwise specified. Cell lines and 10% fetal bovine serum were
acquired from the American Type Culture Collection (ATCC; Manassas,
VA, USA).
Extraction and preparation of plant
extract
Finely powdered dried bark (2.5 kg) was subjected to
sequential extraction using hexane, chloroform, ethyl acetate and
methanol (thrice with each solvent) by sonicating for 3 h at room
temperature. All resulting extracts were filtered and evaporated
using an R-3 rotary evaporator (BÜCHI Labortechnik AG, Flawil,
Switzerland) under reduced pressure at 40°C to obtain crude
extracts of hexane, chloroform, ethyl acetate and methanol. Stock
solutions were prepared by dissolving in dimethyl sulfoxide (DMSO),
and diluted to working solutions prior to use (the final DMSO
concentration was 0.5% v/v).
Preliminary phytochemical analysis,
determination of total flavonoid and polyphenol content and free
radical scavenging activity
Hexane extract of M. zeylanica was tested for the
presence of polyphenols (11),
flavonoids (12), lipids, sterols and
saponins (13,14) using previously described methods with
minor modifications as required. Polyphenol content was expressed
as gallic acid equivalent, and flavonoid content as quercetin
equivalent, per 1 g of plant extract.
Free radical scavenging activity of the extract was
investigated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
(15) with minor modifications.
Hexane extract (0.5 ml) was added at various concentrations (25,
50, 100, 200 and 400 µg/ml) to 0.5 ml of DPPH (Sigma-Aldrich)
solution (5.9 g in 100 ml methanol) and incubated in the dark for
30 min, followed by absorbance (A) reading at 517 nm (Synergy™ HT
Multi-Mode Microplate Reader; Bio-Tek Instruments, Inc., Winooski,
VT, USA). Percentage scavenging ability was calculated as half
maximal effective concentration (EC50) using the
following equation: EC50= (Acontrol -
Asample)/Acontrol × 100. Ascorbic acid was
utilized as the positive control.
Cell culture and cytotoxicity
assay
MCF-7 human ER-positive breast cancer cells,
MDA-MB-231 triple-negative breast cancer cells, SKOV-3 ovarian
epithelial cancer cells and MCF-10A normal mammary epithelial cells
were maintained in ATCC-recommended medium [MCF-7 cells, Dulbecco's
modified Eagle's medium (DMEM; ATCC 30–2002); MDA-MB-231 cells:
Leibovitz's L-15 medium (ATCC 30–2008); SKOV-3 cells, McCoy's 5A
medium (ATCC 30–2007); and MCF-10A cells, DMEM (ATCC 30–2002)] with
10% fetal bovine serum, insulin (Sigma-Aldrich; 0.01 mg/ml),
streptomycin (Sigma-Aldrich; 0.1 mg/ml) and penicillin
(Sigma-Aldrich; 100 U/ml). All cells were cultured at 37°C in an
atmosphere of 5% CO2, with the exception of MDA-MB-231
cells, which were cultured without CO2. Cells were
harvested by trypsinization and seeded into 96-well plates (product
no. 3860-096; Iwaki Cell Biology, Iwaki, Japan) at a density of
5×103 cells/well. Following 24 h incubation, cells were
treated with various doses (25, 50, 100, 200 or 400 µg/ml) of
hexane, chloroform, ethyl acetate or methanol extracts, or
mangiferin. The cytotoxic effect of the extracts was assessed by
sulforhodamine B (SRB) assay following 24 h incubation (16). Briefly, cells were fixed using 50 µl
of ice-cold 50% trichloroacetic acid, incubated for 60 min at 4°C,
washed with tap water five times and stained using 0.4% SRB
solution (100 µl stain/well). Plates were subsequently incubated at
room temperature for 15 min, SRB solution was decanted and unbound
dye was removed by washing with 1% acetic acid five times, followed
by air-drying. Unbuffered Tris-base solution (200 µl/well) was
added to the wells to solubilize unbound SRB dye. The contents were
mixed on an agitator for 1 h at room temperature. Absorbance was
read at optical density 540 nm (Synergy™ HT Multi-Mode Microplate
Reader) and percentage cell viability was calculated (mean of
control group - mean of treated group/control group × 100%). All
experiments were performed in triplicate. Paclitaxel
(Sigma-Aldrich) was utilized as the positive control. Negative
controls received ATCC-recommended medium and DMSO.
Identification of active fractions of
the M. zeylanica bark extract
The crude hexane extract, which was cytotoxic to
cancer cells and less cytotoxic to normal cells, was subjected to a
series of solvent-solvent partitions. It was initially partitioned
between hexane and MeOH/H2O (9:1, v/v) and subsequently,
following separation of the hexane layer, the aqueous layer was
diluted with water to a composition of MeOH/H2O (6:4,
v/v) and extracted with chloroform. The aqueous layer was
subsequently concentrated under reduced pressure and partitioned
between ethyl acetate and water. A total of four fractions, namely
hexane-, chloroform-, ethyl acetate- and water-soluble fractions,
were thus obtained. Cytotoxicity was contained in the
chloroform-soluble fraction. The dried chloroform layer (1.1 g) was
subjected to silica gel column chromatography (230–400 mesh; cat
no. 177/03; Daihan Labtech India Pvt. Ltd., Delhi, India) and
eluted with 100 ml each of hexane-ethyl acetate (8:2, 7:3, 6:4,
1:1, 4:6, 3:7, 2:8, 1:9, v/v), ethyl acetate-methanol (1:1, v/v)
and methanol. All the solvents for chromatography separations were
purchased from Sigma-Aldrich. Active fractions identified by SRB
assay were monitored by normal-phase thin-layer chromatography
(TLC) using hexane-ethyl acetate (1:1, v/v) as the mobile phase. As
all cytotoxic fractions produced almost a clear spot during
normal-phase TLC, all fractions were pooled and concentrated to
give T1. T1 was monitored on reversed-phase
TLC using methanol-water (9.5:0.5, v/v) as the mobile phase,
fractionated in a reversed-phase column (C18), and
eluted with 10 ml each of methanol-water (7:3, 8:3, 9:3, v/v) and
methanol. Fractions identified as most cytotoxic by SRB assay were
monitored by reversed-phase TLC using methanol-water (9:1, v/v) as
the mobile phase. Following observation of the behaviour of these
fractions in reversed phase-TLC, 500 µl from each active fraction
was pooled to give the final fraction (M1) and its
cytotoxicity to cancer cells and normal mammary epithelial cells
was assessed.
