Introduction
Oral squamous cell carcinoma (OSCC) is among the 10
most common cancers worldwide and it is often associated with a
poor prognosis despite the marked progress in surgical methods,
particularly the introduction of microvascular reconstructive
techniques, and the significant progress of chemotherapy and
radiotherapy (1,2). It should be emphasized that
approximately two-thirds of cases are diagnosed at an advanced
stage of disease (stage III–IV) and that tumor-node-metastasis
(TNM) staging often does not explain the clinical behavior of the
tumor (3–6). For this reason, several studies have
been performed on the biological patterns of the tumor, which are
closely connected with its behavior (greater or lesser
aggressiveness) and can predict the prognosis. Oncogenes expressed
at varying percentages and associated modifications of chromosomal
sites have been identified in oral tumors (4–13). The p53
tumor suppressor gene (expressed in 4–50% of cases) is the most
studied oncogene detected on chromosome 17, and it is clear that
preliminary knowledge of the p53 status may be of great assistance
in managing OSCC (14–18).
Following previous studies on p53, we were recently
able to verify that p53 overexpression >50% indicates a poor
prognosis in advanced oral tumors (19–21).
Indeed, p53 overexpression >50%, detected by the simple,
reliable, routine examination of immunohistochemical analysis, is
associated with a high probability of mutation in this tumor
suppressor gene, with a poor response to sequential multimodality
treatments (21).
However, it appears equally important to know the
pattern of this marker in all stages of OSCC. The evolution of
stage I–II is not yet well known with regard to the behavior of the
advanced stages, with data concerning this derived only from
clinical experience (22,23).
It is not sufficient to know that clinically, an
advanced stage of the tumor is more serious than an early stage.
The risk of mortality must be quantifiable for all stages.
The purpose of the present study was to perform a
survival analysis and investigate the risk of mortality with regard
to variables such as tumor stage (stages I, II, IVa and IVb), oral
tumor site and p53 expression in OSCC, in order to obtain useful
information in addition to the classical factors of screening.
Patients and methods
A retrospective study of 150 non-consecutive cases
of stage I, II, IVa and IVb OSCC that were observed and treated by
resection between January 1992 and January 2012 in the
Maxillofacial Surgery Operative Unit (San Salvatore City Hospital,
L' Aquila, Italy), were selected from a total of 580 patients,
according to the inclusion criteria of the homogeneity of G2
histopathological grading (G1, well-differentiated; G2,
moderately-differentiated; and G3, undifferentiated). The G2
grading diagnosis was based on moderate cellular differentiation.
The patients were all smokers.
Immunohistochemical staining procedures were
performed according to the manufacturer's protocols. Paraffin
sections (4-µm thick) were mounted on poly-L-lysine-coated glass
slides, dewaxed, dehydrated, placed in 0.1 M citrate buffer (pH,
0.6) and microwaved twice for 15 min. Endogenous peroxidase
activity was then blocked with 0.3% hydrogen peroxide in methanol
for 30 min. Incubation with monoclonal mouse anti-human antibodies
against p53 (clone DO-7; catalog no. GA616; 1:50 dilution; Dako,
Glostrup, Denmark) was then performed. Next, the sections were
treated with streptavidin-biotin-peroxidase complex, follwed by
immersion in diaminobenzidine-H2O2 substrate,
for 5 min at room temperature, for chromogen development. The
sections were finally counterstained with hematoxylin. A known
p53-positive OSCC specimen was used as a positive control.
Substitution of the primary antibody with non-immune serum served
as a negative control. The p53 labeling index was calculated by
assessing the nuclear immunopositivity in 1,000 neoplastic cell in
10 high-power fields using a double-headed micropscope (BX51;
Olympus, Tokyo, Japan; this was considered positive when there was
≥10% immunostaining.
Cancer staging was performed in accordance with the
2002 American Joint Committee on Cancer sixth edition staging
criteria (24).
Statistical analysis
Continuous variables are presented as mean ±
standard deviation and range. The differences in p53 expression
levels according to the tumor site were evaluated using the
non-parametric Kruskal-Wallis test.
Survival analysis was performed to describe the
overall mortality, and prognostic factors were analyzed by
univariate analysis. The product limit method (Kaplan-Meier) was
used to evaluate the probability of survival to 24 months with
respect to the following variables: Age, gender, site of tumor, TNM
stage and p53 overexpression >50%. Differences between survival
curves were assessed using the log-rank test. Multivariate
(proportional hazard model) analysis was used to estimate
prognostic variables and associated risks of mortality. The results
have been expressed as the conditional relative risk of mortality
and 95% confidence intervals. P<0.05 was used to indicate a
statistically significant difference, and statistical tests were
performed using SAS/STAT 9.2 (SAS Institute Inc., Cary, NC,
USA).
Results
The mean ages and age ranges of the patients were as
follows: Stage I, 37 males (64.7±5.7 years; range, 54–75 years) and
11 females (70.0±3.37 years; range, 65–75 years); stage II, 15
males (64.5±5.6 years; range, 57–74 years) and 12 females (69.2±3.9
years; range, 63–74 years); stage IVa, 42 males (66.9±5.3 years;
range, 55–75 years), 16 females (64.2±6.5 years; range, 54–75
years); and stage IVb, 16 males (65.7±5.4 years; range, 57–74
years) and 1 female (69 years).
