Introduction
Prostate cancer is the most prevalent malignancy and
the second leading cause of cancer-associated mortality in men in
Western countries (1). Several
autopsy studies have revealed, however, that the majority of
prostate cancers remain latent (2–4). These
small and well-differentiated cancers, termed clinically
insignificant (5), may not affect the
health of patients. If these lesions are detected by
prostate-specific antigen (PSA) screening and an extended biopsy,
it is recommended that the tumours not be treated with radical
treatments or treatments that lead to morbidity (6), but be managed by active surveillance.
Distinguishing these latent cancers from aggressive cancers, which
may spread and develop distant metastasis, remains a critical
issue.
The molecular mechanisms that differentiate cancers
with an invasive phenotype from those that remain latent within the
gland continue to be largely unknown. Numerous factors have been
implicated in the process of prostate cancer invasion and
metastasis, but the initial molecular transforming events that
occur in prostatic epithelial cells and the local microenvironment
remain unknown (7,8). The capacity of tumour cells to migrate
and invade the prostate tissue is crucial in the initial steps of
tumour progression (9).
Ras homolog gene family, member A (RhoA) guanosine
triphosphatase (GTPase) is a well-established regulator of the
dynamic and spatiotemporal rearrangement of the cell cytoskeleton
during cell migration (10). Previous
studies have revealed that RhoA is active at the rear of cells and
promotes tail retraction, and RhoA is also localised at the leading
edge of migrating cells, where it regulates protrusion at the front
of cells (10–12). RhoA promotes the invasive behaviour of
tumour cells through invadopodia and bleb-driven amoeboid invasion
(13–15). This has been demonstrated to be
upregulated and participate in the tumour invasion process of
numerous solid tumours, including ovarian carcinoma (16), ameloblastoma (17) and breast cancer (18).
Although a previous study has indicated that
overexpression of RhoA is present in prostate cancer tissues
(19), the activity of RhoA has been
poorly studied in localised and locally advanced prostate cancer.
Notably, the pattern of RhoA expression has not been evaluated in
distinct tumours. Considering the potential role of RhoA in local
tumour invasion, RhoA expression may differ throughout the prostate
gland.
The aim of the present study was to evaluate and
compare the expression and activity of RhoA in specimens obtained
from radical prostatectomy (RP) performed on patients treated for
localised or locally advanced prostate cancer, and to assess the
value of RhoA as a prognostic marker of aggressiveness in prostate
lesions. Three zones were compared, consisting of the tumour
centre, the tumour front and peritumoural tissue.
Patients and methods
Patients
All human tissues were obtained from the Department
of Surgical Pathology, Cochin Hospital (Paris, France), in
accordance with the ethical policies and procedures of the
Institutional Review Board of Cochin Hospital. All patients
provided informed consent prior to participation in the present
study. Details that may disclose the identity of the enrolled
subjects have been omitted. Tumour tissues were graded according to
the modified Gleason grading system (20). The pathological stage was determined
according to the 2009 tumour-node-metastasis classification for
prostate cancer (21).
In total, 34 patients diagnosed with clinically
localised prostate cancer that had been treated with RP were
enrolled in the present study. PSA relapse was experienced by 16
patients during the median follow-up duration of 52 months.
Clinical data records included the patient age, PSA level,
pathological stage, Gleason score and follow-up times.
Immunohistochemistry
Fresh-cut sections (5-µm) were selected from
formalin-fixed paraffin-embedded tissue blocks that contained
tissue with the highest density of tumour cells and the highest
Gleason scores, termed the index tumour. The sections were
deparaffinised with xylene (Sigma-Aldrich, St. Louis, MO, USA)
twice for 5 min, rehydrated through an alcohol gradient, blocked
with 0.3% H2O2/methanol (Sigma-Aldrich) at
room temperature for 30 min, and heated for 40 min at 96°C in 10 mM
sodium-citrate solution (pH 6.0; Sigma-Aldrich) for antigen
retrieval. The slides were then incubated with goat anti-human
polyclonal anti-RhoA antibody (dilution, 1:50; catalogue number,
SC32039, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight
at 4°C, and were then incubated with biotinylated rabbit anti-goat
secondary antibody (dilution, 1:200; catalogue number, BA-5000;
Vector Laboratories, Burlingame, CA, USA) at room temperature for
60 min. This was followed by incubation with Vectastain Elite ABC
Reagent (catalogue number, PK-7100; Vector Laboratories) for 30
min. Peroxidase activity was examined using 3,3′-diaminobenzidine
(catalogue number, D4418; Sigma-Aldrich). The sections were
counterstained using hemalum and mounted using Permount (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Two pathologists independently scored the tissue
staining for RhoA. The central region, which was fully composed of
cancerous glands, was defined as the tumour centre. The edges of
the tumour foci were termed the tumour front. Peritumoural benign
prostate glands that did not exhibit intraepithelial prostatic
neoplasia and were located on the same slide acted as the control,
and were termed the peritumoural prostatic tissues. A
semi-quantitative ordinal and categorical method (22) was used to evaluate staining across
each region. The expression of RhoA was graded as follows: 0, no
staining; 1, weak staining; 2, moderate staining; or 3, strong
staining.