Evaluation of apoptotic effects
The potential apoptotic effects of the hexane
extract were assessed by investigating its effect on caspase-3 and
−7 activity, morphological changes and DNA fragmentation. The
effect on caspase-3 and −7 activity was determined in the three
cancer cell lines. Cells were treated with the hexane extract for 4
h (25, 50, 100, 150 and 200 µg/ml) or 24 h (5, 10, 25, 50 and 100
µg/ml). Caspase activity was assessed using ApoTox-Glo™ triplex
assay according to the manufacturer's protocol (Promega
Corporation, Madison, WI, USA) and compared with untreated
controls.
The three cancer cell lines (5×105
cells/ml) were treated with 200 and 400 µg/ml of the hexane extract
for 24 h and harvested by trypsinization and centrifugation. The
resulting cell pellets were subsequently incubated for 1 h at 55°C
in freshly prepared lysis buffer (5 mM Tris-HCl, pH 8; 1 M NaCl and
5 mM ethylenediaminetetraacetic acid, pH 8; 0.5% sodium dodecyl
sulfate and proteinase K; 200 µg/ml). Following incubation with
RNaseA (200 µg/ml) for 2 h at 50°C, DNA was extracted using
phenol-chloroform-isoamyl alcohol. Extracted DNA was visualised
under ultraviolet light to assess the effect on DNA fragmentation
(Quantum-ST4 1100/20 M; Fisher Biotec Pty Ltd., Wembley, Australia)
following electrophoresis on a 2.0% agarose gel stained with
ethidium bromide (EB).
Cell morphology was assessed by examining acridine
orange (AO)/EB-stained (17) treated
cells. Cells at 70–80% confluence were harvested by trypsinization,
seeded into 24-well plates (Iwaki Cell Biology) on cover slips
(5×104 cells/well) and incubated for 24 h in a
humidified atmosphere at 37°C in 5% CO2. Cells were
subsequently treated with 25, 50, 100, 200 and 400 µg/ml hexane
extract, incubated for 24 h, rinsed with cold phosphate-buffered
saline and fixed with 4% formaldehyde at room temperature. AO/EB
solution (10–20 µl) was added to each well and cells were observed
under a fluorescence microscope (BX51 TRF; Olympus Corporation,
Tokyo, Japan).
RNA isolation and reverse
transcriptase quantitative polymerase chain reaction (RT-qPCR)
The three cancer cell lines (200,000 cells/ml) were
cultured in cell culture flasks, treated with the hexane extract at
100 or 150 µg/ml for 4 h, and 50 or 75 µg/ml for 24 h. Following
treatment, cells were harvested and total RNA was extracted with
TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA) according to the manufacturer's protocol. Total
extracted RNA (2 µg) and 50 ng of random primers (Integrated DNA
Technologies, Coralville, USA) were mixed in a PCR tube (0.2 ml)
and the total volume was made up to 13.5 µl with
diethylpyrocarbonate (DEPC)-treated ultrapure water for reverse
transcription. The resulting RNA-random primer mixture was
denatured at 70°C for 5 min and subsequently quenched on ice for 2
min to prevent formation of secondary structures. Complementary
(c)DNA was synthesized by adding 5 µl 5X buffer, 5 µl 10 mM
deoxynucleotide mixture (deoxyadenosine triphosphate,
deoxyguanosine triphosphate, deoxycytidine triphosphate and
deoxythymidine triphosphate), 25 units of RNasin and 200 units of
Moloney murine leukemia virus reverse transcriptase (all Thermo
Fisher Scientific, Inc.), and the reaction mixture (25 µl) was
incubated at 37°C for 60 min by using a thermal cycler. RT-qPCR was
performed in Stratagene Mx3000P using the MESA Green qPCR Master
Mix Plus for SYBR Assay (Eurogentec, Seraing, Liège, Belgium) with
the primers listed in Table I (except
for p53 in SKOV-3 cancer cells, which are p53-null; Integrated DNA
Technologies). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was
utilized as the housekeeping gene. The reaction was performed in a
total volume of 25 µl, containing 2 µl cDNA sample, 0.5 µl of each
primer (0.5 µM), 12.5 µl SYBR Green reaction mix and DEPC-treated
ultrapure water (9.5 µl). PCR amplification was performed in
duplicate wells. The cycling conditions were as follows:
Denaturation step (95°C for 10 min), and 40 cycles of three-step
amplification (denaturation, 95°C for 30 sec; annealing, 56°C for 1
min; and extension, 72°C for 1 min). In addition, the real-time
reaction of the products was examined by analyzing the melting
point following each reaction. The formula ΔCq = Cqtarget
gene - CqGAPDH was used to determine the ΔCq
values. Following this initial calculation, ΔΔCq values were
calculated using the formula ΔΔCq = ΔCqtreated -
ΔCquntreated. Expression of the gene of interest in the
treated cells was measured relative to that of the untreated
control cells. Results were quantified using the formula
2−ΔΔCq (18).
 | Table I.Primers used for reverse
transcription-quantitative PCR and the PCR product size. |
Table I.
Primers used for reverse
transcription-quantitative PCR and the PCR product size.
| Gene | Forward primer,
5′-3′ | Reverse primer,
5′-3′ | Size, bp |
|---|
| Bcl-2-associated X
protein |
TCCAGGATCGAGCAGGGCGAA |
CGATGCGCTTGAGACACTCGCT | 109 |
| Tumor protein
p53 |
TCTGGCCCCTCCTCAGCATCTT |
TTGGGCAGTGCTCGCTTAGTGC | 369 |
| Survivin |
TGGCCGCTCCTCCCTCAGAAAA |
GCTGCTGCCTCCAAAGAAAGCG | 190 |
| GAPDH |
GGCATTGCCCTCAACGACCAC |
ACATGACAAGGTGCGGCTCCCTA | 283 |
Gas chromatography-mass spectrometry
(GC-MS) analysis of crude hexane extract and fraction
M1
Agilent GC-MS (7890A GC, 5975C MS; Agilent
Technologies, Inc., Santa Clara, CA, USA) was used for
chromatographic analysis. An ionization voltage of 70 eV, injector
and detector temperatures of 260°C and 320°C, respectively, and
J&W DB-5 MS capillary columns (30 m length, 250 µm internal
diameter and 0.25 µm thickness) were used. The oven temperature was
initiated at 110°C (isothermal for 5 min), increased to 280°C at
20°C/min (isothermal for 1 min) and increased again to 320°C at
20°C/min (isothermal for 5 min). Helium was the carrier gas and
this was used at a flow rate of 1.5 ml/min, with an injector volume
of 1 µl with splitless mode. The hexane extract and M1
fraction were dissolved in hexane (1 mg/ml), filtered through 0.2
µm syringe filters (Sigma-Aldrich) and injected into the GC-MS. The
mass spectrum of each compound was identified by comparison to the
National Institute of Standards and Technology library (http://www.nist.gov/).
