Introduction
Worldwide, esophageal cancer (EC) is the eighth most
common cancer and sixth most common cause of cancer-associated
mortality (1). It is generally
diagnosed at a late stage of disease and has a poor prognosis, with
a 5-year survival rate of <10% (2). The majority of patients with EC in Asia,
including China and Japan, have esophageal squamous cell carcinoma
(ESCC), while the majority of patients in Western countries have
esophageal adenocarcinoma (EAC) (3).
Surgery and preoperative chemoradiotherapy are optional treatments
for patients with resectable tumors to treat ESCC and EAC (4). The most commonly utilized chemotherapy
agents are fluoropyrimidine, taxanes (paclitaxel or docetaxel) and
platinum compounds. Although ESCC and EAC are responsive to
chemotherapy, the response rates are low, particularly for patients
with advanced diseases (5).
Therefore, for ES patients, novel therapies are required.
Gambogic acid (GA;
C38H44O8; molecular weight,
628.75) is the major active ingredient in gamboges, and is a
brownish orange dry resin secreted from Garcinia hanburyi, a
plant that primarily grows in South China, Cambodia and Thailand
(6). Gamboge resin has been used as a
coloring material and in traditional Chinese medicine (TCM) for the
treatment of human diseases. Previous studies have demonstrated
that GA has anticancer effects and inhibits the growth of multiple
types of human cancer cells in vitro and in vivo
(7,8).
In animal tumor models and clinical trials, GA efficiently inhibits
tumor growth with minimal side effects, with little toxicity on the
immune and hemopoietic systems (7).
GA exhibits notable inhibition of proliferation, induction of
apoptosis, reversion of multidrug resistance and antiangiogenesis
(6,8,9). Although
the underlying mechanism is not fully understood, studies have
demonstrated that GA induces cytotoxity in a variety of tumor cells
by cell cycle arrest, interaction with the oncogene v-myc avian
myelocytomatosis viral oncogene homolog and inhibition of
telomerase activity (9). In addition,
GA has shown to downregulate the expression of nuclear receptor
coactivator 3 and inhibit the protein kinase B (AKT) pathway in
leukemia K562 cells (10). Therefore,
investigation into the mechanisms responsible for GA-mediated
antitumor effects is likely to have great clinical significance.
The present study aimed to elucidate the underlying antitumor
activity of GA in ESCC and provided a novel insight into ESCC
treatment.
Materials and methods
Cell culture
ESCC TE-1 cells were purchased from the Health
Science Research Resources Bank (Osaka, Japan). The cells were
cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in
an atmosphere containing 5% CO2.
Cell proliferation assay
GA was purchased from Sigma-Aldrich. A total of
1×104 TE-1 cells per well were plated in 96-well plates
(Corning Incorporated, Corning, NY, USA) and cultured for 24 h at
37°C with 5% CO2 in a humidified atmosphere. At the
indicated time points (12, 24 and 36 h) following various
concentrations of GA treatment (2–10 µg/ml), 10 µl of Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) reagent was added to each well and incubated at 37°C for 3
h. Absorbance was measured at 450 nm in a spectrophotometer
(UV-2802PCU; CRAIC Technologies, Inc., San Dimas, CA, USA). Each
experiment was performed in triplicate and repeated at least two
times.
Preparation of cytosolic
fractions
TE-1 cells were harvested and then disrupted in
lysis buffer A [0.33 M sucrose, 10 mM Hepes (pH 7.4;
Sigma-Aldrich), 1 mM MgCl2, 0.1% Triton X-100
(Sigma-Aldrich), protease inhibitor cocktail (PIC; Sigma-Aldrich)
and phenylmethanesulfonyl fluoride (PMSF)]. The cell lysates were
centrifuged for 5 min at 800 × g, and the supernatants were
collected to use as the cytosolic fractions. The resulting pellets
were resuspended in lysis buffer B [0.45 M NaCl, 10 mM Hepes (pH
7.4), PIC and PMSF] and centrifuged for 5 min at 18,000 × g. The
supernatants were collected to use as the cytosolic fractions.
Samples were frozen in aliquots in liquid nitrogen and stored at
−80°C. PMSF, sodium orthovanadate, sodium fluoride and PIC were
purchased from Roche Diagnostics (Indianapolis, IN, USA).
