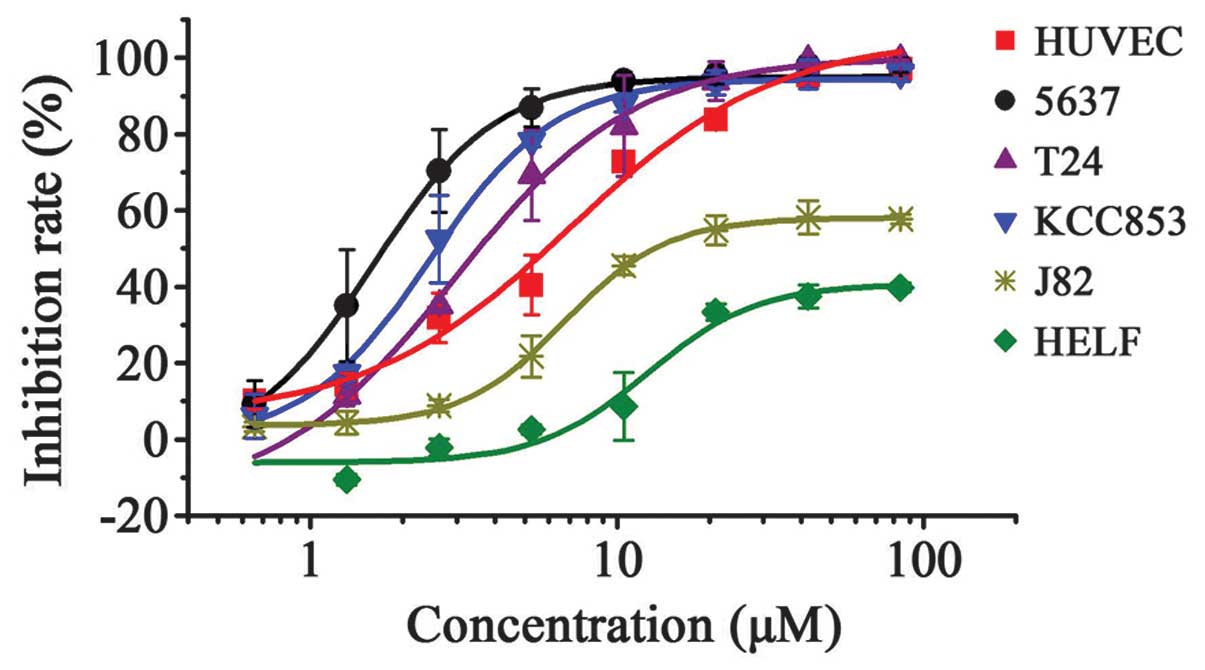
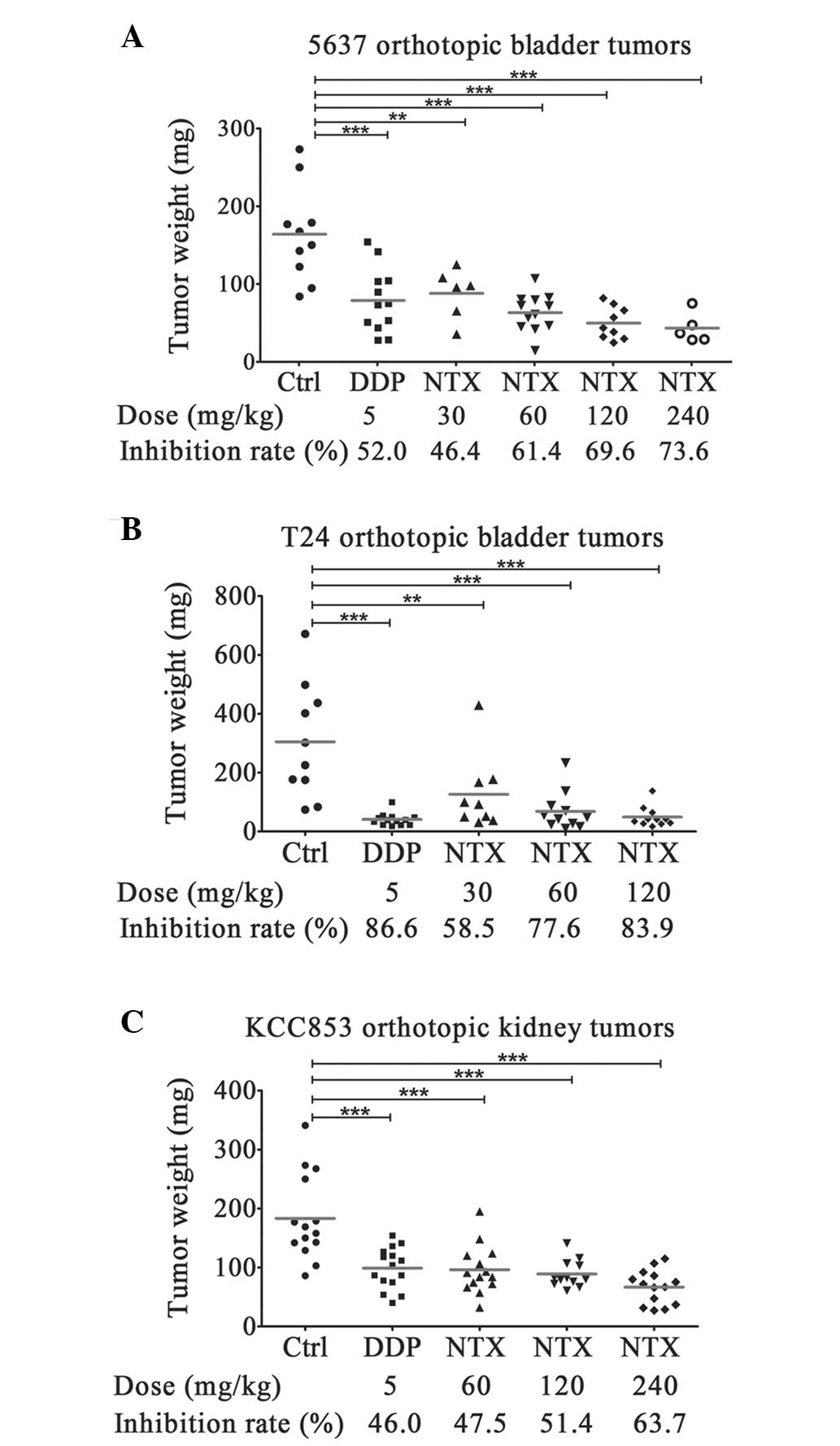
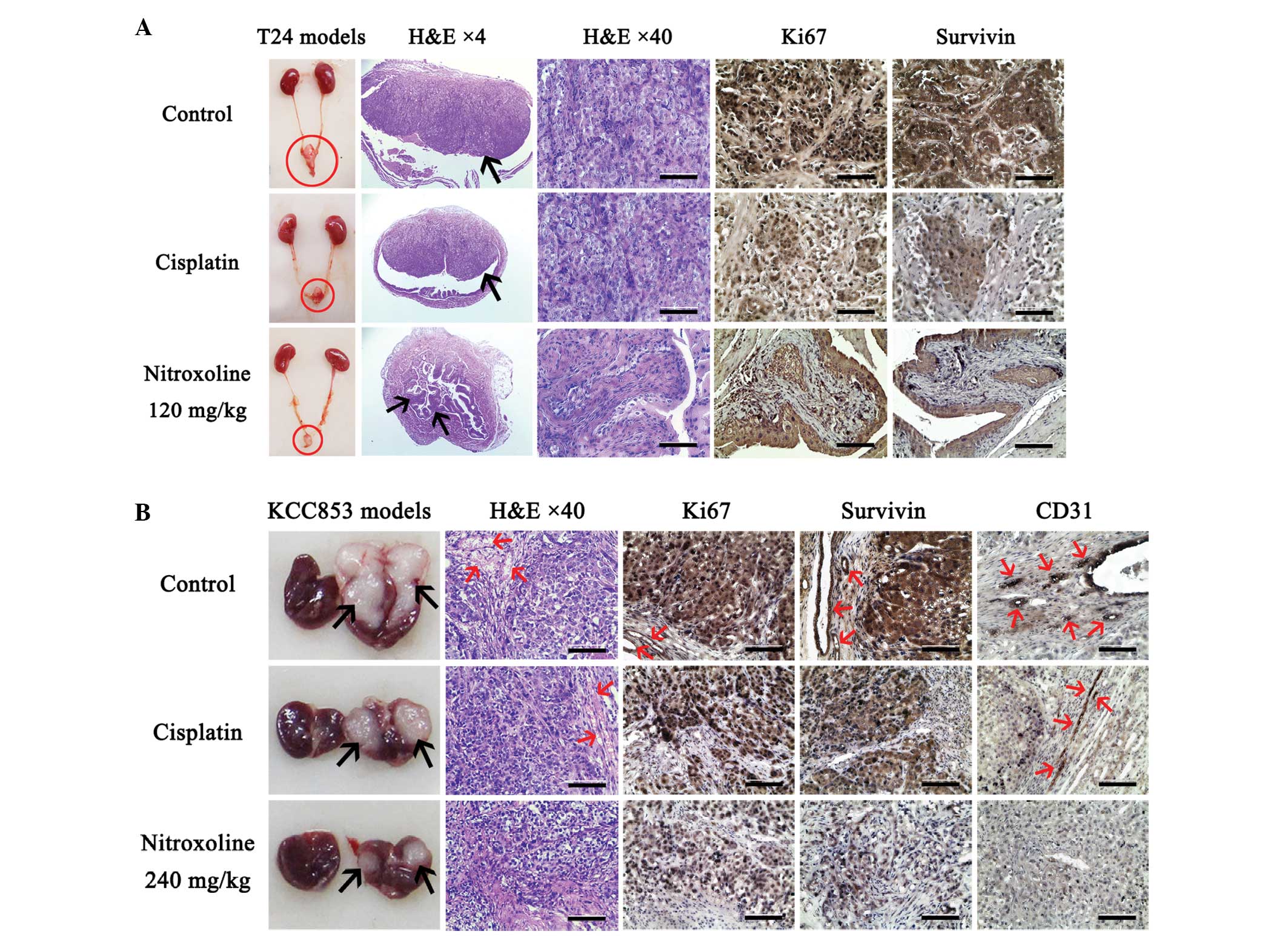
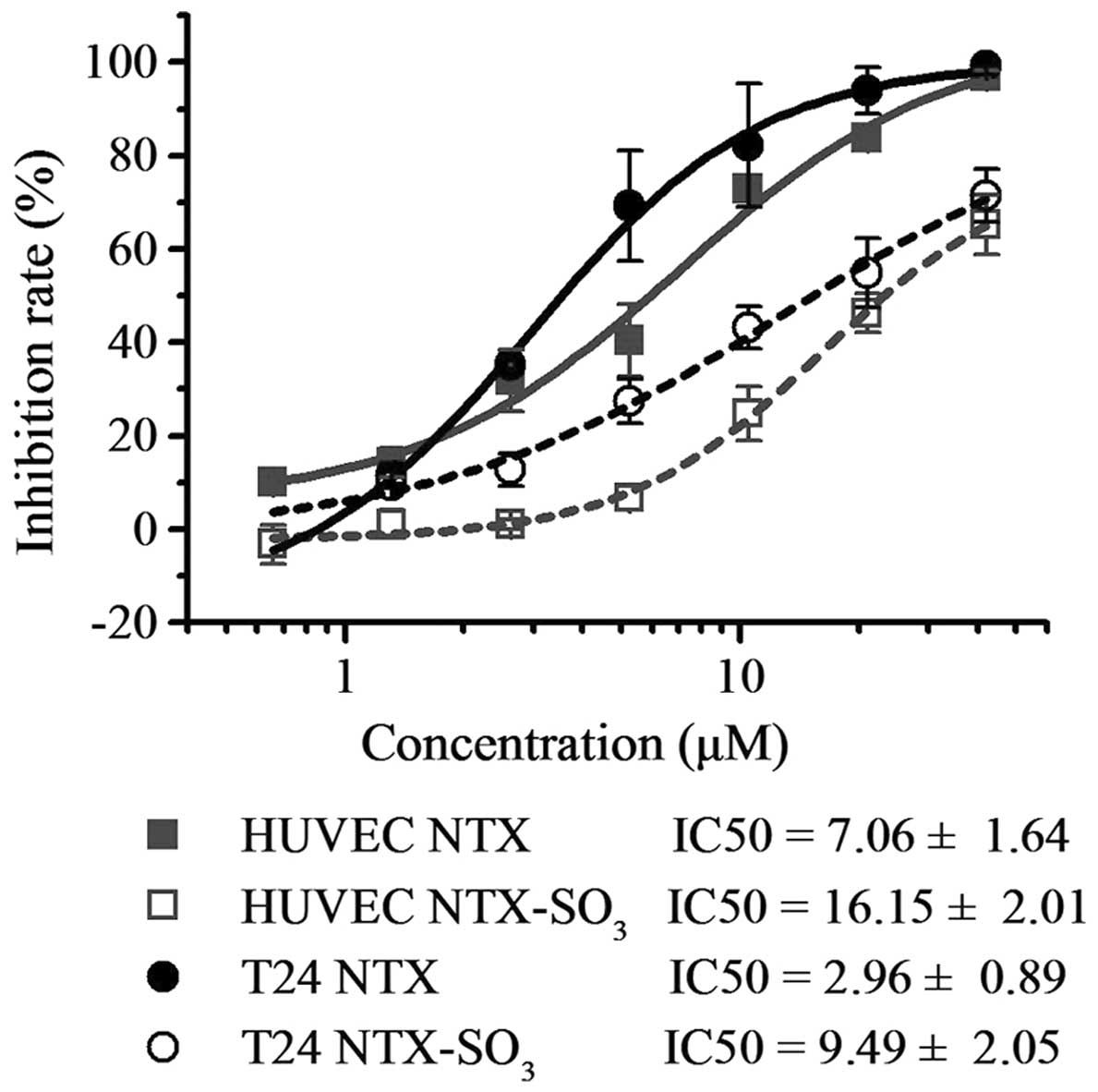
|
1
|
Shim JS and Liu JO: Recent advances in
drug repositioning for the discovery of new anticancer drugs. Int J
Biol Sci. 10:654–663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mrhar A, Kopitar Z, Kozjek F, Presl V and
Karba R: Clinical pharmacokinetics of nitroxoline. Int J Clin
Pharmacol Biopharm. 17:476–481. 1979.PubMed/NCBI
|
|
3
|
Shim JS, Matsui Y, Bhat S, Nacev BA, Xu J,
Bhang HE, Dhara S, Han KC, Chong CR, Pomper MG, et al: Effect of
nitroxoline on angiogenesis and growth of human bladder cancer. J
Natl Cancer Inst. 102:1855–1873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lazovic J, Guo L, Nakashima J, Mirsadraei
L, Yong W, Kim HJ, Ellingson B, Wu H and Pope WB: Nitroxoline
induces apoptosis and slows glioma growth in vivo. Neuro Oncol.