Statistical analyses
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla,
CA, USA) was used for statistical analysis. Results are expressed
as the mean ± standard deviation of three independent experiments.
One way analysis of variance with Dunnett's post hoc test was used
to compare groups, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Phytochemical investigation, extract
yields, and total polyphenol and flavonoid content in hexane
extract of M. zeylanica bark
From 2.5 kg of powdered bark material, 5.20, 5.89,
13.42 and 138.92 g of hexane, chloroform, ethyl acetate and
methanol extracts were obtained, corresponding to yields of 0.208,
0.236, 0.5368 and 5.568%, respectively. Qualitative phytochemical
investigation revealed that the hexane extract contained steroids,
flavonoids, phenolic compounds, tannins and reducing sugars, while
saponins were not detected (Table
II). The total polyphenolic and flavonoid content in the
n-hexane extract was 113.2 mg gallic acid equivalent and 30.4 mg
quercetin equivalent, respectively, per 1 g of dried hexane
extract.
 | Table II.Qualitative phytochemical screening
of the hexane extract of Mangifera zeylanica. |
Table II.
Qualitative phytochemical screening
of the hexane extract of Mangifera zeylanica.
| Phytochemical |
Presence/absence |
|---|
| Steroids | ++++ |
| Flavonoids | + |
| Phenolic
compounds | ++++ |
| Tannins | + |
| Reducing
sugars | + |
| Saponins | − |
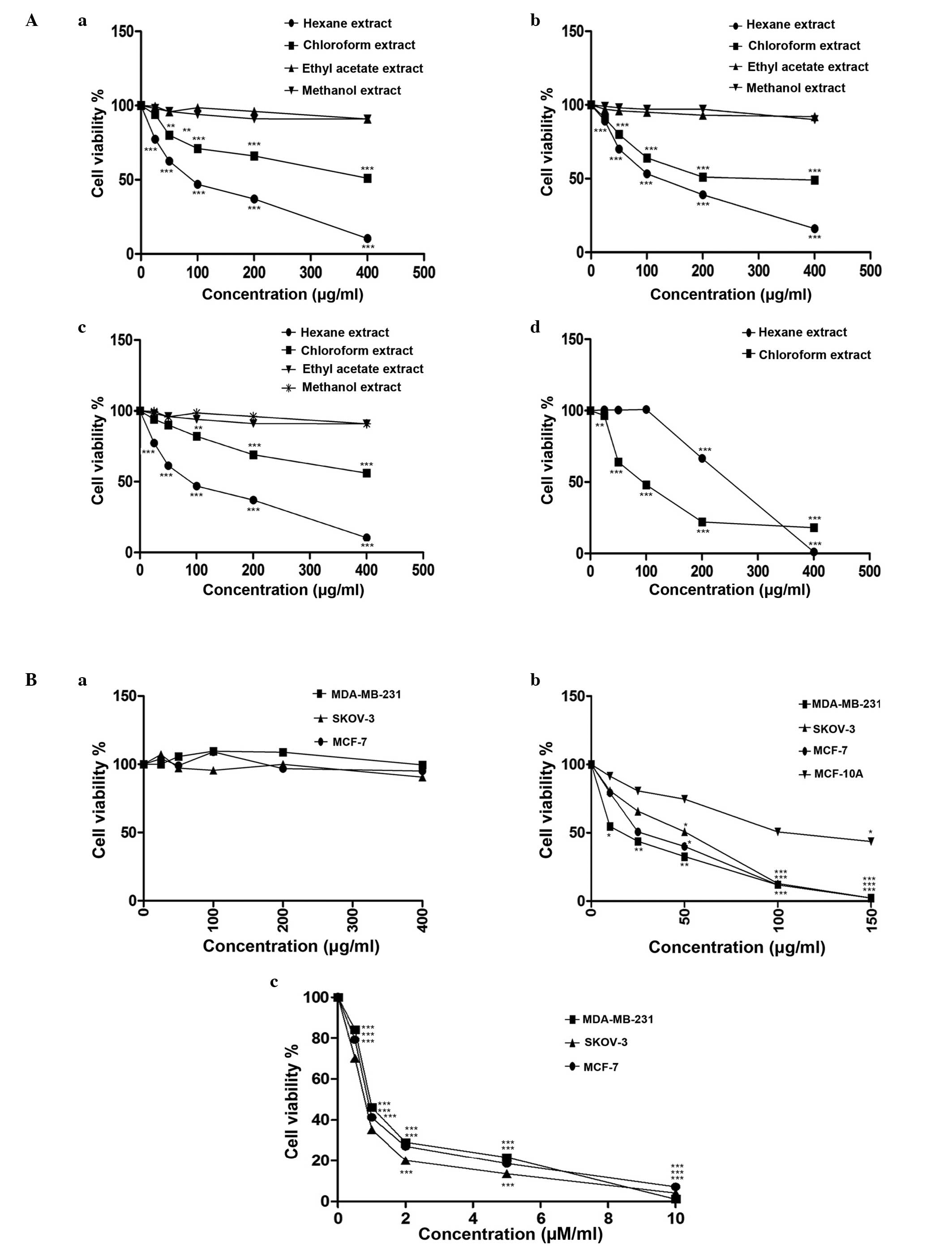
Varying levels of cytotoxicity of
extracts and M1 fraction are observed in distinct cell
lines
IC50 values of the four solvent extracts
of M. zeylanica bark, fraction M1, mangiferin and
paclitaxel are provided in Table
III. Of the four solvent extracts, only the hexane and
chloroform extracts demonstrated significant cytotoxicity to all
three cancer cell lines in a dose-dependent manner, following 24 h
of incubation [MCF-7 (87.64±0.37 µg/ml hexane, P<0.0001;
422.9±0.40 µg/ml chloroform, P<0.0001); MDA-MB-231 (116.5±0.32
µg/ml hexane, P<0.0001; 280.1±3.44 µg/ml chloroform,
P<0.0001); SKOV-3 (86.6±0.48 µg/ml hexane, P<0.0001;
506.5±1.17 µg/ml chloroform, P<0.0001) and MCF-10A cells
(217.2±0.33 µg/ml hexane, P<0.0001; 92.86±0.53 µg/ml chloroform,
P<0.0001)]. The hexane extract was highly cytotoxic to the three
cancer cell lines and less cytotoxic to normal mammary epithelial
cells. By contrast, the chloroform extract was less cytotoxic to
the cancer cell lines and highly cytotoxic to normal cells.