Apoptosis assays
Apoptosis was determined by terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay,
using an In Situ Cell-Death Detection kit (Boehringer
Mannheim; Roche Diagnostics), according to the manufacturer's
protocol. Briefly, 5,000 TE-1 cells/well were treated with various
concentrations (0, 4, 6, 8 and 10 µg/ml) of GA for 24 h in 96-well
plates (Corning Incorporated). Following treatment, cells were
trypsinized (Thermo Fisher Scientific, Inc.) and cytospin (9,000 ×
g at 4°C; Eppendorf 5810R; Eppendorf, Hamburg, Germany)
preparations were obtained. Cells were fixed with freshly prepared
paraformaldehyde (Thermo Fisher Scientific, Inc.) [4% in
phosphate-buffered saline (PBS; Thermo Fisher Scientific, Inc.); pH
7.4], rinsed with PBS three times (5 min each time), and incubated
in permeabilization solution (Sigma-Aldrich). Subsequent to
cross-reaction with the TUNEL reaction mixture for 60 min at 37°C
and cross-reaction with converter-alkaline phosphatase solution
(Sigma-Aldrich) for 30 min at 37°C in a humidified chamber, the
slides were reacted with alkaline phosphatase substrate solution
for 5–10 min (Vector Laboratories, Inc., Burlingame, CA, USA),
rinsed with PBS three times (5 min each time) and mounted under a
coverslip for analysis with a light microscope (CX23; Olympus
Corporation, Tokyo, Japan). The number of TUNEL-positive cells was
assessed at ×40 magnification, and representative fields were
photographed (using a Nikon L200 camera; Nikon Corporation, Tokyo,
Japan). The percentages of apoptotic cells were calculated from the
ratio of apoptotic cells to total cells counted. A minimum of 500
cells was counted in five isolated fields, and assays were
performed in duplicate, three times (six times in total). An AKT
selective inhibitor, GSK690693, was purchased from Selleck
Chemicals (Houston, TX, USA).
Caspase activity assay
The activity of caspase-3 was measured using a
caspase-3 assay kit (#KA0740; Abnova, Walnut, CA, USA), according
to the manufacturer's protocol. Briefly, TE-1 cells were treated
with various concentrations (0, 4, 6, 8 and 10 µg/ml) of GA with or
without z-VAD for 24 h, and then the cell lysis buffer containing
50 µg of protein was incubated with 5 µl of 4 mM peptide nucleic
acid (pNA)-conjugated substrate at 37°C for 2 h. The amount of pNA
released was measured at 405 nm using a spectrophotometer
(UV-2802PCU; CRAIC Technologies). Samples without cell lysates or
substrates acted as controls. All measurements of caspase activity
were performed in duplicate and only samples in which the
differences between the values obtained across replicates did not
exceed 10% were used to calculate the mean value.
Western blot analysis
Culture cells were lysed in cell lysis buffer
containing phosphatase inhibitor cocktail and proteinase inhibitor
cocktail (Sigma-Aldrich), and the protein concentrations were
determined using the BCA Protein Assay kit (Pierce Biotechnology,
Inc., Rockford, IL, USA). Total protein (20–40 mg) was subjected to
sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) under reducing conditions
and transferred to polyvinylidene difluoride membranes (Bio-Rad
Laboratories, Inc.). The membranes were blocked with Tris-buffered
saline (Sigma-Aldrich) containing 0.05% Tween-20 (Sigma-Aldrich)
and either 5% skim milk or 5% bovine serum albumin (Sigma-Aldrich),
and incubated with monoclonal rabbit antibodies against NF-κB
(#4764; dilution, 1:1,000), AKT (#8596; dilution, 1:1,000) and
phosphorylated-AKT (p-AKT; clone Ser473; #4060; dilution, 1:1,000),
which were all purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Subsequent to washing three times with PBS, the
membranes were incubated for 1 h at room temperature with
species-specific horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G secondary antibody (#7074; dilution, 1:2,000; Cell
Signaling Technology, Inc.). Immunoreactive bands were visualized
using SuperSignal West Dura Extended Duration Substrate Enhanced
Chemiluminescent Substrate (Pierce Biotechnology, Inc.). Each
experiment was performed at least 3 times independently.
Autophagy assay
Monodansylcadaverine (MDC) is also a specific marker
for autophagic vacuoles. To confirm that GA treatment induces
autophagy, TE-1 cells were treated with various concentrations (0,
4, 6, 8 and 10 µg/ml) of GA for 24 h. The autophagic vacuoles were
labeled with MDC by incubating with 0.05 mM/l MDC (Sigma-Aldrich)
in PBS at 37°C for 2 h. Cells were then washed three times (5 min
each time) with cold PBS buffer and immediately measured under a
flow cytometer (FACScan; BD Biosciences, San Jose, CA, USA) using
the Cell Quest software version 4.0 (BD Biosciences) to determine
the percentage of cells undergoing autophagy that recruited
MDC-positive particles.