Western blot analysis
A total of 20 tissue samples were obtained from 11
patients that had undergone RP for the treatment of clinically
localised or locally advanced prostate cancer. Immediately
subsequent to surgical removal, small sections of tissue were
dissected, snap-frozen and stored in liquid nitrogen. Histological
analysis of the frozen sections was then performed. The percentage
of tumour cells in each sample was evaluated. In total, 12 samples
contained 50–90% cancerous cells and were labelled as tumour
tissues, and 8 samples containing no cancerous tissue were termed
peritumoural prostatic tissue. These samples were then subjected to
western blot analysis for RhoA expression and G-LISA analysis for
RhoA activity. The proteins were extracted on ice with cold RIPA
lysis buffer (catalogue number, 9806; Cell Signaling Technology,
Inc., Danvers, MA, USA) containing a protease and phosphatase
inhibitor (catalogue number, 88669; Thermo Fisher Scientific,
Inc.).
Lysates were centrifuged at 12,000 × g for 20 min at
4°C, and the supernatant was collected. Total protein
concentrations were determined using a bicinchoninic acid assay
(BCA) protein-assay kit (catalogue number, 23250; Thermo Fisher
Scientific, Inc.). Equal quantities of protein were subjected to
12% SDS-PAGE and then electrically transferred onto nitrocellulose
membranes (Hybond-C; Amersham Biosciences, Uppsala, Sweden). The
membranes were blocked for 1 h with 5% (w/v) non-fat milk in
phosphate-buffered saline with 0.1% (v/v) Tween-20 (PBST;
Sigma-Aldrich), and incubated with polyclonal goat anti-human RhoA
(dilution, 1:250; catalogue number, SC32039; Santa Cruz
Biotechnology, Inc.) and monoclonal mouse anti-human β-actin
(dilution, 1:500; catalogue number, SC47778; Santa Cruz
Biotechnology, Inc.) primary antibodies overnight at 4°C. Finally,
the membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibodies, consisting of the polyclonal
rabbit anti-goat immunoglobulin G (IgG)-HRP antibody (dilution,
1:5,000; catalogue number, SC2768; Santa Cruz Biotechnology, Inc.)
and polyclonal goat anti-mouse IgG-HRP antibody (dilution, 1:5,000;
catalogue number, SC2005; Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature.
Subsequent to washing three times with PBST, the
proteins were visualised using an enhanced chemiluminescence Prime
Western Blotting Detection kit (GE Healthcare Bio-Sciences,
Pittsburgh, PA, USA). Images of the protein bands were captured
using a digital imaging system (Image Quant LAS; GE Healthcare
Bio-Sciences), and densitometric measurements of band intensity in
the western blots were performed using ImageJ software (National
Institutes of Health, Bethesda, MA, USA). The results are
representative of three independent experiments.
G-LISA analysis
RhoA activity was assayed using a G-LISA RhoA
Activation Assay Biochem kit (cat. no. BK124; Cytoskeleton, Inc.,
Denver, CO, USA), according to the manufacturer's instructions.
Briefly, the prostate tissue samples were homogenised in ice-cold
lysis buffer with a protease-inhibitor cocktail, and then
centrifuged at 450 × g at 4°C for 1 min. The supernatants were
harvested and protein concentrations were measured using the
Precision Red Advanced Protein Assay Reagent (catalogue number,
ADV02; Cytoskeleton, Inc.) and were finally equalised with ice-cold
lysis buffer to 1.0 mg/ml. Equalised prostate-tissue protein
extractions were transferred to a Rho-GTP-binding protein
pre-coated plate (Cytoskeleton, Inc.). The plate was placed on an
orbital microplate shaker (SSM1; Bibby Scientific Limited Group,
Staffordshire, UK) at 0.72 × g for 30 min at 4°C, and then
incubated with monoclonal mouse anti-human anti-RhoA primary
antibody (cat. no. GL01A; 1:250; Cytoskeleton, Inc.), followed by a
polyclonal goat anti-mouse horseradish-conjugated secondary
antibody (cat. no. GL02; 1:62.5; Cytoskeleton, Inc.), on an orbital
microplate shaker (SSM1; Bibby Scientific Limited Group) at 0.72 ×
g at room temperature, for 45 min each. The plate was then
incubated with the HRP detection reagent at 37°C for 15 min.
