Introduction
Myoferlin protein is associated with cell membrane
repair (1). Cancer cells demonstrate
an increased proliferative rate compared with normal cells. As the
cell membrane is a vital organelle, there is an increased
occurrence of membrane repair events in cancer cells compared with
normal cells (1). Therefore,
myoferlin may possess a significant role in tumorigenesis.
The ferlin family of proteins, which contains
myoferlin, is a mammalian protein family named due to its members'
homology with the Fer-1 protein of Caenorhabditis elegans
(2). A defective Fer-1 gene
results in infertility, due to abnormal membrane fusion processes
during the development of sperm (3).
The ferlin family of proteins possesses multiple C2 domains, and is
able to anchor to the cell membrane using a carboxyl terminal. C2
domains participate in protein-protein or protein-membrane
interactions (4); they are calcium
sensing, and mediate membrane fusion, repair and vesicle
trafficking in skeletal muscles. The ferlin family contains six
members: Dysferlin, myoferlin, otoferlin, Fer-1-like 4, Fer-1-like
5 and Fer-1-like 6 (1,4).
Myoferlin has been well-studied in muscle cells due
to its correlation with musculopathy. During the normal embryonic
development of muscle or the regeneration of mature muscle cells
following injury, myoblasts possessing a single nucleus fuse and
form large syncytial myofibers. Myoferlin is highly expressed
during myoblast fusion, although the specific function of myoferlin
in this process remains to be elucidated (5). In endothelial cells, myoferlin regulates
angiogenesis, which is associated with vascular endothelial growth
factor receptor-2 (VEGFR-2) expression (6,7).
Myoferlin expression by cancer cells has received
attention in a number of studies. The MDA-MB-231 breast cancer cell
line exhibits high myoferlin expression and is frequently used in
studies of myoferlin in cancer. In vitro, depletion of
myoferlin induces a mesenchymal to epithelial transition and
reduces cancer cell invasiveness (8,9). Although
the mechanism via which myoferlin impacts breast cancer cell
invasiveness remains to be elucidated, a number of studies have
suggested that matrix metalloproteinases may possess a significant
role in the myoferlin-mediated regulation of invasion (10). In vivo, myoferlin-depleted
breast cancer cells demonstrate reduced cellular proliferation, are
smaller and form less invasive tumors (1,9).
Furthermore, myoferlin is hypothesized to be a significant
modulator of epidermal growth factor receptor (EGFR) expression in
breast cancer cells (11). In
pancreatic cancer, patients exhibiting myoferlin-expressing tumors
possess relatively poor clinical outcomes (12).