17:53–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang H, Taggart JE, Zhang X, Benbrook DM,
Lind SE and Ding WQ: Nitroxoline (8-hydroxy-5-nitroquinoline) is
more a potent anti-cancer agent than clioquinol
(5-chloro-7-iodo-8-quinoline). Cancer Lett. 312:11–17. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mirković B, Renko M, Turk S, Sosič I,
Jevnikar Z, Obermajer N, Turk D, Gobec S and Kos J: Novel mechanism
of cathepsin B inhibition by antibiotic nitroxoline and related
compounds. Chem Med Chem. 6:1351–1356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta SC, Sung B, Prasad S, Webb LJ and
Aggarwal BB: Cancer drug discovery by repurposing: Teaching new
tricks to old dogs. Trends Pharmacol Sci. 34:508–517. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Research Council: Guide for the
Care and Use of Laboratory Animals (8th). The National Academies
Press. Washington DC: 2011.
|
|
9
|
Hadaschik BA, Black PC, Sea JC, Metwalli
AR, Fazli L, Dinney CP, Gleave ME and So AI: A validated mouse
model for orthotopic bladder cancer using transurethral tumour
inoculation and bioluminescence imaging. BJU Int. 100:1377–1384.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang MR, Yang G, Charisse K,
Epstein-Barash H, Manoharan M and Li LC: An orthotopic bladder
tumor model and the evaluation of intravesical saRNA treatment. J
Vis Exp pii. 4207:2012
|
|
11
|
Zhao H, Nolley R, Chen Z and Peehl DM:
Tissue slice grafts: An in vivo model of human prostate androgen
signaling. Am J Pathol. 177:229–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thong AE, Zhao H, Ingels A, Valta MP,
Nolley R, Santos J, Young SR and Peehl DM: Tissue slice grafts of
human renal cell carcinoma: An authentic preclinical model with
high engraftment rate and metastatic potential. Urol Oncol.
32:43.e23–e30. 2014. View Article : Google Scholar
|
|
13
|
Murugasu-Oei B and Dick T: In vitro
activity of the chelating agents nitroxoline and oxine against
Mycobacterium bovis BCG. Int J Antimicrob Agents. 18:579–582. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reagan-Shaw S, Nihal M and Ahmad N: Dose
translation from animal to human studies revisited. FASEB J.
22:659–661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montironi R and Lopez-Beltran A: The 2004
WHO classification of bladder tumors: A summary and commentary. Int
J Surg Pathol. 13:143–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santos L, Amaro T, Costa C, Pereira S,
Bento MJ, Lopes P, Oliveira J, Criado B and Lopes C: Ki-67 index
enhances the prognostic accuracy of the urothelial superficial
bladder carcinoma risk group classification. Int J Cancer.
105:267–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shariat SF, Ashfaq R, Karakiewicz PI,
Saeedi O, Sagalowsky AI and Lotan Y: Survivin expression is
associated with bladder cancer presence, stage, progression, and
mortality. Cancer. 109:1106–1113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lopez-Beltran A, Scarpelli M, Montironi R
and Kirkali Z: 2004 WHO classification of the renal tumors of the
adults. Eur Urol. 49:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albelda SM, Muller WA, Buck CA and Newman
PJ: Molecular and cellular properties of PECAM-1 (endoCAM/CD31): A
novel vascular cell-cell adhesion molecule. J Cell Biol.
114:1059–1068. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wagenlehner FM, Münch F, Pilatz A, Bärmann
B, Weidner W, Wagenlehner CM, Straubinger M, Blenk H, Pfister W,
Kresken M and Naber KG: Urinary concentrations and antibacterial
activities of nitroxoline at 250 milligrams versus trimethoprim at
200 milligrams against uropathogens in healthy volunteers.
Antimicrob Agents Chemother. 58:713–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boguski MS, Mandl KD and Sukhatme VP: Drug
discovery. Repurposing with a difference. Science. 324:1394–1395.
2009. View Article : Google Scholar : PubMed/NCBI
|