Subsequent to the addition of HRP stop buffer, absorbance was read
at 490 nm using an ELx808 Absorbance Microplate Reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Statistical analysis
Comparisons between data were performed using the
χ2 test with a Yates continuity correction for
independent samples, and the McNemar test was conducted for paired
samples. Continuous data from two independent groups were compared
using the Mann-Whitney U test. All tests performed were two tailed.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
The clinicopathological features of the study
population are summarised in Table I.
A total of 34 patients were included in the IHC cohort and 11
patients were included in the WB and G-LISA cohort. The median age,
median PSA level, pathological stages and Gleason scores in the two
cohorts were comparable (Table I).
The patients in the IHC cohort were followed up for a median
duration of 52 months. A total of 16 (47.1%) patients in the IHC
cohort exhibited PSA relapses.
 | Table I.Demographics of patients with prostate
cancer and the characteristics of tissue specimens assessed by IHC
or WB and G-LISA. |
Table I.
Demographics of patients with prostate
cancer and the characteristics of tissue specimens assessed by IHC
or WB and G-LISA.
|
| Cohort, n (%) |
|---|
|
|
|
|---|
| Characteristics | IHC | WB and G-LISA |
|---|
| Total | 34
(100.0) | 11
(100) |
| Median age, years
(range) | 62 (50–73) | 61 (49–71) |
| Median PSA level,
ng/ml (range) | 8.66 (2.5–30) | 9.2 (4.4–30) |