To the best of our knowledge, the expression of
myoferlin in lung cancer surgical specimens has not been previously
investigated. A recent study concerning myoferlin expression in
normal lung parenchyma and bronchial epithelium stimulated the
present study to investigate whether myoferlin is expressed in
non-small cell lung cancer (NSCLC) (13).
The aim of the present study was to elucidate the
clinicopathological characteristics of myoferlin expression in
NSCLC.
Materials and methods
Case selection
Data from primary NSCLC patients treated at
Gyeongsang National University Hospital (Jinju, South Korea) was
collected between January 2002 and December 2009. A total of 148
patients were enrolled in the present study. The clinical data and
survival period of all patients were obtained by reviewing clinical
charts and National Statistical Office of Korea (Seoul, South
Korea) records. Current smokers and ex-smokers were included in the
positive-smoking history group. All of the included patients were
clinically operable; pneumonectomies, lobectomies or sleeve
lobectomies were performed. The tumor-node-metastasis (TNM) stage
of each patient was assessed using the American Joint Committee on
Cancer Staging Manual, 7th edition (14).
Gross images and hematoxylin and eosin (HE)-stained
sections of surgical specimens were reviewed, and the tumor
pathological characteristics were described by two pathologists.
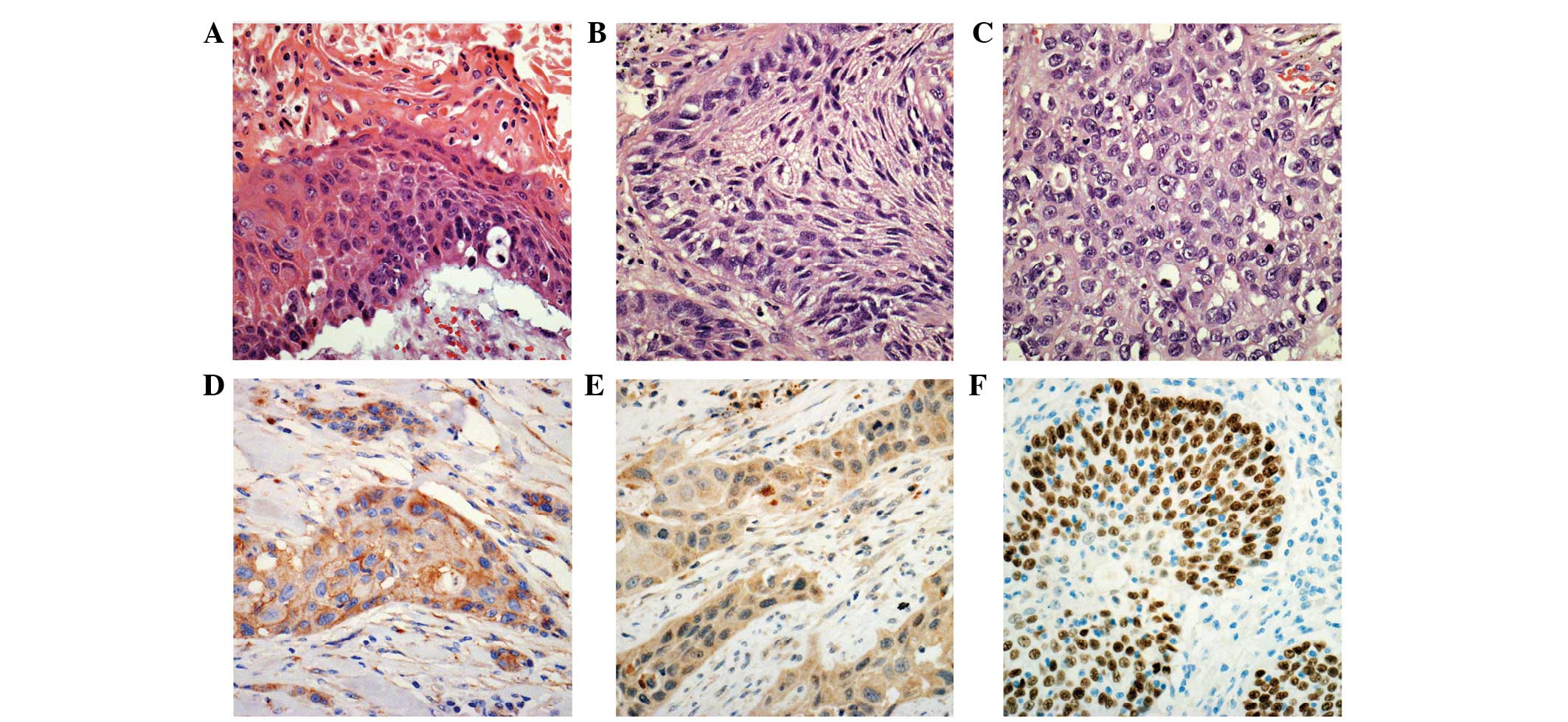
The degree of differentiation in squamous cell carcinoma was
classified, using a three-tiered system, as either well-,
moderately- or poorly-differentiated. Well-differentiated squamous
cell carcinomas maintained the characteristic morphology of
squamous cells, including keratinization, a plump cytoplasm and
distinct cellular borders with intercellular bridges.
Poorly-differentiated squamous cell carcinomas did not exhibit
these histological features (Fig. 1).
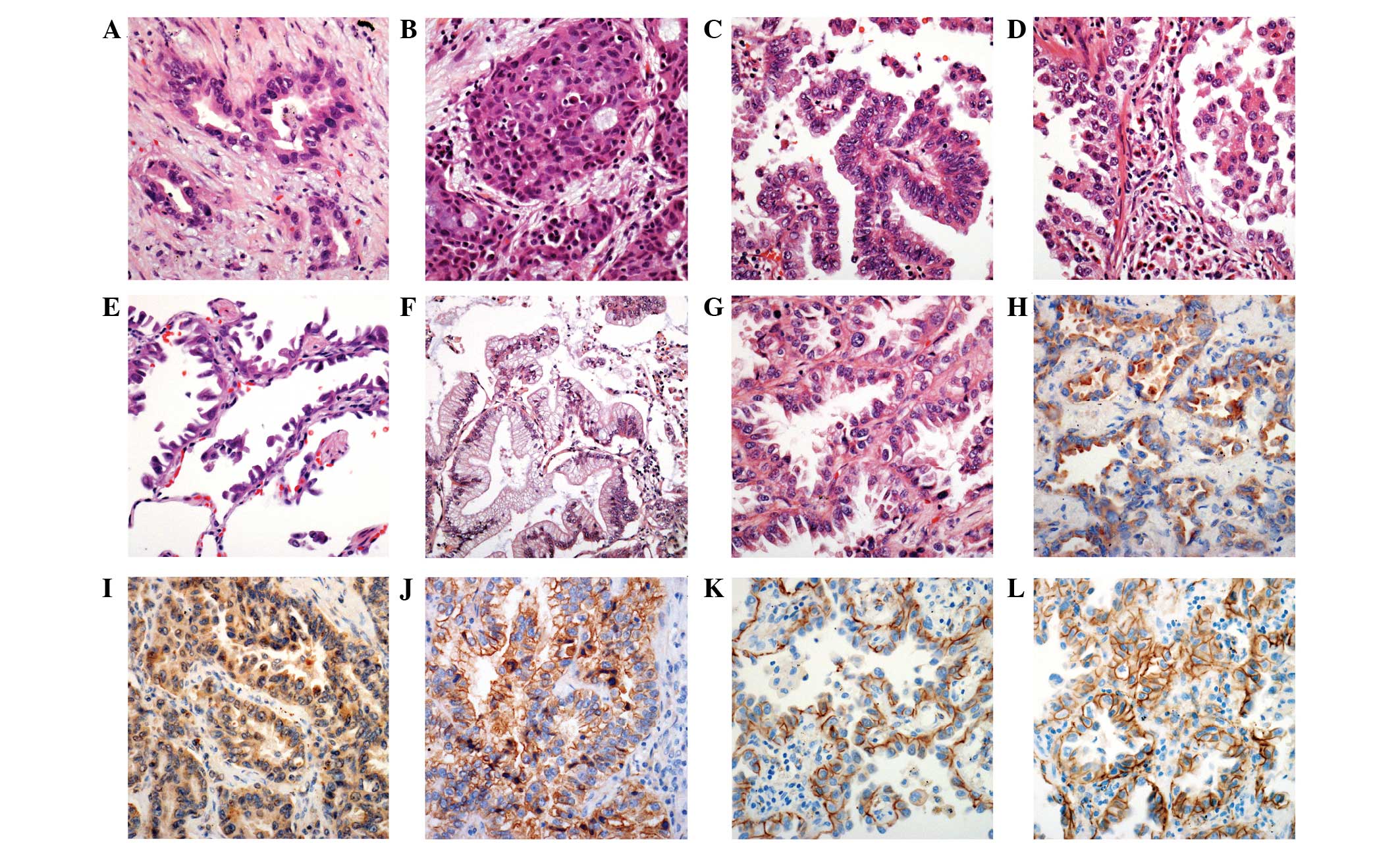
The histological characteristics of adenocarcinoma were described
using the classification system of the International Association
for the Study of Lung Cancer/American Thoracic Society/European
Respiratory Society (15,16). The nuclear grade of adenocarcinomas
was defined, using a two-tiered system, as either high- or
low-grade. High-grade adenocarcinomas demonstrated atypical nuclei,
macronucleoli and a coarse chromatin pattern in >10% of the
tumor cells on the whole slide. Low-grade adenocarcinomas
demonstrated relatively regular-sized and -shaped nuclei, and
evenly distributed chromatin in almost all tumor cells. The
Institutional Review Board of Gyeongsang National University
Hospital (Jinju, Korea) approved the present study.