Statistical analysis
All data and results presented were confirmed in at
least three independent experiments, unless otherwise indicated.
Data were expressed as the mean ± standard error unless otherwise
noted. The differences between groups were analyzed using a
two-tailed Student's t-test and the null hypothesis was rejected at
the 0.05 level. Significant differences were considered to be
indicated by P<0.05.
Results
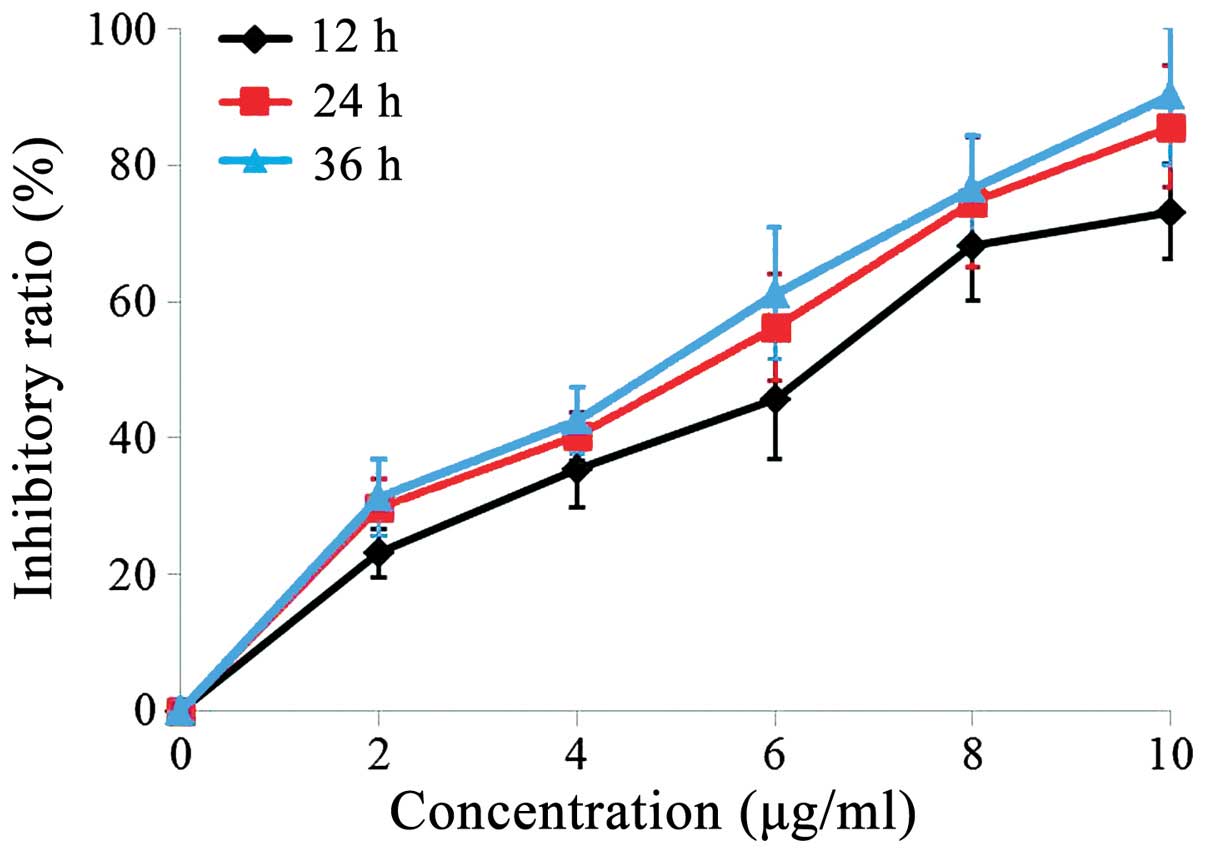
GA inhibits TE-1 cell growth in
vitro
To analyze the effect of GA on cell growth, the
CCK-8 assay was used to measure TE-1 cell viability following
exposure to various concentrations (0, 4, 6, 8 and 10 µg/ml) of GA
for 12, 24 and 36 h. The result showed that GA significantly
inhibited the growth of TE-1 cells in a dose- and time-dependent
manner (Fig. 1). The maximum cell
growth inhibition was evident at 36 h exposure to 10 µg/ml GA. The
half maximal inhibitory concentrations (IC50) of 12, 24
and 36 h of GA treatment for TE-1 cells were 6.5, 5.8 and 5.3 µg/ml
respectively.