| Tumour stage |
|
|
| T2 | 17 (50.0) | 6
(55) |
| T3 | 17 (50.0) | 5
(45) |
| Gleason score |
|
|
| 3+3 | 15 (44.0) | 5
(45) |
| 3+4 | 11 (32.0) | 3
(27) |
| 4+3 | 6
(18.0) | 2
(18) |
| 4+4 | 2 (6.0) | 1 (9) |
| Median follow-up
period, months (range) | 52 (7–112) | N/A |
| PSA relapse | 16 (47.1) | N/A |
Expression and activity of RhoA in
prostate tissues
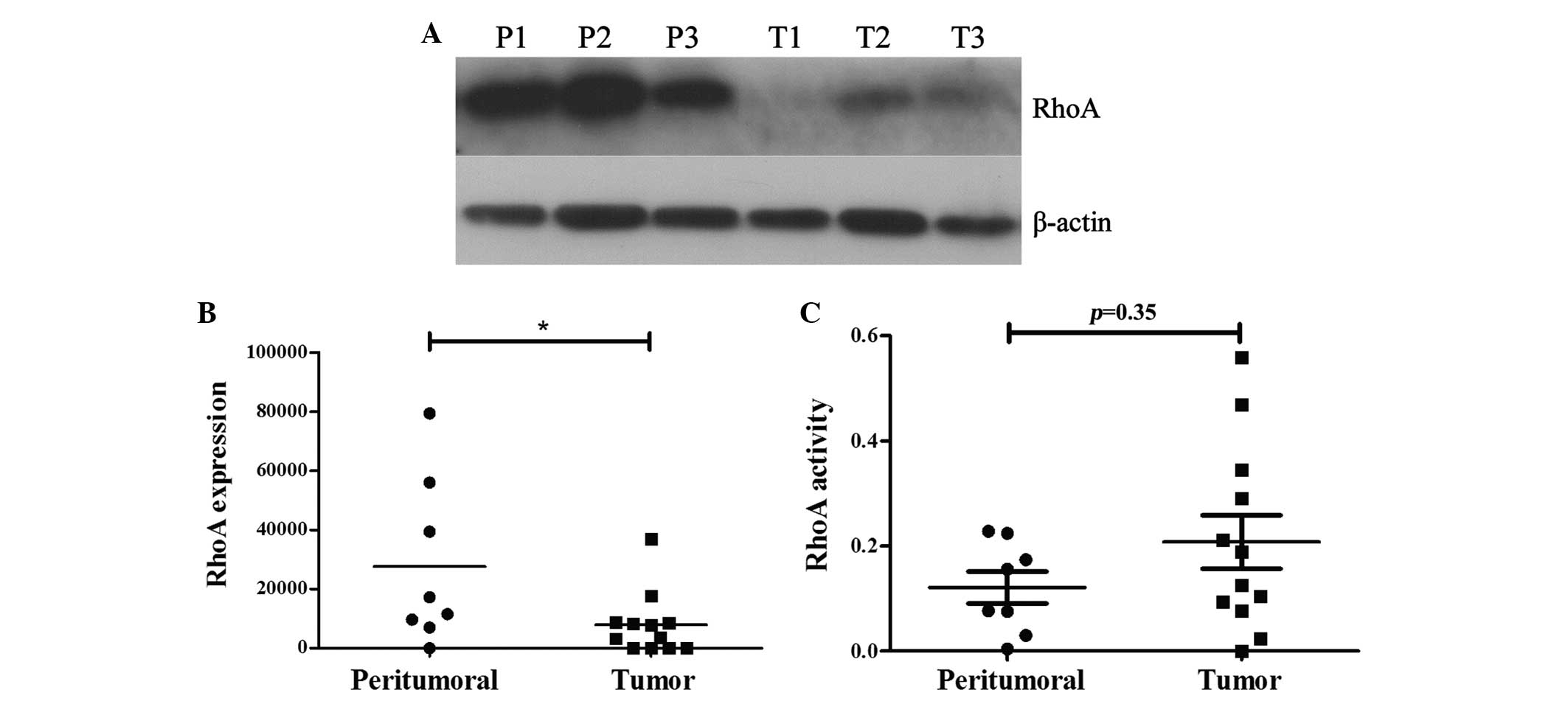
RhoA expression was significantly decreased in
prostate cancer tissue specimens compared with the peritumoural
tissue specimens (P<0.05; Fig. 1A and
B). As the activation of RhoA is a prerequisite for the
execution of its effects, the activity of RhoA was evaluated by
G-LISA in the same tissue samples. Although RhoA activity was
increased in cancer tissues (optical density, 0.20) compared with
non-cancer tissues (optical density, 0.12), the difference was not
statistically significant (P=0.35).
Expression patterns of RhoA in
cancerous prostate glands
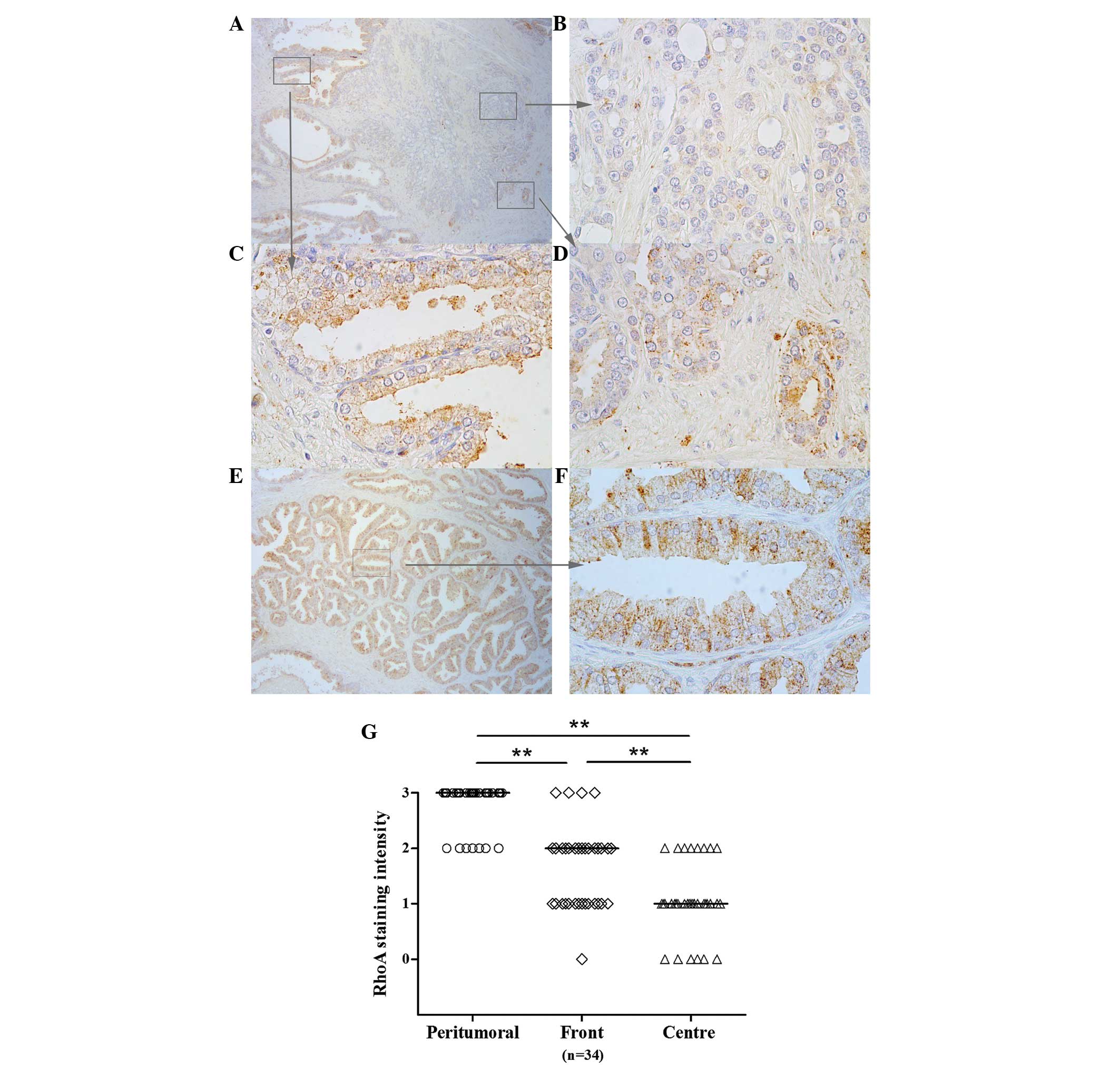
To further evaluate the patterns of RhoA expression
in prostate tissue specimens, immunohistochemistry was performed in
34 paraffin-embedded tissue specimens excised by RP. Analysis of
the intensity of staining enabled the detection of various patterns
of expression throughout the tissues. In the distant peritumoural
tissue, immunohistological analysis revealed moderate or strong
staining for RhoA expression in all tissues (Fig. 2). In the tumour centre, RhoA
expression was significantly decreased, with moderate staining