Mangiferin was not cytotoxic to the cancer cell lines investigated
in the present study. Fraction M1 was strongly cytotoxic
to the three cancer cell lines and less cytotoxic to normal cells
(Fig. 1A and B). Among the cancer
cell lines studied, the highest cytotoxic response was observed in
the MDA-MB-231 triple-negative cell line (15.42±0.41 µg/ml).
 | Table III.IC50 values of solvent
extracts of Mangifera zeylanica, mangiferin, paclitaxel and
M1 fraction on MCF-7 and MDA-MB-231 breast cancer cell
lines, SKOV-3 ovarian cancer cell line and MCF-10A normal mammary
epithelial cells. |
Table III.
IC50 values of solvent
extracts of Mangifera zeylanica, mangiferin, paclitaxel and
M1 fraction on MCF-7 and MDA-MB-231 breast cancer cell
lines, SKOV-3 ovarian cancer cell line and MCF-10A normal mammary
epithelial cells.
|
| IC50
valuea |
|---|
|
|
|
|---|
|
Extract/compound | MCF-7
cellsb | MDA-MB-231
cellsb | SKOV-3
cellsb | MCF-10A
cellsc |
|---|
| Hexane extract,
µg/ml | 87.64±0.37 | 116.5±0.32 | 86.6±0.48 | 217.2±0.33 |
| Chloroform extract,
µg/ml | 422.9±0.40 | 280.1±3.44 | 506.5±1.17 | 92.86±0.53 |
| Ethyl acetate
extract, µg/ml | >1000 | >1000 | >1000 | >1000 |
| Methanol extract,
µg/ml | >1000 | >1000 | >1000 | >1000 |
| Mangiferin,
µg/ml | >1000 | >1000 | >1000 | Not assessed |
| Paclitaxel, µM | 0.9959±0.04 | 1.129±0.08 | 0.7807±0.03 | Not assessed |
| M1
fraction, µg/ml | 28.05±0.84 | 15.42±0.41 | 38.66±0.42 | 114.6±0.32 |
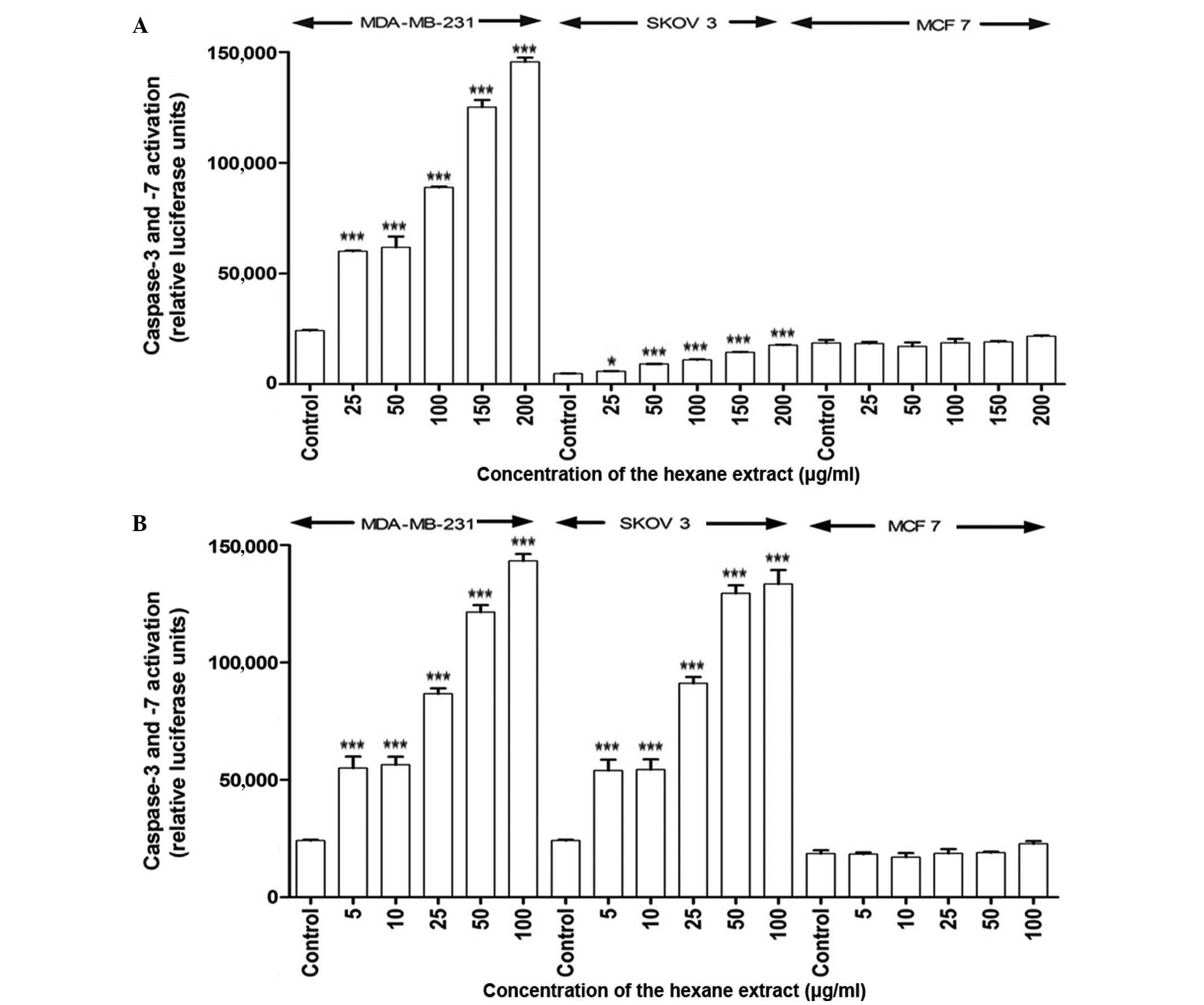
Apoptosis is induced by the hexane
extract of M. zeylanica bark
In response to treatment with the hexane extract,
caspase-3 and −7 activity significantly increased in MDA-MB-231 and
SKOV-3 cells in a time- and dose-dependent manner (P<0.001)
compared with the positive control (ascorbic acid;
EC50=4.2 µg/ml); however, caspase-7 was not activated in
MCF-7 cells at 4 or 24 h post-incubation (Fig. 2). The EC50 values obtained
for the hexane extract indicate that it has free radical scavenging
activity, although its activity is lower than that of ascorbic acid
(values higher than the positive control have a lower activity).