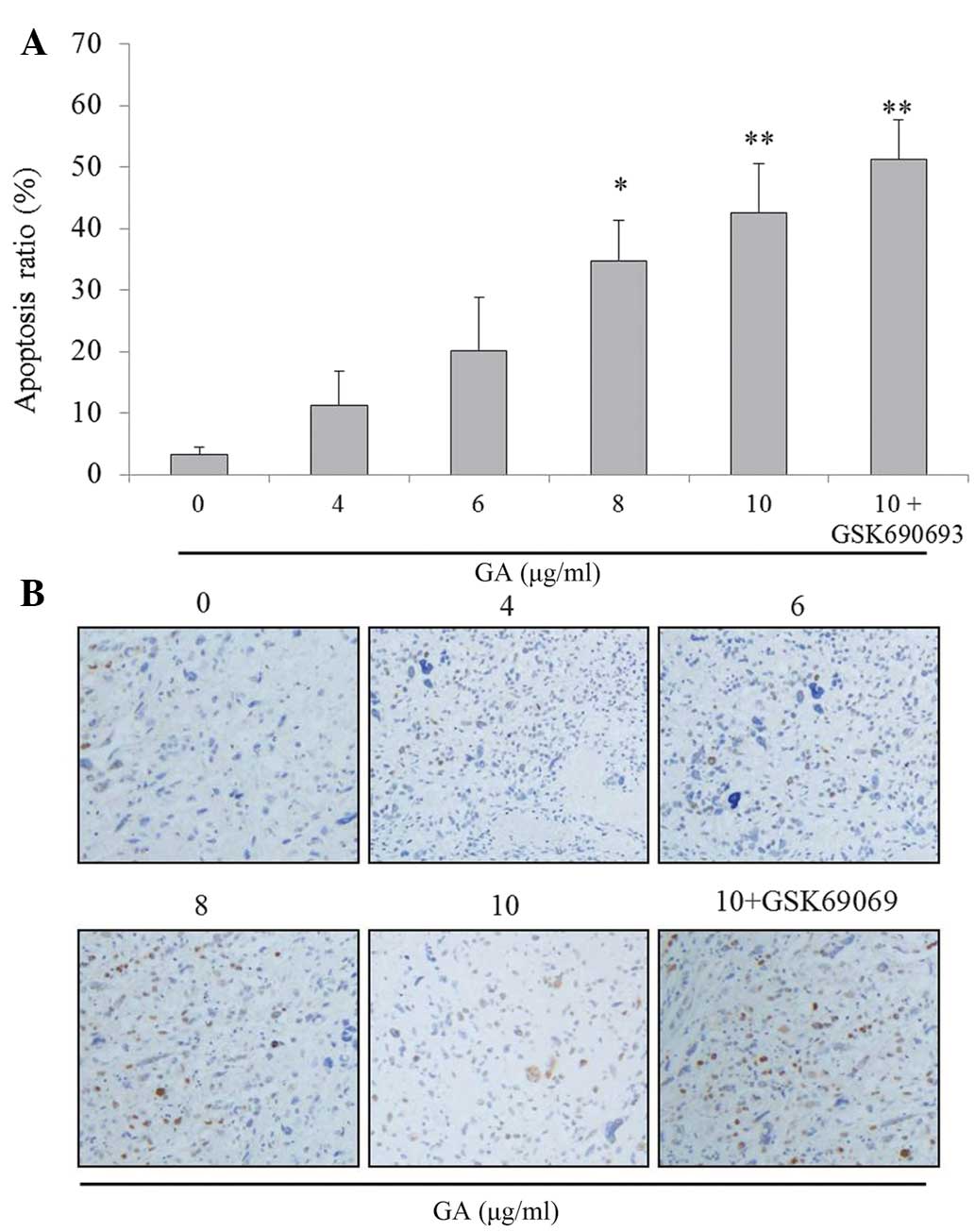
GA induces TE-1 cells apoptosis in
vitro
The inhibition of cell proliferation may result from
the induction of apoptosis. Apoptosis rate was first observed using
TUNEL analysis in TE-1 cells following treatment with various
concentrations (0, 4, 6, 8 and 10 µg/ml) of GA. The result
indicated that GA may significantly increase the percentage of TE-1
cell apoptosis in a dose-dependent manner (between 11.2 and 42.6%),
which may be augmented by the AKT inhibitor, GSK690693 (Fig. 2A and B).