observed in only 8 tissues (23%; P<0.001). Strong staining was
not observed in this region in any of the specimens. At the tumour
front, staining was moderate or strong in 19 tissues (56%), which
was significantly increased compared with the incidence of moderate
or strong staining in the centre of tumours (P<0.01; Fig. 2).
Association betweeen RhoA expression
and adverse oncological features and outcomes
In the prostate cancer tissue specimens, RhoA
expression was significantly increased in high-grade tumours that
demonstrated a Gleason score >3+4, regardless of the location of
the tumour cells, such as the tumour centre or front (Fig. 3A). Subsequent to a median follow-up
duration of 52 months, 62.5% of the patients that experienced PSA
relapse exhibited increased expression of RhoA at the tumour front
compared with the expression in the tumour centre, while only 35%
of patients without PSA relapse demonstrated increased RhoA
expression at the tumour front (Fig.
3B; P=0.09). No association was identified between the RhoA
expression or PSA level prior to surgery and the pathological stage
of tumours.
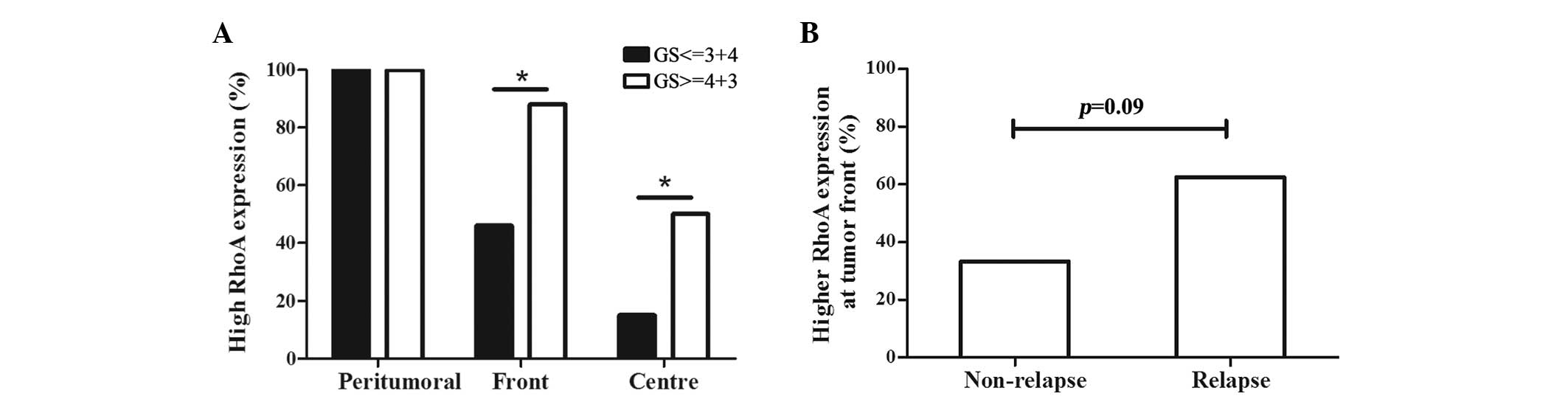 | Figure 3.Association between the GS, PSA
relapse and RhoA expression. (A) In the tumour centre and front,
patients with poor tumor differentiation demonstrated a
significantly increased expression of RhoA, as indicated by a GS of
4+3 or higher and a percentage of RhoA staining score ≥2 (tumour
centre, 15 vs. 50%, P=0.044; tumour front, 46 vs. 88%, P=0.039).