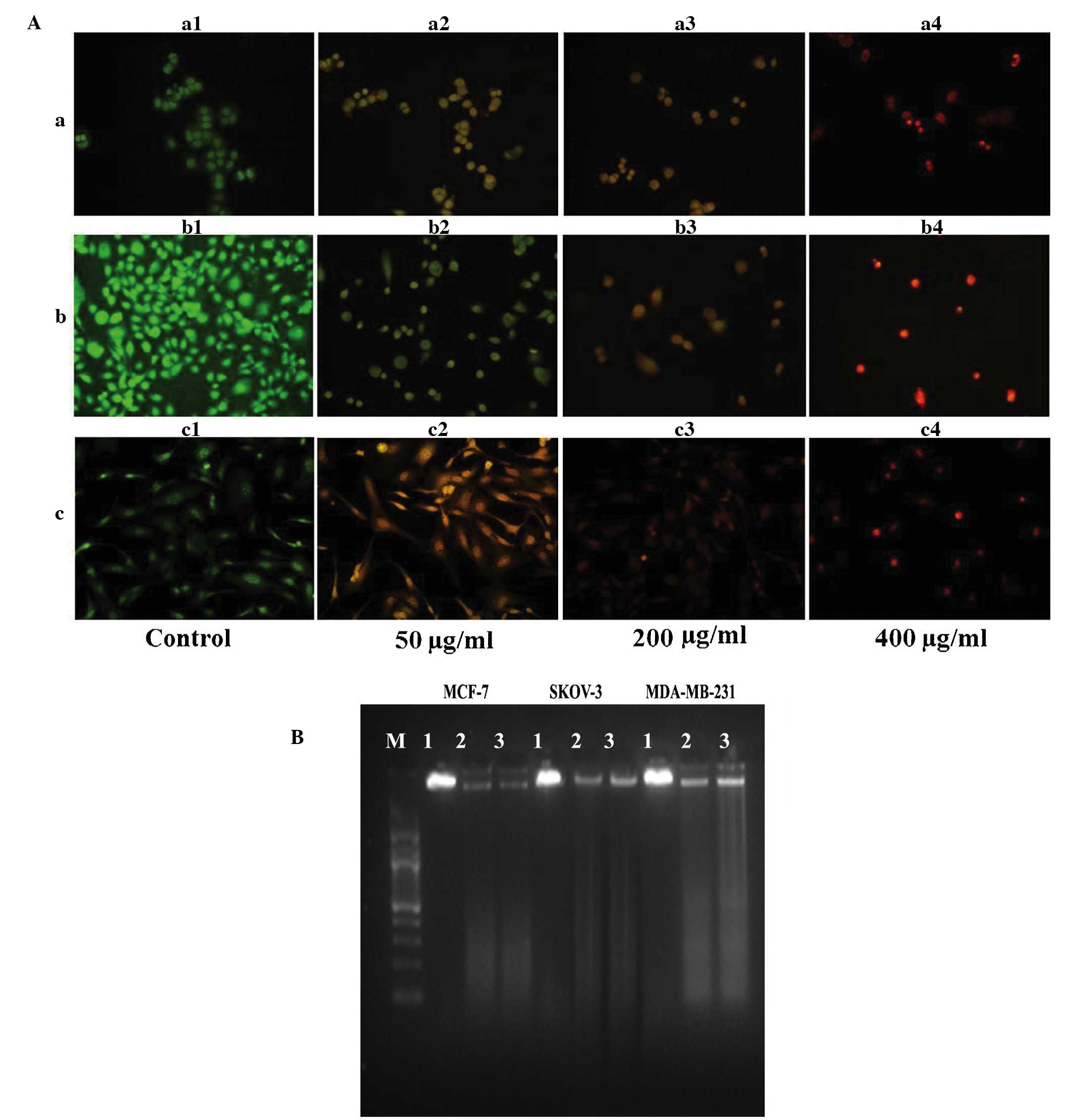
AO/EB staining (Fig. 3A) revealed
primary morphological evidence of apoptosis (including chromatin
condensation, nuclear fragmentation and changes in the size and
shape of cells) in the three cancer cell lines at 24 h
post-incubation. DNA fragmentation, a characteristic of late
apoptosis, was observed in the three cancer cell lines exposed to
the hexane extract for 24 h, with no such evidence observed in
control cells (Fig. 3B).