GA promotes caspase-3 activities in
TE-1 cells in vitro
Since caspase-3, a member of the family of
aspartate-specific cysteinyl proteases, has been identified as a
key mediator of apoptosis in mammalian cells by cleaving a variety
of key cellular proteins (11), the
activation of caspase-3 was investigated following treatment with
various concentrations (0, 4, 6, 8 and 10 µg/ml) of GA in TE-1
cells. The result showed that caspase-3 activity may be promoted by
GA in a dose-dependent manner, which may be reduced by z-VAD
(Fig. 3A). Another CCK-8 assay was
performed to ascertain whether z-VAD reverses GA-induced growth
inhibition in TE-1 cells. The results indicated that z-VAD
attenuated GA-induced growth inhibition in the cells (Fig. 3B).
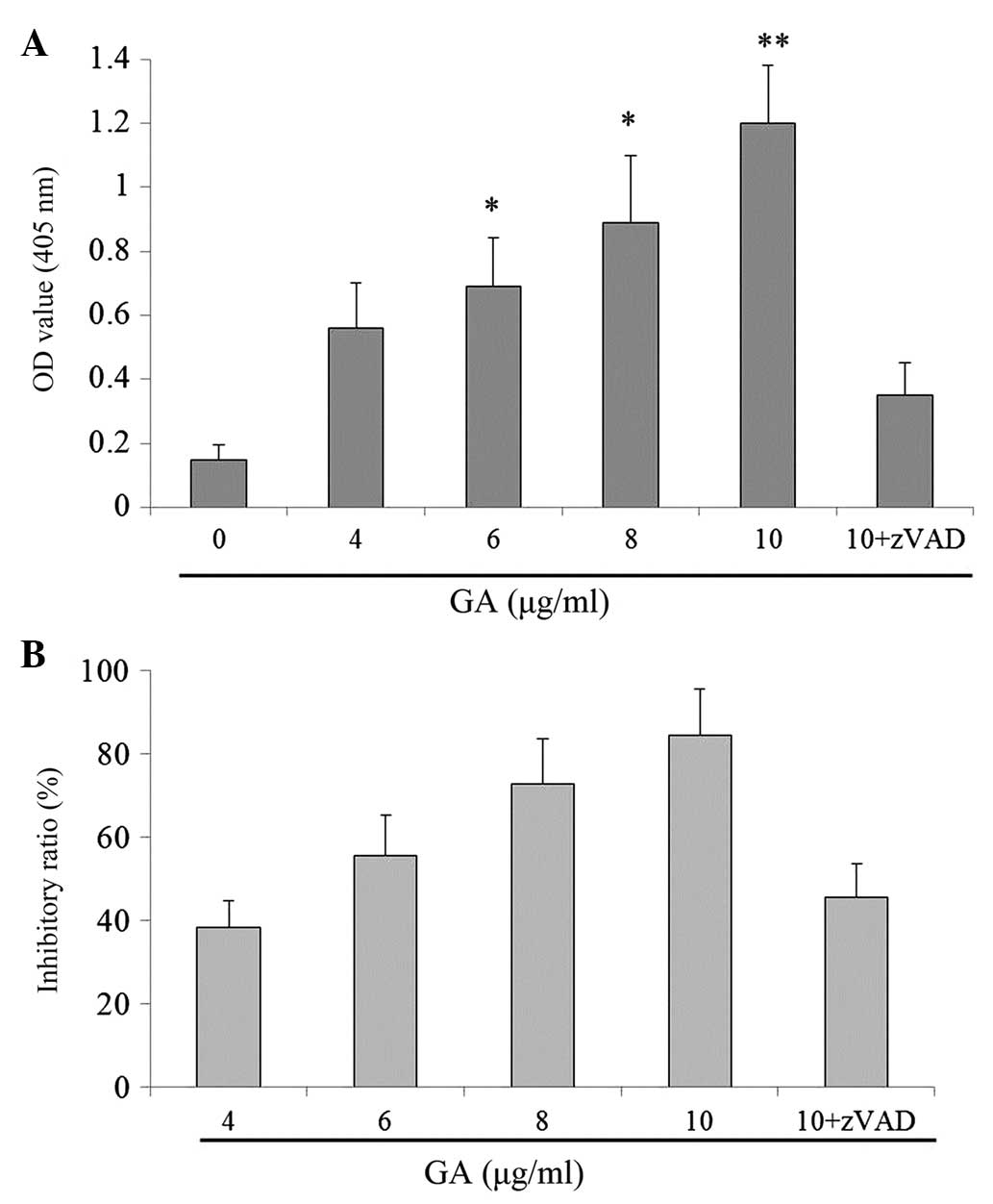 | Figure 3.GA promoted caspase-3 activities in
TE-1 cells in vitro. (A) Cells were treated with various
concentrations of GA (0, 4, 6, 8, 10 and >10 µg/ml) for 24 h,