*P<0.05, high-grade differentiation vs. low-grade
differentiation. (B) Patients that developed relapse possessed
increased RhoA expression in tumour fronts compared to those that
did not relapse. However, this difference was not statistically
significant (62.5 vs. 35.0%; P=0.089). RhoA, Ras homolog gene
family, member A; PSA, prostate-specific antigen; GS, Gleason
score. |
Discussion
To the best of our knowledge, the present study is
the first to evaluate RhoA expression not only in the centre of
tumours, but also at the tumour front and in peritumoural tissues.
The current study identified significantly increased RhoA
expression at the tumour front compared with the tumour centre.
RhoA has previously been reported to be involved in
prostate cancer invasion. Hodge et al reported increased
expression of RhoA in highly invasive variants of PC-3 prostate
cancer cells compared with minimally invasive variants (23). Neuropeptide-stimulated migration in
prostate-cancer cells has been revealed to be mediated by RhoA
(24). RhoA has also been reported to
induce migration towards monocyte chemoattractant protein 1 in PC-3
cells (25). Several inhibitors of
the migration of prostate cancer cells, such as transmembrane
protein with epidermal growth factor-like and two follistatin-like
domains 2 (26), microRNA-34a
(27), exchange protein directly
activated by cyclic adenosine monophosphate (28), FTY720 (29) and WIN55,21–2 (30), have been reported to inhibit RhoA
activity in prostate cancer cell lines.
The microenvironment of a tumour is highly complex
and varies between locations (31).
Several studies have addressed this issue and demonstrated that the
tumour front and tumour centre exhibit different characteristics,
resulting in different behaviours (32,33). In
colorectal carcinomas, Cianchi et al revealed that cells at
the invasive front exhibit more aggressive behaviour compared to
cells within the central regions of tumours (32).
The present study indicates that prostate cancer
cells at the tumour front demonstrate a more aggressive phenotype,
and express specific and different features compared with cells at
the centre of the tumour. Chemokine (C-X-C motif) receptor 4
(CXCR4), which is implicated in tumour invasion through the
extracellular matrix, is specifically expressed at the tumour front
of prostate tumours, whereas the expression of CXCR4 at the centre
of tumours is low (33). The present
results indicate that RhoA expression in the centre of the tumour
is low. The current findings also clearly highlight the importance
of investigating the tumour front and the surrounding peritumoural
tissue prior to undertaking large assays, to avoid biased
interpretations.
As RhoA is implicated in cancer-cell invasion, it
was hypothesized that cancer cells expressing RhoA at the tumour
front may demonstrate increased mobility and aggressiveness
(10). This hypothesis is also
supported by the present finding that the probability of PSA
relapse subsequent to surgery was increased in patients with high
RhoA expression at tumour fronts, although this was not
statistically significant. The present results also reveal that
high RhoA expression was associated with high-grade prostate cancer
in the tumour centre and the tumour front, indicating that poorly
differentiated tumours were more likely to express RhoA, which may
also facilitate tissue invasion.
Although a previous study by Schmidt et al
has suggested that the expression of RhoA in benign prostate glands
is decreased (19), the present study
revealed opposite findings. In the study by Schmidt et al,
the authors used biopsy material from 91 patients with localised
prostate cancer. This study observed decreased expression of RhoA
in benign regions compared with cancerous regions. However, the
true location of these benign regions may not be accurately
identified on biopsy materials. The benign regions may have been
located close to tumour foci that were not sampled by the biopsy.
It is also challenging to determine whether the tumoural tissue
sampled by the biopsy was located at the tumour front or the
centre. Notably, significantly increased RhoA expression was
identified in the distant peritumoural region, whereas the activity
of RhoA, as determined by G-LISA, was decreased in these regions
compared with tumoural areas. As RhoA is a ubiquitous protein with
numerous functions, the present results may indicate that the
expression and activity of RhoA in the distant peritumoural region
was not associated with the presence of cancer.
RhoA is also involved in secretory granule
trafficking and exocytosis (34,35), and
may execute these functions in the epithelial cells of distant
tissues. Although RhoA expression was decreased in the tumour
cells, the increased activity of RhoA may consolidate the
hypothesis that RhoA performs a specific role in prostate cancer
progression. As RhoA targets several downstream effectors, with
Rho-associated, coiled-coil containing protein kinase 1 and 2 being
the most important, a comparison between the specific activities of
these effectors within the various tissue locations may improve the
present understanding of the RhoA pathways and the implications of
these pathways on prostate cancer progression.
The present study has several limitations, the most
important being the small sample size, which may have biased the
statistical analysis. A larger cohort is required to reliably test
the association between RhoA expression and other adverse clinical
and pathological features. Another limitation of the current study
is the absence of whole gland analysis. Only the expression in the
index tumour was focused on, and contiguous or distant tumours may
not have exhibited the same profile of RhoA expression. Additional
analyses should be conducted on more samples to assess the
downstream effectors for the whole prostatic gland.