Tissue microarray (TMA)
Resected tumor samples were fixed overnight in 20%
buffered neutral formalin. The samples were grossly examined,
dissected and embedded in paraffin blocks. Representative blocks
were selected following microscopic examination of HE-stained
sections from each tumor (BX-51, light microscope, Olympus
Corporation, Tokyo, Japan). A 3-mm representative core of tissue
was obtained from each paraffin block and arranged in new recipient
TMA paraffin blocks. A representative area of the donor blocks was
selected based on its major differentiation and location near the
invasive front.
Immunohistochemical (IHC)
analysis
Immunohistochemistry was performed on the TMA block
sections for 7 distinct proteins. Primary antibodies for myoferlin,
VEGFR-2, EGFR, E-cadherin, β-catenin, tumor protein p63 and thyroid
transcription factor-1 (TTF-1) were used to investigate protein
expression, and the association between the expression of certain
proteins was examined. VEGFR-2 is a well-known drug target for the
prevention of angiogenesis, and its expression has been
demonstrated to correlate with myoferlin expression in endothelial
cells (6). The expression of EGFR,
E-cadherin and β-catenin in cancer cells is known to be associated
with patient prognosis (17,18). TTF-1 and p63 are generally known as
specific markers for pulmonary adenocarcinoma and squamous cell
carcinoma (19).
IHC staining was performed on 4-µm sections from the
TMA blocks. Once attached to glass slides, the sections were
deparaffinized, rehydrated and incubated in 3% hydrogen peroxide
for 10 min in order to block endogenous peroxidase activity, which
may cause non-specific background staining. Sections were
subsequently heated for 20 min in 10 mM citrate buffer (pH 6.0) in
a microwave oven (700 W). Following incubation with Ultra V Block
(Lab Vision; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for
7 min at room temperature in order to block background staining,
slides were incubated with a mouse monoclonal primary antibody
specific to myoferlin (1:100 dilution; 7D2; ab76746; Abcam,
Cambridge, UK) according to the manufacturer's protocols. The
compound 3,3′-diaminobenzidine was utilized in order to detect the
proteins, and the sections were counterstained using hematoxylin.
IHC staining was additionally performed using the same protocol
with primary antibodies for VEGFR-2 (1:200 dilution; rabbit
polyclonal; PA5-16487; Thermo Fisher Scientific, Waltham, MA, USA),
EGFR (1:100 dilution; mouse monoclonal; 3C6; 790–2988; Ventana
Medical Systems, Inc., Tuscon, AZ, USA), E-cadherin (1:200
dilution; rabbit monoclonal; EP700Y; MA5-14458; Thermo Fisher
Scientific, Inc.), β-catenin (1:400 dilution; mouse monoclonal;
E-5; sc-7963; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), p63
(1:50 dilution; mouse monoclonal; 4A4; Ventana Medical Systems,
Inc.), and TTF-1 (1:1,500 dilution; rabbit monoclonal; SP141;
790–4756; Ventana Medical Systems, Inc.). The positive control used
for myoferlin comparison was normal bronchiolar epithelium; tissues
with equal to or more intense staining compared with normal
bronchiolar epithelium were regarded as positive. For EGFR, tissue
with equivocal expression was classified as negative, and focal or
diffuse membrane staining was classified as positive for myoferlin
expression. Almost all EGFR-positive specimens demonstrated
cytoplasmic staining. In addition, VEGFR-2 was expressed in the
cytoplasm. Tissues demonstrating β-catenin or E-cadherin expression
in the cellular membrane were regarded as demonstrating positive
expression.
Western blot analysis
Fresh tumor samples were obtained from the central
potion of the mass, in an area that appeared to be homogeneous and
was neither necrotic nor fibrotic. The western blot analysis method
was used as previously described to determine myoferlin and VEGFR-2
expression (20).
Statistical analysis
The overall survival of patients with NSCLC was
compared using univariate and multivariate Cox proportional hazard
model analyses. The analysis of correlations was performed using
χ2 tests. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL,
USA).
Results
Clinicopathological features of 148
NSCLC patients
The clinicopathological features of the 148 NSCLC
patients are summarized in Table I.
Of these patients, 125 were male and 95 possessed a history of
smoking (current or ex-smoker). The mean patient age was 64.85
years. The majority of the patients (83/148; 56.1%) were evaluated
as TNM stage I following surgery, while 51 (34.5%), 12 (8.1%), and
2 (1.4%) patients were evaluated as TNM stages II, III and IV,
respectively. A lobectomy was performed in 130 (87.8%) patients. A
bilobectomy or sleeve lobectomy was performed in 3 (2.0%) patients,
and a pneumonectomy was performed in 15 (10.1%) patients. Squamous
cell carcinoma accounted for 96 (64.9%) of the total NSCLC cases,
59 of which demonstrated moderate differentiation (Fig. 1A–C). A total of 15 and 22 patients
exhibited well- and poorly-differentiated squamous cell carcinomas,
respectively. Adenocarcinomas accounted for 37 (25.0%) of the total
NSCLC cases. Acinar, solid, papillary, micropapillary, lepidic and
mucinous growth patterns were observed in 15, 6, 8, 3, 3 and 2
patients, respectively (Fig. 2A–G).