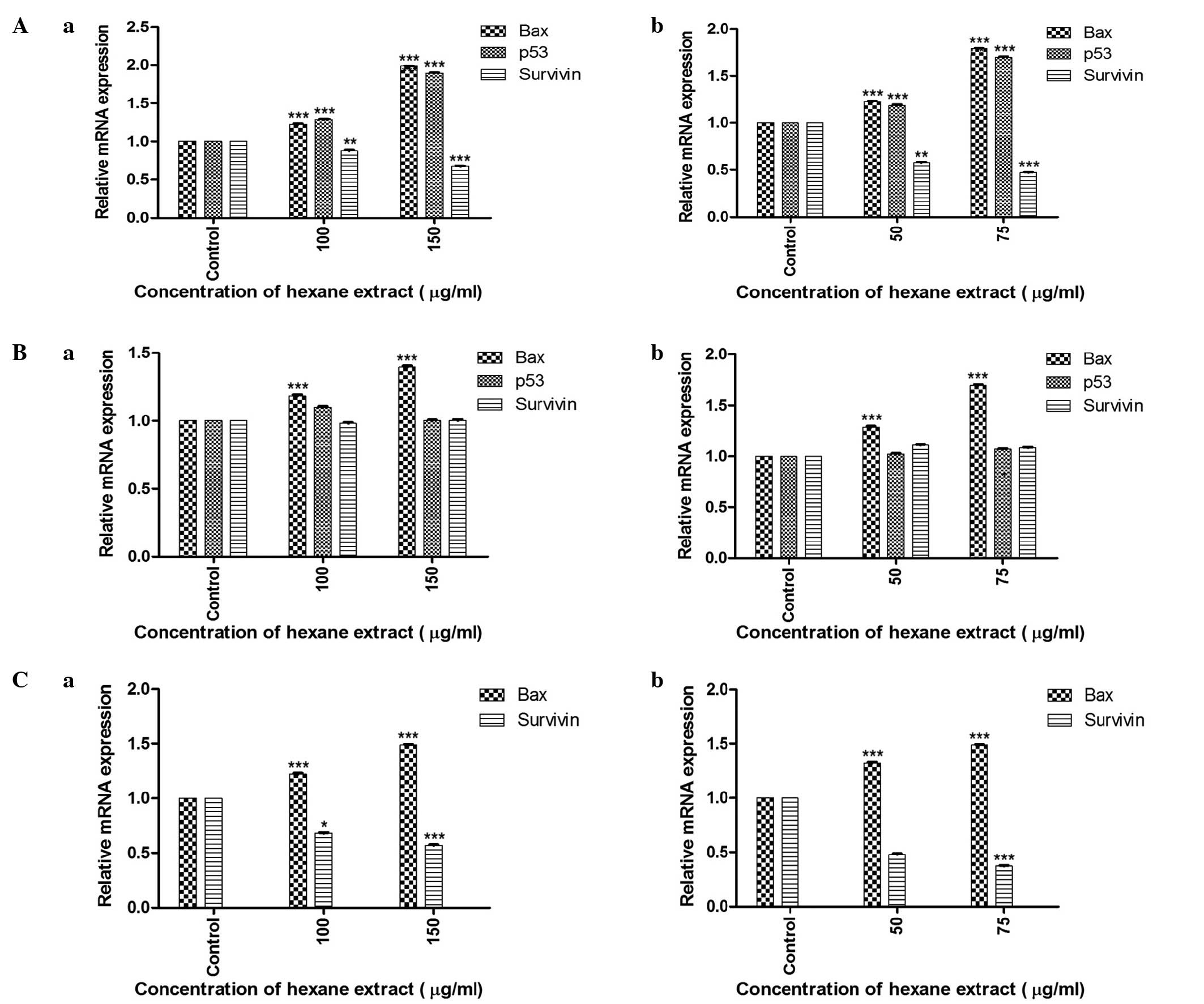
RT-qPCR analysis of p53,
Bcl-2-associated X protein (Bax) and survivin genes reveals
differential expression of various tumor-associated factors
The relative mRNA expression of the genes
investigated in the three cancer cell lines is shown in Fig. 4. RT-qPCR evaluation of cells treated
with the hexane extract of M. zeylanica bark demonstrated that this
extract significantly increased the expression of p53 and Bax mRNA,
and decreased the expression of survivin mRNA in MCF-7 cells. In
MDA-MB-231 cells, Bax expression was increased; however, p53 and
survivin expression were not affected. In SKOV-3 cells,
upregulation of Bax and downregulation of survivin was
observed.
Hexane extract of M. zeylanica bark
demonstrates free radical scavenging activity
The DPPH free radical scavenging assay of the hexane
extract gave an EC50 value of 33.1 µg/ml.
Phytochemical analysis by GC-MS
identifies 11 lipophilic compounds
GC-MS analysis of the hexane extract of M. zeylanica
bark tentatively identified 11 lipophilic compounds. The hexane
extract was rich in sterols and long-chain hydrocarbons.
Compositional analysis of the M1 fraction by GC-MS
revealed that it contained 7 unknown compounds along with a small
number of known compounds (Table
IV).
 | Table IV.Major lipophilic compounds of the
hexane extract and M1 fraction obtained from
bioactivity-guided fractionation of the hexane extract of
Mangifera zeylanica, identified by gas chromatography-mass
spectrometry analysis. |
Table IV.
Major lipophilic compounds of the
hexane extract and M1 fraction obtained from
bioactivity-guided fractionation of the hexane extract of
Mangifera zeylanica, identified by gas chromatography-mass
spectrometry analysis.
| A, Hexane
extract |
|---|
|
|---|
| Retention time,
min | Area, % | Compound name |
|---|
| 6.389 | 0.42 | 3-methyl
heptadecane |
| 11.454 | 5.64 | Hexacosane |
| 13.650 | 0.42 | Campesterol |
| 13.816 | 0.47 | Stigmasterol |
| 14.222 | 2.90 | β-sitosterol |
| 14.224 | 2.90 | γ-sitosterol |
| 14.394 | 1.16 | Lanosterol |
| 14.892 | 6.76 |
9,19-cyclolanost-24-en-3-ol
(cycloartenol) |
| 14.962 | 2.15 | Lanosterol |
| 15.026 | 1.21 | β-amyrin |
| 16.262 | 6.61 |
4,4-dimethyl-2-nonadecyl-5H-1,3-oxazole |
|
| B, M1
fraction |
|
| Retention time,
min | Area, % | Compound name |
|
|
3.845 |
6.25 | Unknown |
|
4.315 |
1.36 | Unknown |
|
4.754 | 19.99 | Unknown |
|
5.883 |
3.49 |
Oleana-2,12-dien-29-oic acid |
|
5.932 |
4.27 | Unknown |
|
6.942 |
1.27 | Unknown |
| 14.283 | 10.12 | Unknown |
| 22.754 |
1.48 |
2-ethylacridine |
| 31.593 |
1.25 |
2-oxo-n-propyl-2-(veratrylidenehydrazino)
acetamide |
Discussion
Of the four organic extracts of M. zeylanica
bark, the percentage yield was lowest for the hexane extract.