In conclusion, the present study has identified
increased RhoA expression in prostate tumour fronts and has
determined associations between increased RhoA expression and
cancer relapse, increased RhoA expression and increased Gleason
score. This indicates an association between RhoA expression and
aggressiveness of the prostate cancer. The present findings may
provide a foundation for novel therapeutic approaches that may
inhibit the clinical aggressiveness of prostate cancer.
Acknowledgements
The authors would like to thank Dr T. Hua-Huy
(Service de Physiologie, Cochin Hospital, Paris, France) for aiding
with the present study and comments on the manuscript. This study
was supported by the Program of International Science &
Technology Cooperation (grant no. 2012DFG31440), awarded by the
Ministry of Science and Technology, People's Republic of China. The
authors thank NewMed Publishing Services for providing pro
bono final editing services.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haas GP, Delongchamps NB, Jones RF,
Chandan V, Serio AM, Vickers AJ, Jumbelic M, Threatte G, Korets R,
Lilja H and de la Roza G: Needle biopsies on autopsy prostates:
Sensitivity of cancer detection based on true prevalence. J Natl
Cancer Inst. 99:1484–1489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Breslow N, Chan CW, Dhom G, Drury RA,
Franks LM, Gellei B, Lee YS, Lundberg S, Sparke B, Sternby NH and
Tulinius H: Latent carcinoma of prostate at autopsy in seven areas.
The International Agency for Research on Cancer, Lyons, France. Int
J Cancer. 20:680–688. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thompson IM: Latent carcinoma of the
prostate. Eur Urol. 39(Suppl 4): S41–S42. 2001. View Article : Google Scholar
|
|
5
|
Stamey TA, Yemoto CM, McNeal JE, Sigal BM
and Johnstone IM: Prostate cancer is highly predictable: A
prognostic equation based on all morphological variables in radical
prostatectomy specimens. J Urol. 163:1155–1160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gulati R, Wever EM, Tsodikov A, Penson DF,
Inoue LY, Katcher J, Lee SY, Heijnsdijk EA, Draisma G, de Koning HJ
and Etzioni R: What if i don't treat my PSA-detected prostate
cancer? Answers from three natural history models. Cancer Epidemiol
Biomarkers Prev. 20:740–750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Putzke AP, Ventura AP, Bailey AM, Akture
C, Opoku-Ansah J, Celiktaş M, Hwang MS, Darling DS, Coleman IM,
Nelson PS, et al: Metastatic progression of prostate cancer and
e-cadherin regulation by zeb1 and SRC family kinases. Am J Pathol.
179:400–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomita K, van Bokhoven A, van Leenders GJ,
Ruijter ET, Jansen CF, Bussemakers MJ and Schalken P: Cadherin
switching in human prostate cancer progression. Cancer Res.
60:3650–3654. 2000.PubMed/NCBI
|
|
9
|
Gotzmann J, Mikula M, Eger A,
Schulte-Hermann R, Foisner R, Beug H and Mikulits W: Molecular
aspects of epithelial cell plasticity: Implications for local tumor
invasion and metastasis. Mutat Res. 566:9–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pertz O, Hodgson L, Klemke RL and Hahn KM:
Spatiotemporal dynamics of RhoA activity in migrating cells.
Nature. 440:1069–1072. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Machacek M, Hodgson L, Welch C, Elliott H,
Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM and Danuser G:
Coordination of Rho GTPase activities during cell protrusion.
Nature. 461:99–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurokawa K and Matsuda M: Localized RhoA
activation as a requirement for the induction of membrane ruffling.
Mol Biol Cell. 16:4294–4303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakurai-Yageta M, Recchi C, Le Dez G,
Sibarita JB, Daviet L, Camonis J, D'Souza-Schorey C and Chavrier P:
The interaction of IQGAP1 with the exocyst complex is required for
tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol.