Large cell neuroendocrine carcinomas were observed in 8 of the
NSCLC patients. Pleomorphic and mucoepidermoid carcinomas were
observed in 6 and 1 patient(s), respectively. The median survival
time was 37 months and the 5-year survival rate was 22.3%.
 | Table I.Clinicopathogical features of 148
non-small cell lung cancer patients. |
Table I.
Clinicopathogical features of 148
non-small cell lung cancer patients.
| Clinicopathological
feature | Value |
|---|
| Male gender, n
(%) | 125 (84.5) |
| Smoking history, n
(%) | 97
(65.5) |
| Mean age, years | 64.85 |
| Tumor-node-metastasis
stage, n (%) |
|
| I | 83
(56.1) |
| II | 51
(34.5) |
| III | 12 (8.1) |
| IV | 2
(1.4) |
| Surgical procedure, n
(%) |
|
|
Lobectomy | 130 (87.8) |
|
Bilobectomy or sleeve
lobectomy | 3
(2.0) |
|
Pneumonectomy | 15
(10.1) |
| Histological type,
n (%) |
|
|
Squamous cell carcinoma | 96
(64.9) |
|
Well-differentiated | 15 |
|
Moderately-differentiated | 59 |
|
Poorly-differentiated | 22 |
|
Adenocarcinoma | 37
(25.0) |
|
Acinar | 15 |
|
Solid | 6 |
|
Papillary | 8 |
|
Micropapillary | 3 |
|
Lepidic | 3 |
|
Mucinous | 2 |
| Large
cell neuroendocrine carcinoma | 8
(5.4) |
|
Other | 7
(4.7) |
| Median survival,
months | 37 |
| Five-year survival
rate, n (%) | 33
(22.3) |
| Myoferlin
expression, n/total n (%) |
|
|
Squamous cell carcinoma | 37/96
(38.5) |
|
Adenocarcinoma | 31/37
(83.8) |
| Large
cell neuroendocrine carcinoma | 3/8
(37.5) |
|
Pleomorphic carcinoma | 3/6
(50.0) |
|
Mucoepidermoid carcinoma | 1/1
(100.0) |
|
Total | 75/148
(50.7) |
Myoferlin is expressed in NSCLC
The expression of myoferlin in each pathological
subtype is summarized in Table I.
Myoferlin expression in the cytoplasm of cancer cells was observed
in 75/148 NSCLC cases. Myoferlin expression in the cytoplasm of
cancer cells was observed in 37/96 (38.5%) squamous cell carcinoma
cases (Fig. 1D). Normal bronchial
epithelium, lung parenchyma and a number of capillary endothelial
cells surrounding the cancer cells additionally expressed
cytoplasmic myoferlin. Myoferlin protein expression was observed in
the cytoplasm in 31/37 (83.8%) adenocarcinomas cases (Fig. 2H). Myoferlin expression was
additionally observed in 3/8 large cell neuroendocrine carcinoma, 3
pleomorphic carcinoma and 1 mucoepidermoid carcinoma case(s).
Differential expression of 7 distinct
proteins in NSCLC cells
The expression of myoferlin, VEGFR-2, E-cadherin,
β-catenin, EGFR, TTF-1 and p63 in the cancer cells was examined and
is summarized in Table II (Fig. 1 and 2).
Initially, the expression of each protein in association with TNM
stage was investigated; however, no statistically significant
correlations were observed. Myoferlin-positive cancer cells were
more abundant in adenocarcinoma cases compared with squamous cell
carcinoma cases (P<0.001). A larger proportion of
VEGFR-2-positive cancers were adenocarcinoma cases (P<0.001).
E-cadherin and β-catenin were more frequently expressed in
adenocarcinoma compared with squamous cell carcinoma (P=0.002 and
P=0.001, respectively). EGFR demonstrated increased expression in
squamous cell carcinoma compared with adenocarcinoma (P=0.040).
VEGFR-2 expression was predominantly cytoplasmic; however,
capillary endothelial cells demonstrated only weak VEGFR-2
cytoplasmic staining. EGFR, β-catenin and E-cadherin were
predominantly expressed in the cellular membrane, although focal
cytoplasmic expression was additionally observed. There was a
significant correlation between p63 expression and squamous cell
carcinoma differentiation (P=0.009). A total of 69/73 patients with
well- or moderately-differentiated squamous cell carcinoma
exhibited p63 expression in IHC. A total of 16/22 patients with
poorly-differentiated squamous cell carcinoma demonstrated p63
expression. The nuclear grade of the adenocarcinoma did not exhibit
a significant correlation with any of the proteins investigated in
the present study.
 | Table II.Clinicopathological features of 148
non-small cell lung carcinoma patients. |
Table II.