However, the hexane extract was selectively cytotoxic to the cancer
cells investigated in the present study and contained secondary
metabolites, including flavonoids, tannins, steroids, reducing
sugars and phenolic compounds, while saponins were absent. The
polyphenol content of the hexane extract was greater than the
flavonoid content.
The cytotoxicity of the hexane extract to
ER-positive (MCF-7) and triple-negative breast cancer cells
(MDA-MB-231), and to ovarian epithelial cells (SKOV-3) was
dose-dependent, and this extract demonstrated reduced cytotoxicity
to normal mammary epithelial cells. By contrast, the chloroform
extract demonstrated reduced cytotoxicity in the cancer cells and
increased cytotoxicity in the normal cells investigated in the
present study. The M1 fraction, obtained from
fractionation of the hexane extract, additionally demonstrated high
levels of cytotoxicity in the three cancer cell lines and reduced
cytotoxicity in normal mammary epithelial cells. Notably, the
highest cytotoxicity was exerted on triple-negative cells.
Mangiferin was not observed to exert cytotoxic effects on any of
the cancer cell lines investigated in the present study.
García-Rivera et al (19)
failed to identify any significant cytotoxicity of mangiferin in
MDA-MB-231 cells. Thus, compound(s) other than mangiferin in M.
zeylanica appear to mediate the cytotoxic and apoptotic effects
observed in the present study.
The processes of homeostasis of organs and tissues
depends upon the vital role of apoptosis, the dysregulation of
which may be observed in cancer (20,21).
Apoptosis involves the sequential activation of a cascade of
proteases, known as caspases. There are two classes of caspase,
initiators and effectors, and the latter class includes caspase-3
and −7 (22). The extrinsic and
intrinsic pathways of apoptosis merge to form a common pathway,
which is mediated by these effector caspases (23).
In the present study, characteristic features of
apoptosis, including activation of caspase-3 and −7 (except in
MCF-7 cells), nuclear fragmentation and chromatin condensation were
clearly observed in the three cancer cell lines in response to
treatment with the hexane extract of M. zeylanica bark.
Activation of caspase-7 was not observed in MCF-7 cells, and these
cells do not express caspase-3. Thus, it is possible that the
hexane extract caused caspase-independent apoptosis in MCF-7 cells
through the intrinsic pathway, potentially via activation of
apoptosis-inducing factor or endonuclease G, which are responsible
for DNA fragmentation (24).
Triple-negative breast cancer cells and ovarian epithelial cancer
cells demonstrated typical activation of caspase-3 and −7 following
exposure to the hexane extract. As the presence of caspase-3 and −7
alone is not able to signify whether the intrinsic or extrinsic
pathway has been activated, additional components require
investigation in order to ascertain the pathways activated.
Bax and p53 genes have significant roles in
apoptosis; increased expression of Bax is known to induce apoptosis
(25), while p53, in addition to
mediating apoptosis, regulates the antiapoptotic gene survivin
(26). In the present study, the
upregulation of Bax and p53, with concomitant downregulation of
survivin, observed in MCF-7 breast cancer cells in response to the
hexane extract suggested that apoptosis in these cells may be
mediated via the intrinsic pathway. Triple-negative breast cancer
cells, which carry a mutant p53, demonstrated upregulation of Bax,
while p53 and survivin expression was not altered in these cells
following treatment with the hexane extract; this suggested that a
p53-independent pathway may mediate apoptosis in these cells. In
the ovarian epithelial cancer cells, which are p53 null,
proapoptotic Bax was upregulated and antiapoptotic survivin was
downregulated. It is likely that a p53-independent pathway, such as
the mitochondria-dependent ‘intrinsic’ cytochrome pathway, is
involved in the mediation of apoptosis in these cells (27). The effect of the hexane extract on the
activation of caspases and on mRNA expression of proapoptotic and
antiapoptotic genes observed in the present study suggested that
M. zeylanica exerts its antiproliferative effects, at least
partly, via apoptosis; however, the underlying mechanism of
apoptosis may differ between the three cancer cell lines
investigated.
Oxidants are able to damage DNA and cause mutations,
which may lead to carcinogenesis, and are additionally able to
stimulate cell division (28).
Antioxidants reduce oxidative damage to DNA and reduce aberrant
increases in cell division (29). The
results of the present study demonstrated that the hexane extract
of M. zeylanica possessed antioxidant ability, as revealed
by the observed free radical scavenging activity.
GC-MS analysis of the hexane extract identified that
it was rich in sterols and long-chain hydrocarbons. β-sitosterol
and β-amyrin detected in the hexane extract have been reported to
be cytotoxic and apoptosis-inducing compounds in MCF-7 breast
cancer cells and HL-60 leukemia cells, respectively (30–32). The
M1 fraction was identified to contain 7 unknown
compounds. It additionally contained a small number of known
compounds that are not cytotoxic. GC-MS profiles of active
fractions gave the present study a strong direction for isolation
of phytochemicals from the hexane extract, which is currently being
investigated in additional studies.
In conclusion, the results of the present study
provide confirmatory evidence for the presence of anticancer
compounds in M. zeylanica, an endemic plant used by
traditional practitioners in Sri Lanka for the treatment of cancer.
Of the two solvent extracts identified to be cytotoxic (hexane and
chloroform extracts), the hexane extract demonstrated a greater
cytotoxicity in the three cancer cell lines and reduced
cytotoxicity in normal mammary epithelial cells. Furthermore, the
hexane extract exerted apoptotic and antioxidant effects. The
greater cytotoxic effect exerted by the active fraction,
particularly on triple-negative cells, warrants additional studies
investigating the anticancer effects of M. zeylanica.
Acknowledgements
The present study was supported by the National
Research Council (Colombo, Sri Lanka; grant no. NRC 11-018).