181:985–998. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hotary K, Li XY, Allen E, Stevens SL and
Weiss SJ: A cancer cell metalloprotease triad regulates the
basement membrane transmigration program. Genes Dev. 20:2673–2686.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berton S, Belletti B, Wolf K, Canzonieri
V, Lovat F, Vecchione A, Colombatti A, Friedl P and Baldassarre G:
The tumor suppressor functions of p27(kip1) include control of the
mesenchymal/amoeboid transition. Mol Cell Biol. 29:5031–5045. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horiuchi A, Imai T, Wang C, Ohira S, Feng
Y, Nikaido T and Konishi I: Up-regulation of small GTPases, RhoA
and RhoC, is associated with tumor progression in ovarian
carcinoma. Lab Invest. 83:861–870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Modolo F, Biz MT, de Sousa SM, Fachinelli
Rde L and Crema VO: Immunohistochemical expression of Rho GTPases
in ameloblastomas. J Oral Pathol Med. 41:400–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fritz G, Brachetti C, Bahlmann F, Schmidt
M and Kaina B: Rho GTPases in human breast tumours: Expression and
mutation analyses and correlation with clinical parameters. Br J
Cancer. 87:635–644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmidt LJ, Duncan K, Yadav N, Regan KM,
Verone AR, Lohse CM, Pop EA, Attwood K, Wilding G, Mohler JL, et
al: RhoA as a mediator of clinically relevant androgen action in
prostate cancer cells. Mol Endocrinol. 26:716–735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL: ISUP Grading Committee: The 2005 International Society
of Urological Pathology (ISUP) Consensus Conference on Gleason
Grading of Prostatic Carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobin LH, Gospodarowicz MK and Wittekind
C: Prostate. TNM Classification of Malignant Tumors (7th).
(Hoboken, NJ). 243–248. 2009.
|
|
22
|
Taylor CR and Levenson RM: Quantification
of immunohistochemistry - issues concerning methods, utility and
semiquantitative assessment II. Histopathology. 49:411–424. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hodge JC, Bub J, Kaul S, Kajdacsy-Balla A
and Lindholm PF: Requirement of RhoA activity for increased nuclear
factor kappaB activity and PC-3 human prostate cancer cell
invasion. Cancer Res. 63:1359–1364. 2003.PubMed/NCBI
|
|
24
|
Zheng R, Iwase A, Shen R, Goodman OB Jr,
Sugimoto N, Takuwa Y and Lerner DJ: Neuropeptide-stimulated cell
migration in prostate cancer cells is mediated by RhoA kinase
signaling and inhibited by neutral endopeptidase. Oncogene.
25:5942–5952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loberg RD, Tantivejkul K, Craig M, Neeley
CK and Pienta KJ: PAR1-mediated RhoA activation facilitates
CCL2-induced chemotaxis in PC-3 cells. J Cell Biochem.
101:1292–1300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Corbin JM, Tipton GJ, Yang LV,
Asch AS and Ruiz-Echevarría MJ: The TMEFF2 tumor suppressor
modulates integrin expression, RhoA activation and migration of
prostate cancer cells. Biochim Biophys Acta. 1843:1216–1224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamamura S, Saini S, Majid S, Hirata H,
Ueno K, Deng G and Dahiya R: MicroRNA-34a modulates c-Myc
transcriptional complexes to suppress malignancy in human prostate
cancer cells. PLoS One. 7:e297222012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grandoch M, Rose A, ter Braak M,
Jendrossek V, Rübben H, Fischer JW, Schmidt M and Weber P: Epac
inhibits migration and proliferation of human prostate carcinoma
cells. Br J Cancer. 101:2038–2042. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou C, Ling MT, Lee Kin-Wah T, Man K,
Wang X and Wong YC: FTY720, a fungus metabolite, inhibits invasion
ability of androgen-independent prostate cancer cells through
inactivation of RhoA-GTPase. Cancer Lett. 233:36–47. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nithipatikom K, Gomez-Granados AD, Tang
AT, Pfeiffer AW, Williams CL and Campbell WB: Cannabinoid receptor
type 1 (CB1) activation inhibits small GTPase RhoA activity and
regulates motility of prostate carcinoma cells. Endocrinology.
153:29–41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Joyce JA: Therapeutic targeting of the
tumor microenvironment. Cancer Cell. 7:513–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cianchi F, Cuzzocrea S, Vinci MC,
Messerini L, Comin CE, Navarra G, Perigli G, Centorrino T, Marzocco
S, Lenzi E, et al: Heterogeneous expression of cyclooxygenase-2 and
inducible nitric oxide synthase within colorectal tumors:
Correlation with tumor angiogenesis. Dig Liver Dis. 42:20–27. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Delongchamps NB, Beuvon F, Mathieu JR,
Delmas S, Metzger I, Prats H and Cabon F: CXCR4 is highly expressed
at the tumor front but not in the center of prostate cancers. World
J Urol. 33:281–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gasman S, Chasserot-Golaz S, Hubert P,
Aunis D and Bader MF: Identification of a potential effector
pathway for the trimeric Go protein associated with secretory
granules. Go stimulates a granule-bound phosphatidylinositol
4-kinase by activating RhoA in chromaffin cells. J Biol Chem.
273:16913–16920. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frantz C, Coppola T and Regazzi R:
Involvement of Rho GTPases and their effectors in the secretory
process of PC12 cells. Exp Cell Res. 273:119–126. 2002. View Article : Google Scholar : PubMed/NCBI
|