Clinicopathological features of 148
non-small cell lung carcinoma patients.
|
| Myoferlin | VEGFR-2 | E-cadherin | β-catenin | EGFR | TTF-1 | p63 |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Factor | + | − | P-value | + | − | P-value | + | − | P-value | + | − | P-value | + | − | P-value | + | − | P-value | + | − | P-value |
|---|
| Stage |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| I | 44 | 39 | 0.632 | 39 | 44 | 0.558 | 64 | 19 | 0.391 | 70 | 13 | 0.424 | 29 | 54 | 0.721 | 25 | 58 | 0.643 | 51 | 32 | 0.876 |
| II | 25 | 26 |
| 18 | 33 |
| 37 | 14 |
| 39 | 12 |
| 19 | 32 |
| 6 | 45 |
| 31 | 20 |
|
|
III | 4 | 8 |
| 4 | 8 |
| 7 | 5 |
| 9 | 3 |
| 4 | 8 |
| 3 | 9 |
| 10 | 2 |
|
| IV | 2 | 0 |
| 2 | 0 |
| 2 | 0 |
| 2 | 0 |
| 0 | 2 |
| 2 | 0 |
| 0 | 2 |
|
| Path type |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sqcc | 37 | 59 | <0.0001 | 28 | 68 | <0.0001 | 67 | 29 | 0.002 | 74 | 22 | 0.001 | 39 | 57 | 0.040 | 4 | 92 | <0.0001 | 86 | 10 | <0.0001 |
|
Adc | 31 | 6 |
| 28 | 9 |
| 35 | 2 |
| 37 | 0 |
| 8 | 29 |
| 28 | 9 |
| 3 | 34 |
|
Correlations exist between the
expression of specific proteins in 2 major histological subtypes:
Squamous cell carcinoma and adenocarcinoma
Squamous cell carcinoma and adenocarcinoma accounted
for 89.9% of the NSCLC cases in the present study. There were 133
patients with squamous cell carcinoma or adenocarcinoma, and
correlations between protein expression and tumor stage or
pathological type were investigated (Table III). VEGFR-2, TTF-1 and p63
expression were significantly correlated with myoferlin expression
(P<0.001, P<0.001 and P=0.006, respectively). Although the
expression of all 7 investigated proteins was significantly
correlated with the histological subtypes of squamous cell
carcinoma and adenocarcinoma (Table
III), E-cadherin, β-catenin and EGFR expression was only weakly
correlated with myoferlin expression. (P=0.114, P=0.726 and
P=0.461, respectively). VEGFR-2, TTF-1 and p63 expression was
strongly correlated with myoferlin expression in low-stage tumors.
In squamous cell carcinomas, a highly significant correlation
between myoferlin and VEGFR-2 expression was identified
(P=0.001).
 | Table III.Correlation between expression of
myoferlin and other proteins in 133 squamous cell carcinoma and
adenocarcinoma patients. |
Table III.
Correlation between expression of
myoferlin and other proteins in 133 squamous cell carcinoma and
adenocarcinoma patients.
|
|
| VEGFR-2 | E-cadherin | β-catenin | EGFR | TTF-1 | p63 |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Factor | Myoferlin | + | − | P-value | + | − | P-value | + | − | P-value | + | − | P-value | + | − | P-value | + | − | P-value |
|---|
| Sqcc+Adc | + | 43 | 25 | 0.000 | 56 | 46 | 0.114 | 56 | 12 | 0.726 | 22 | 46 | 0.461 | 25 | 43 | 0.000 | 38 | 30 | 0.006 |
|
| − | 13 | 52 |
| 12 | 19 |
| 55 | 10 |
| 25 | 40 |
| 7 | 58 |
| 51 | 14 |
|
| Stage of
Sqcc+Adc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| I | + | 28 | 13 | 0.000 | 35 | 6 | 0.057 | 36 | 5 | 0.525 | 13 | 28 | 0.882 | 18 | 23 | 0.008 | 22 | 19 | 0.024 |
|
| − | 6 | 27 |
| 22 | 11 |
| 27 | 6 |
| 11 | 22 |
| 5 | 28 |
| 26 | 7 |
|
| II | + | 11 | 10 | 0.220 | 16 | 5 | 1.000 | 16 | 5 | 0.439 | 6 | 15 | 0.108 | 5 | 16 | 0.015 | 12 | 9 | 0.174 |
|
| − | 5 | 20 |
| 20 | 5 |
| 22 | 3 |
| 13 | 12 |
| 0 | 25 |
| 19 | 6 |
|
|
III | + | 2 | 2 | 0.576 | 3 | 1 | 1.000 | 2 | 2 | 0.491 | 3 | 1 | 0.088 | 0 | 4 | 0.491 | 4 | 0 | 1.000 |
|
| − | 2 | 5 |
| 4 | 3 |
| 6 | 1 |
| 1 | 3 |
| 2 | 5 |
| 6 | 1 |
|
| Path type |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sqcc | + | 18 | 19 | 0.001 | 27 | 10 | 0.591 | 25 | 12 | 0.079 | 16 | 21 | 0.679 | 2 | 35 | 0.638 | 35 | 2 | 0.308 |
|
| − | 10 | 49 |
| 40 | 19 |
| 49 | 10 |
| 23 | 36 |
| 2 | 57 |
| 51 | 8 |
|
|
Adc | + | 25 | 6 | 0.140 | 29 | 2 | 1.000 | 31 | 0 | N/I | 6 | 25 | 0.591 | 23 | 8 | 1.000 | 3 | 28 | 1.000 |
|
| − | 3 | 3 |
| 6 | 0 |
| 6 | 0 |
| 2 | 4 |
| 5 | 1 |
| 0 | 6 |
|
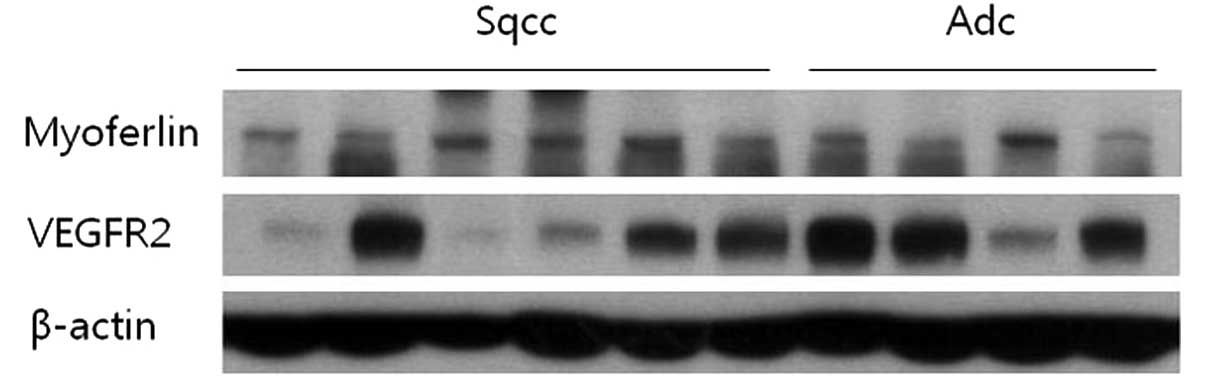
Western blot analysis shows myoferlin
and VEGFR-2 expression
Specimens from 6 patients with squamous cell
carcinoma and 4 patients with adenocarcinoma were analyzed using
western blotting. All specimens demonstrated myoferlin expression
via immunohistochemical staining. Upon western blotting, myoferlin
protein was detected in all cases. In addition, VEGFR-2 protein was
identified in several cases (Fig.