References
|
1
|
National Breast Cancer Coalition: Ending
Breast Cancer: A Baseline Status Report. 2011 Progress Report
(Washington DC, USA). National Breast Cancer Coalition. 2011.
|
|
2
|
Rochefort H, Glondu M, Sahla ME, Platet N
and Garcia M: How to target estrogen receptor-negative breast
cancer? Endocr Relat Cancer. 10:261–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Humphreys K, Darabai H, Rosin G,
Hannelius U, Heikkinen T, Aittomäki K, Blomqvist C, Pharoah PD,
Dunning AM, et al: A genome-wide association scan on estrogen
receptor-negative breast cancer. Breast Cancer Res. 12:R932010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verma S, Provencher L and Rent R: Emerging
trends in the treatment of triple-negative breast cancer in Canada:
A survey. Curr Oncol. 18:180–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hurvitz SA and Kakkar R: Role of lapatinib
alone or in combination in the treatment of HER-2 positive breast
cancer. Breast Cancer (Dove Med Press). 4:35–51. 2012.PubMed/NCBI
|
|
6
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coates A, Abraham S, Kaye SB, Sowerbutts
T, Frewin C, Fox RM and Tattersall MH: On the receiving end -
patient perception of the side-effects of cancer chemotherapy. Eur
J Cancer Clin Oncol. 19:203–208. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weerarathne WAPG, Samarajeewa PK and
Nilanthi RMR: Genetic diversity of Etamba in Sri Lanka. Trop Agric
Res Ext. 8:107–112. 2005.
|
|
10
|
Herath P, Karunanayake E, Selliah SS and
Wannigama GP: Isolation of mangiferin from the bark of Mangifera
zeylanica. Phytochemistry. 9:11411970. View Article : Google Scholar
|
|
11
|
Gouveia S, Gonçalves J and Castilho PC:
Characterization of phenolic compounds and antioxidant activity of
ethanolic extracts from flowers of Andryala glandulosa ssp. Varia
(Lowe ex DC). R. Fern an Endemic Species of Macaronesia Region.
Indian Crop Prod. 42:573–582. 2013. View Article : Google Scholar
|
|
12
|
Zhishen J, Mengcheng T and Jianming W: The
determination of flavonoid contents in mulberry and their
scavenging effects on superoxide radicals. Food Chem. 64:555–559.
1999. View Article : Google Scholar
|
|
13
|
Kokate CK: Practical Pharmacognosy (4th).
Vallabh Prakashan, New Delhi: 107–111. 2005.
|
|
14
|
Raman N: Phytochemical Techniques (1st).
New Delhi: New India Publishing Agency. 19–24. 2006.
|
|
15
|
Chan EW, Soh EY, Tie PP and Law YP:
Antioxidant and antibacterial properties of green, black, and
herbal teas of Camellia sinensis. Pharmacognosy Res.
3:266–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samarakoon SR, Thabrew I, Galhena PB, De
Silva D and Tennekoon KH: A comparison of the cytotoxic potential
of standardized aqueous and ethanolic extracts of a polyherbal
mixture comprised of Nigella sativa (seeds), Hemidesmus
indicus (roots) and Smilax glabra (rhizome).
Pharmacognosy Res. 2:335–342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ribble D, Goldstein NB, Norris DA and
Shellman YG: A simple technique for quantifying apoptosis in
96-well plates. BMC Biotechnol. 5:122005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
García-Rivera D, Delgado R, Bougarne N,
Haegeman G and Berghe WV: Gallic acid indanone and mangiferin
xanthone are strong determinants of immunosuppressive anti-tumour
effects of Mangifera indica L. bark in MDA-MB231 breast
cancer cells. Cancer Lett. 305:21–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sankari SL, Masthan KM, Babu NA,
Bhattacharjee T and Elumalai M: Apoptosis in cancer - an update.
Asian Pac J Cancer Prev. 13:4873–4878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bates DJ and Lewis LD: Manipulating the
apoptotic pathway: Potential therapeutics for cancer patients. Br J
Clin Pharmacol. 76:381–395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pirnia F, Schneider E, Betticher DC and
Borner MM: Mitomycin C induces apoptosis and caspase-8 and −9
processing through a caspase-3 and Fas-independent pathway. Cell
Death Differ. 9:905–914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bajt ML, Knight TR, Lemasters JJ and
Jaeschke H: Acetaminophen-induced oxidant stress and cell injury in
cultured mouse hepatocytes: Protection by N-acetyl cysteine.
Toxicol Sci. 80:343–349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Findley HW, Gu L, Yeager AM and Zhou M:
Expression and regulation of Bcl-2, Bcl-xl, and Bax correlate with
p53 status and sensitivity to apoptosis in childhood acute
lymphoblastic leukemia. Blood. 89:2986–2993. 1997.PubMed/NCBI
|
|
26
|
Mirza A, McGuirk M, Hockenberry TN, Wu Q,
Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR, et al:
Human survivin is negatively regulated by wild-type p53 and
participates in p53-dependent apoptotic pathway. Oncogene.
21:2613–2622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abeysinghe RD, Greene BT, Haynes R,
Willingham MC, Turner J, Planalp RP, Brechbiel MW, Torti FM and
Torti SV: p53-independent apoptosis mediated by tachpyridine, an
anti-cancer iron chelator. Carcinogenesis. 22:1607–1614. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rahman K: Studies on free radicals,
antioxidants, and co-factors. Clin Interv Aging. 2:219–236.
2007.PubMed/NCBI
|
|
29
|
Ames BN, Shigenaga MK and Hagen TM:
Oxidants, antioxidants, and the degenerative diseases of aging.
Proc Natl Acad Sci USA. 90:7915–7922. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chai JW, Kuppusamy UR and Kanthimathi MS:
Beta-sitosterol induces apoptosis in MCF-7 cells. Malays J Biochem
Mol Biol. 16:28–30. 2008.
|
|
31
|
Barros FW, Bandeira PN, Lima DJ, Meira AS,
de Farias SS, Albuquerque MR, dos Santos HS, Lemos TL, de Morais
MO, Costa-Lotufo LV and Pessoa Cdo Ó: Amyrin esters induce cell
death by apoptosis in HL-60 leukemia cells. Bioorg Med Chem.
19:1268–1276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fabiyi OA, Atolani O, Adeyemi OS and
Olatunji GA: Antioxidant and cytotoxicity of β-Amyrin acetate
fraction from Bridelia ferruginea leaves. Asian Pac J Trop
Biomed. 2(Suppl): S981–S984. 2012. View Article : Google Scholar
|