and then subjected to caspase-3 colorimetric protease kits. (B)
Cells were cultured in RPMI-1640 medium for 24 h and then incubated
with various concentrations of GA (4, 6, 8 and 10 µg/ml) with or
without z-VAD for 24 h. The viability was determined by the CCK-8
assay. Data are presented as the mean ± standard error of th mean
of the results for three independent experiments. *P<0.05 and
**P<0.01 vs. vehicle group (0 µg/ml GA). OD, optical density;
GA, gambogic acid; zVAD, z-VAD-fmk. |
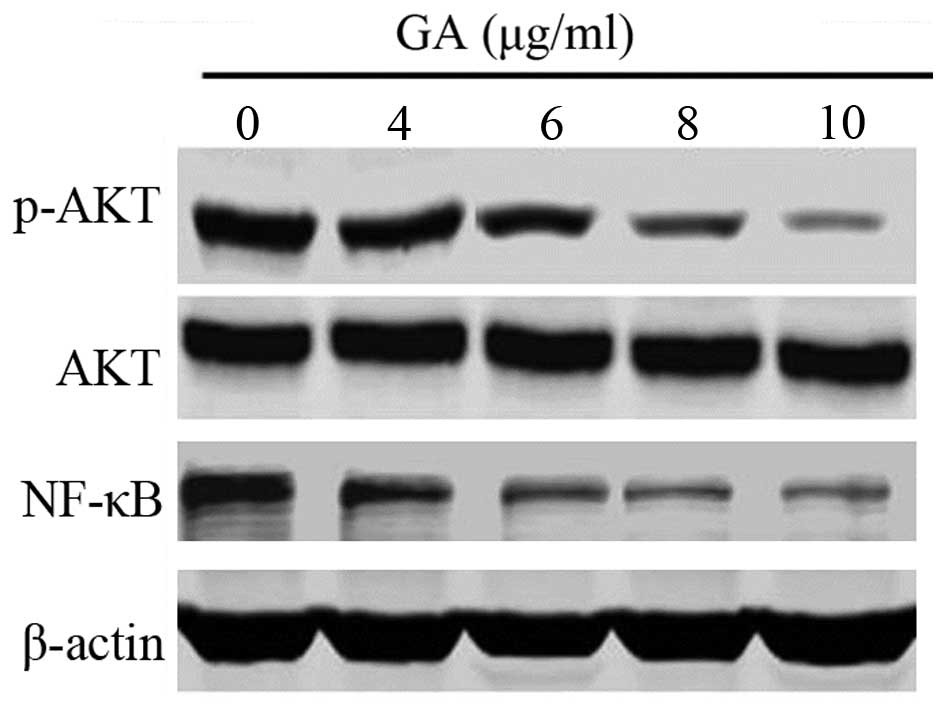
GA decreases the expression of p-AKT
and NF-κB in TE-1 cells
Since p-AKT and transcription factor NF-κB mainly
mediate cell apoptosis, western blot analysis was conducted to
ascertain the expression status of p-AKT and NF-κB following
treatment with GA in TE-1 cells. In the current study, GA was
indicated to decrease the expression of p-AKT and NF-κB in TE-1
cells in a dose-dependent manner (Fig.
4).