3).
Survival analysis of squamous cell
carcinoma
As revealed in Table
IV, univariate analysis of squamous cell carcinoma patients
indicated that p63 expression and pathological differentiation
possessed relatively high odds ratios of 1.908 [95% confidence
interval (CI), 0.804–4.528; P=0.143] and 2.010 (95% CI, .074–3.762;
P=0.029), respectively. Myoferlin and VEGFR-2 possessed odds ratios
of 1.221 (95% CI, 0.672–2.221; P=0.512) and 1.219 (95% CI,
0.644–2.306; P=0.542), respectively. Using multivariate analysis,
the parameter of stage demonstrated an odds ratio of 1.765 (95% CI,
0.908–3.429; P=0.094), procedure had an odds ratio of 1.487 (95%
CI, 0.684–3.232; P=0.316), pathologic differentiation had an odds
ratio of 1.561 (95% CI, 0.730–3.337; P=0.250) and loss of p63
expression demonstrated an odds ratio of 1.680 (95% CI,
0.541–5.219; P=0.370).
 | Table IV.Cox proportional hazard model
analysis of squamous cell carcinoma and adenocarcinoma
patients. |
Table IV.
Cox proportional hazard model
analysis of squamous cell carcinoma and adenocarcinoma
patients.
|
| Sqcc | Adc |
|---|
|
|
|
|
|---|
| Analysis | OR | P-value | OR | P-value |
|---|
| Univariate |
|
|
|
|
|
Myoferlin, negative vs.
positive | 1.221 | 0.512 | 1.556 | 0.677 |
| VEGFR2,
negavite vs. positive | 1.219 | 0.542 | 0.682 | 0.589 |
|
β-catenin, positive vs.
negative | 0.919 | 0.821 | N/A | N/A |
|
E-cadherin, positive vs.
negative | 1.139 | 0.695 | N/A | N/A |
| EGFR,
negative vs. positive | 0.871 | 0.656 | 0.347 | 0.320 |
| p63,
positive vs. negative | 1.908 | 0.143 | N/A | N/A |
| TTF-1,
positive vs. negative | N/A | N/A | 1.560 | 0.530 |
|
Differentiation of Sqcc, M/D
and W/D vs. P/D | 2.010 | 0.029 | N/A | N/A |
| Pattern
of Adc, others vs. solid and micropapillary | N/A | N/A | 3.111 | 0.092 |
| Nuclear
grade of Adc, low vs. high | N/A | N/A | 0.771 | 0.714 |
| Median
age, <67 vs. ≥67 in Sqcc; <65 vs. ≥65 in Adc | 1.080 | 0.842 | 2.912 | 0.286 |
| Smoking
history, non-smoker vs. ex- or current | 0.579 | 0.114 | 3.155 | 0.188 |
| Stage,
<IIa vs. ≥IIb | 1.765 | 0.094 | 6.721 | 0.057 |
|
Procedure, L vs. P, bi and
sleeve | 1.487 | 0.316 |
N/Aa |
N/Aa |
|
Differentiation of Sqcc, M/D
and W/D vs. P/D | 1.561 | 0.250 | N/A | N/A |
| Multivariate |
|
|
|
|
| Pattern
of Adc, others vs. solid and micropapillary | N/A | N/A | 1.639 | 0.570 |
|
Myoferlin, negative vs.
positive | 1.028 | 0.938 | 2.942 | 0.339 |
| EGFR,
negative vs. positive | 0.990 | 0.978 | 0.248 | 0.298 |
| VEGFR2,
negative vs. positive | 1.101 | 0.833 | 0.145 | 0.055 |
|
E-cadherin, positive vs.
negative | 1.252 | 0.541 | N/A | N/A |
| p63,
positive vs. negative | 1.680 | 0.370 | N/A | N/A |
| TTF-1,
positive vs. negative | N/A | N/A | 0.642 | 0.608 |
Survival analysis of
adenocarcinoma
As shown in Table IV,
univariate analysis of adenocarcinoma patients indicated that a
pathological group of solid and micropapillary growth patterns
possessed relatively high odds ratios of 3.111 (95% CI,
0.832–11.633; P=0.092). The parameter of EGFR expression had an
odds ratio of 0.347 (95% CI, 0.043–2.793; P=0.320). Myoferlin,
VEGFR-2 and TTF-1 expression possessed odds ratios of 1.556 (95%
CI, 0.194–12.472; P=0.677), 0.682 (95% CI, 0.170–2.735; P=0.589)
and 1.560 (95% CI, 0.390–6.251; P=0.530), respectively. Upon
multivariate analysis, VEGFR-2 expression demonstrated an odds
ratio of 0.145 (95% CI, 0.020–1.046; P=0.055). EGFR expression,
myoferlin expression and pathological pattern possessed odds ratios
of 0.248 (95% CI, 0.018–3.429; P=0.298), 2.942 (95% CI,
0.321–26.930; P=0.339) and 1.639 (95% CI, 0.298–9.006; P=0.570),
respectively.
Discussion
Myoferlin expression was identified in 75/148 NSCLC
patients. All NSCLC pathological subtypes contained
myoferlin-positive tumors. Adenocarcinomas possessed the largest
proportion of myoferlin-expressing tumors. Immunohistochemistry
indicated that myoferlin protein was localized to the cytoplasm of
the tumor cells. Leung et al (1,13)
previously described cytoplasmic expression of myoferlin in human
airway epithelium and mouse Lewis lung carcinoma (LCC) cells. In
airway epithelial cells, myoferlin expression was detected in the
cytoplasm, cell membrane and Golgi membrane using confocal
microscopy, immunofluorescent staining and immunohistochemical
staining. In LCC cells, cytoplasmic expression of myoferlin was
detected by studying immunofluorescence. In the present study,
normal bronchial epithelial cells and NSCLC cells demonstrated
similar localization of expressed protein. Dislocation of myoferlin
protein, from the cell membrane to the cytoplasm, requires further
investigation in order to specify the pathophysiological function
of myoferlin.
The present study identified that adenocarcinoma
patients with myoferlin protein expression possess poorer prognoses
(odds ratio, 2.942; P=0.339) upon multivariate analysis. However,
the P-value was not <0.05, therefore this was not a
statistically significant difference. However, there was a higher
odds ratio in the myoferlin-expressing group compared with the
group comprising solid and micropapillary pattern tumors upon
multivariate analysis. Pulmonary adenocarcinoma with a
micropapillary and solid pattern is a well-known indicator of a
poor prognosis, therefore, further evaluation of these factors may
be required in a larger study (21).
In a previous study, Sun et al (22) reported clinically relevant mutations
associated with pulmonary adenocarcinoma in 10 genes, EGFR,
tumor protein p53, KRAS, ribosomal protein S6 kinase β-2,
Ataxin-2, DHX9, tyrosine-protein phosphatase non-receptor
type 13, specificity protein 1*, spectrin α non-erythrocytic 1 and
myoferlin (MYOF) using sequencing analysis. Mutation of the
MYOF gene in lung adenocarcinoma was detected upon exome and
messenger RNA sequencing analysis. As the list of genes identified
by Sun et al (22) included
EGFR and KRAS, which are clinicopathologically
significant mutations in pulmonary adenocarcinomas, further
molecular study of the MYOF gene may be required.
Another notable result of the present study is the
statistically significant correlation identified between myoferlin
and VEGFR-2 expression in adenocarcinoma and squamous cell
carcinoma (P<0.001). This correlation was more pronounced in
stage I patients (P<0.001) compared with stage II (P=0.220) or
stage III (P=0.576) patients; as stage increased, the correlation
became less significant. The correlation between myoferlin and
VEGFR-2 expression demonstrated increased significance in squamous
cell carcinoma (P=0.001) compared with adenocarcinoma (P=0.140). Yu
et al (6) and Bernatchez et
al (7) have previously reported
that the physiological function of myoferlin is to regulate VEGFR-2
stability and activity; loss of myoferlin reduces the expression
and autophosphorylation of VEGFR-2 in endothelial cells. These
functions additionally require the endothelial cell-specific
tyrosine kinase receptors, dynamin-2 and tyrosine kinase with
immunoglobulin and epidermal growth factor homology domains-2.
However, to the best of our knowledge, only two studies have
discussed the physiological functions of myoferlin with respect to
VEGFR-2 (6,7). Although myoferlin is relatively well
studied in skeletal muscle due to its role in musculopathy, its
precise physiological functions remain to be fully elucidated. The
function of myoferlin in tumor cells is also unclear. To the best
of our knowledge, the present study is the first to reveal a marked
correlation between myoferlin and VEGFR-2 expression in vivo
in tumor cells.
Angiogenic factors of cancer, VEGF and VEGFR, are
well-established drug targets. Bevacizumab is a monoclonal antibody
of VEGF (19). It was the first
angiogenic inhibitor to be identified. In patients exhibiting
squamous cell carcinoma, bevacizumab induced the side effect of
hemoptysis (23). However, more
recent clinical trials investigating VEGFR-2-targeted therapy
demonstrated a relatively positive performance, without induction
of hemoptysis, in patients exhibiting NSCLC containing squamous
cell carcinoma (24). In addition,
small molecule inhibitors of receptor tyrosine kinases, which work
with VEGFR-2, nintedanib, sunitinib, sorafenib, vandetanib and
vatalanib, have been tested as potential anticancer therapies
(25,26). Due to the association between
myoferlin and VEGFR-2 observed in the current study, the efficacy
of myoferlin as a therapeutic agent may require further
investigation.
In conclusion, to the best of our knowledge, the
present study is the first to describe myoferlin expression in
NSCLC. In adenocarcinoma cases, myoferlin-positive patients
possessed a poor prognosis (odds ratio, 2.94; P=0.339). In squamous
cell carcinoma cases, myoferlin expression was significantly
associated with VEGFR-2 expression (P=0.001). As VEGFR-2 is a
significant therapeutic target, myoferlin expression in NSCLC may
require further investigation in future studies.
References
|
1
|
Leung C, Yu C, Lin MI, Tognon C and
Bernatchez P: Expression of myoferlin in human and murine carcinoma
tumors, Role in membrane repair, cell proliferation and
tumorigenesis. Am J Pathol. 182:1900–1909. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Achanzar WE and Ward S: A nematode gene
required for sperm vesicle fusion. J Cell Sci. 110:1073–1081.