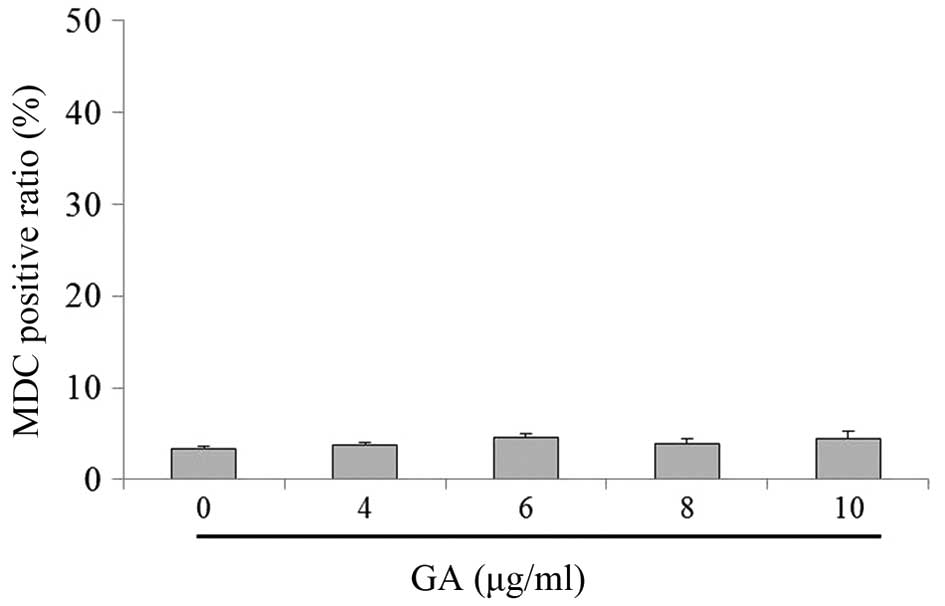
GA does not induce autophagy in TE-1
cells
The involvement of autophagy in GA-treated TE-1
cells was then examined. Compared with the control group, GA only
caused a slight increase in the MDC positive ratio (Fig. 5). In addition, there was no
significant difference between the treatment and the control group,
regarding cell morphology. The results indicated that GA may not
induce autophagy in TE-1 cells.
Discussion
Programmed cell death (PCD), a critical mechanism
for the development and homeostasis of multicellular organisms, and
consists of two major forms: Apoptosis and autophagy (12). Apoptosis (type I PCD) is a
cell-intrinsic suicide mechanism regulated by various cellular
signaling pathways (13).
Accumulating evidence has shown that GA may exert antitumor effects
against a variety of human cancers cell lines, including hepatoma,
breast cancer and gastric and lung carcinoma (6,14,15). To the best of our knowledge, the
present study is the first to suggest that GA may significantly
induce TE-1 cells apoptosis. In addition, the antitumor activity of
GA was accompanied by the decreased expression of p-AKT and NF-κB,
and the inhibition of AKT and NF-κB activation by chemical
inhibitors augmented the apoptotic effect responses to GA in the
TE-1 cells.
As an alternative death pathway to apoptosis,
autophagic cell death has achieved the great prominence, and the
mutual association between apoptotic and autophagic cell death is
under debate. Luo et al reported that GA may induce
apoptosis and autophagy in glioblastoma cells and that autophagy
inhibition promoted apoptosis (16).
Gu et al also reported that reactive oxygen species-mediated
autophagy induced by the dysregulation of lipid metabolism protects
colorectal cancer cells treated with GA (14). However, in the present study, the MDC
positive ratio levels were not affected by GA in TE-1 cells, which
indicated that GA may not induce autophagy in TE-1 cells. Shi et
al reported that another natural product, shikonin, promotes
autophagy in BXPC-3 human pancreatic cancer cells through the
phosphatidylinositol-4,5-bisphosphate 3-kinase/AKT signaling
pathway (17). It is possible that
the AKT/NF-κB pathway activity is also required for autophagy
induction by GA in TE-1 cells (17).
TCMs have been used for more than a millennium in
China to prevent and alleviate a wide variety of diseases. TCM is a
key component of the multidisciplinary treatment for advanced
tumors. A large number of studies showed that the proper use of
TCM-based therapies may enhance immune function, speed up recovery,
alleviate chemoradiotherapy-associated toxicities, improve quality
of life and extend survival (18–20).
However, the majority of TCM-based treatment only would be
categorized as palliative therapy in a clinical setting. The exact
antitumor mechanisms of TCM remain unclear, which hinders the usage
of TCM in clinical cancer treatment. The present study demonstrates
that GA may result in significant arrest of growth in TE-1 cells.
In addition, GA induces apoptosis in ESCC TE-1 cells via
suppression of the NF-ĸB pathway. The present study attempts to
explain the mechanism underlying the antitumor effects of GA in
ESCC, which may aid in the breakthrough of TCM in the radical
treatment of malignancies.
The natural product GA is a promising novel
antitumor agent that acts via various mechanisms in solid tumors
and hematological malignancies. GA may be suitable for exploitation
against various malignancies that are refractory to standard care
as GA is indicated to act through numerous antitumor
mechanisms.
Acknowledgements
The authors would like to thank Dr Ke Wu (Department
of Thoracic Surgery, YueBei People's Hospital, Shaoguan, China) for
providing valuable technical assistance.
References
|
1
|
Dawsey SP, Tonui S, Parker RK, Fitzwater
JW, Dawsey SM, White RE and Abnet CC: Esophageal cancer in young
people: A case series of 109 cases and review of the literature.