1997.PubMed/NCBI
|
|
3
|
Ward S, Argon Y and Nelson GA: Sperm
morphogenesis in wild-type and fertilization-defective mutants of
Caenorhabditis elegans. J Cell Biol. 91:26–44. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Posey AD Jr, Pytel P, Gardikiotes K, et
al: Endocytic recycling proteins EHD1 and EHD2 interact with
fer-1-like-5 (Fer1L5) and mediate myoblast fusion. J Biol Chem.
286:7379–7388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doherty KR, Cave A, Davis DB, Delmonte AJ,
Posey A, Earley JU, Hadhazy M and McNally EM: Normal myoblast
fusion requires myoferlin. Development. 132:5565–5575. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu C, Sharma A, Trane A, Utokaparch S,
Leung C and Bernatchez P: Myoferlin gene silencing decreases Tie-2
expression in vitro and angiogenesis in vivo. Vascul
Pharmacol. 55:26–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bernatchez PN, Acevedo L,
Fernandez-Hernando C, Murata T, Chalouni C, Kim J,
Erdjument-Bromage H, Shah V, Gratton JP, McNally EM, et al:
Myoferlin regulates vascular endothelial growth factor receptor-2
stability and function. J Biol Chem. 282:30745–30753. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li R, Ackerman WE 4th, Mihai C, Volakis
LI, Ghadiali S and Kniss DA: Myoferlin depletion in breast cancer
cells promotes mesenchymal to epithelial shape change and stalls
invasion. PLoS One. 7:e397662012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Volakis LI, Li R, Ackerman WE 4th, Mihai
C, Bechel M, Summerfield TL, Ahn CS, Powell HM, Zielinski R, Rosol
TJ, et al: Loss of myoferlin redirects breast cancer cell motility
towards collective migration. PLoS One. 9:e861102014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eisenberg MC, Kim Y, Li R, Ackerman WE,
Kniss DA and Friedman A: Mechanistic modeling of the effects of
myoferlin on tumor cell invasion. Proc Natl Acad Sci USA.
108:20078–20083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turtoi A, Blomme A, Bellahcène A, Gilles
C, Hennequière V, Peixoto P, Bianchi E, Noel A, De Pauw E, Lifrange
E, et al: Myoferlin is a key regulator of EGFR activity in breast
cancer. Cancer Res. 73:5438–5448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang WS, Liu XH, Liu LX, Lou WH, Jin DY,
Yang PY and Wang XL: iTRAQ-based quantitative proteomics reveals
myoferlin as a novel prognostic predictor in pancreatic
adenocarcinoma. J Proteomics. 91:453–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leung C, Shaheen F, Bernatchez P and
Hackett TL: Expression of myoferlin in human airway epithelium and
its role in cell adhesion and zonula occludens-1 expression. PLoS
One. 7:e404782012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chheang S and Brown K: Lung cancer
staging: Clinical and radiologic perspectives. Semin Intervent
Radiol. 30:99–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ha SY and Roh MS: The new 2011
international association for the study of lung cancer/american
thoracic society/european respiratory society classification of
lung adenocarcinoma in resected specimens: Clinicopathologic
relevance and emerging issues. Korean J Pathol. 47:316–325. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki S, Dobashi Y, Sakurai H, Nishikawa
K, Hanawa M and Ooi A: Protein overexpression and gene
amplification of epidermal growth factor receptor in nonsmall cell
lung carcinomas. Histopathology. Cancer. 103:1265–1273. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim H, Yoo SB, Sun P, Jin Y, Jheon S, Lee
CT and Chung JH: Alteration of the E-Cadherin/beta-Catenin Complex
is an independent poor prognostic factor in lung adenocarcinoma.
Korean J Pathol. 47:44–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rekhtman N, Ang DC, Sima CS, Travis WD and
Moreira AL: Immunohistochemical algorithm for differentiation of
lung adenocarcinoma and squamous cell carcinoma based on large
series of whole-tissue sections with validation in small specimens.
Mod Pathol. 24:1348–1359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jang I, Jeon BT, Jeong EA, Kim EJ, Kang D,
Lee JS, Jeong BG, Kim JH, Choi BH, Lee JE, et al:
Pak1/LIMK1/cofilin pathway contributes to tumor migration and
invasion in human non-small cell lung carcinomas and cell lines.
Korean J Physiol Pharmacol. 16:159–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morales-Oyarvide V and Mino-Kenudson M:
High-grade lung adenocarcinomas with micropapillary and/or solid
patterns: A review. Curr Opin Pulm Med. 20:317–323. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Z, Wang L, Eckloff BW, Deng B, Wang Y,
Wampfler JA, Jang J, Wieben ED, Jen J, You M and Yang P: Conserved
recurrent gene mutations correlate with pathway deregulation and
clinical outcomes of lung adenocarcinoma in never-smokers. BMC Med
Genomics. 7:322014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lauro S, Onesti CE, Righini R and
Marchetti P: The use of bevacizumab in non-small cell lung cancer,
An update. Anticancer Res. 34:1537–1545. 2014.PubMed/NCBI
|
|
24
|
Garon EB, Ciuleanu TE, Arrieta O, Prabhash
K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel
J, et al: Ramucirumab plus docetaxel versus placebo plus docetaxel
for second-line treatment of stage IV non-small-cell lung cancer
after disease progression on platinum-based therapy (REVEL): A
multicentre, double-blind, randomised phase 3 trial. Lancet.
384:665–673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rashdan S and Hanna N: Nintedanib for the
treatment of non-small-cell lung cancer. Expert Opin Pharmacother.
15:729–739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Majeti BK, Lee JH, Simmons BH and Shojaei
F: VEGF is an important mediator of tumor angiogenesis in malignant
lesions in a genetically engineered mouse model of lung
adenocarcinoma. BMC Cancer. 13:2132013. View Article : Google Scholar : PubMed/NCBI
|