PLoS One. 5:e140802010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ando N, Ozawa S, Kitagawa Y, Shinozawa Y
and Kitajima M: Improvement in the results of surgical treatment of
advanced squamous esophageal carcinoma during 15 consecutive years.
Ann Surg. 232:225–232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Strong VE, D'Amico TA, Kleinberg L and
Ajani J: Impact of the 7th Edition AJCC staging classification on
the NCCN clinical practice guidelines in oncology for gastric and
esophageal cancers. J Natl Compr Canc Netw. 11:60–66.
2013.PubMed/NCBI
|
|
5
|
Iwase H, Shimada M, Tsuzuki T, Hirashima
N, Okeya M, Hibino Y, Ryuge N, Yokoi M, Kida Y, Kuno T, et al:
Concurrent chemoradiotherapy with a novel fluoropyrimidine, S-1 and
cisplatin for locally advanced esophageal cancer: Long-term results
of a phase II trial. Oncology. 84:342–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao L, Guo QL, You QD, Wu ZQ and Gu HY:
Gambogic acid induces apoptosis and regulates expressions of Bax
and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol
Pharm Bull. 27:998–1003. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gu H, Rao S, Zhao J, Wang J, Mu R, Rong J,
Tao L, Qi Q, You Q and Guo Q: Gambogic acid reduced bcl-2
expression via p53 in human breast MCF-7 cancer cells. J Cancer Res
Clin Oncol. 135:1777–1782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie H, Qin YX, Zhou YL, Tong LJ, Lin LP,
Geng MY, Duan WH and Ding J: GA3, a new gambogic acid derivative,
exhibits potent antitumor activities in vitro via
apoptosis-involved mechanisms. Acta Pharmacol Sin. 30:346–354.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo QL, You QD, Wu ZQ, Yuan ST and Zhao L:
General gambogic acids inhibited growth of human hepatoma SMMC-7721
cells in vitro and in nude mice. Acta Pharmacol Sin.
25:769–774. 2004.PubMed/NCBI
|
|
10
|
Colo GP, Rubio MF, Nojek IM, Werbajh SE,
Echeverría PC, Alvarado CV, Nahmod VE, Galigniana MD and Costas MA:
The p160 nuclear receptor co-activator RAC3 exerts an
anti-apoptotic role through a cytoplasmatic action. Oncogene.
27:2430–2444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nicholson DW and Thornberry NA: Caspases:
Killer proteases. Trends Biochem Sci. 22:299–306. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Savill J and Fadok VA: Corpse clearance
defines the meaning of cell death. Nature. 407:784–788. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu HY, Guo QL, You QD, Liu W, Qi Q, Li Z,
Yuan ST and Zhang K: Gambogic acid inducing apoptosis in human
hepatoma SMMC-7721 cells with p53 and Bax up-regulated. Chin J Nat
Med. 3:169–171. 2005.
|
|
15
|
Xu J, Zhou M, Ouyang J, Wang J, Zhang Q,
Xu Y, Xu Y, Zhang Q, Xu X and Zeng H: Gambogic acid induces
mitochondria-dependent apoptosis by modulation of Bcl-2 and Bax in
mantle cell lymphoma JeKo-1 cells. Zhongguo Aizheng Yanjiu
(Yingwenban). 25:183–191. 2013.
|
|
16
|
Luo GX, Cai J, Lin JZ, Luo WS, Luo HS,
Jiang YY and Zhang Y: Autophagy inhibition promotes gambogic
acid-induced suppression of growth and apoptosis in glioblastoma
cells. Asian Pac J Cancer Prev. 13:6211–6216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi SQ and Cao HM: Shikonin promotes
autophagy in BXPC-3 human pancreatic cancer cells through the
PI3K/Akt signaling pathway. Oncol Lett. 8:1087–1089.
2014.PubMed/NCBI
|
|
18
|
Zhang BC: Treatment of symptoms and root
causes of disease-management of the top ten symptoms in cancer
patients. Fang Ai Tian Di. 20:17–18. 2007.
|
|
19
|
Smith ME and Bauer-Wu S: Traditional
Chinese Medicine for cancer-related symptoms. Semin Oncol Nurs.
28:64–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang S, Wu X, Tan M, Gong J, Tan W, Bian
B, Chen M and Wang Y: Fighting fire with fire: Poisonous Chinese
herbal medicine for cancer therapy. J Ethnopharmacol. 140:33–45.
2012. View Article : Google Scholar : PubMed/NCBI
|