A significant association was found between the site
of the tumor and p53 overexpression (P<0.0001). The distribution
frequency of tumor site according to p53 overexpression was as
follows: Tongue, 42.9%; floor, 21.4%; retromolar trigone, 11.9%;
attached gingiva, 9.5%; and cheeks and soft palate, 7.1%.
The cumulative survival rate of the entire group
after 24 months of follow-up was 70.3% (standard error, 3.9). The
results of the univariate analysis for the prognostic meaning of
the main explanatory variables are shown in Table I. The log-rank test indicated that 3
prognostic factors were significantly correlated with prognosis.
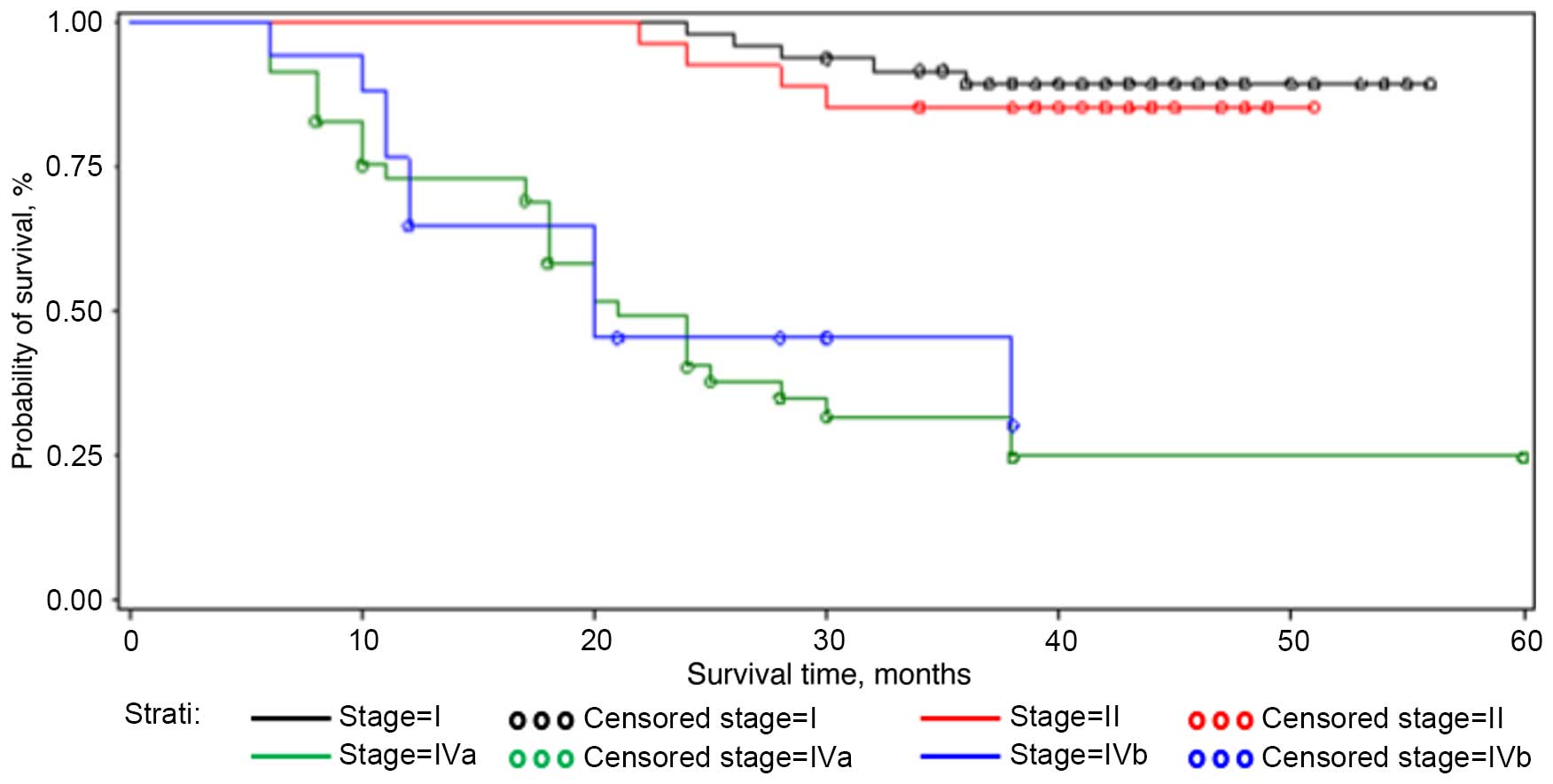
Stage I and stage II tumors showed a higher cumulative probability
of 24 months of survival (97.9 and 96.3%, respectively) compared
with stage IVa and IVb (40.2 and 45.2%, respectively)
(P<0.0001). With regard to the site of tumor in the cohort
examined, the cheek, the floor and the soft palate showed the worst
prognosis when compared with tumors of the attached gingiva, tongue
and retromolar trigone, with a cumulative probability of 24 months
of survival at 22.2, 25.9 and 27.8%, respectively (P<0.0001).
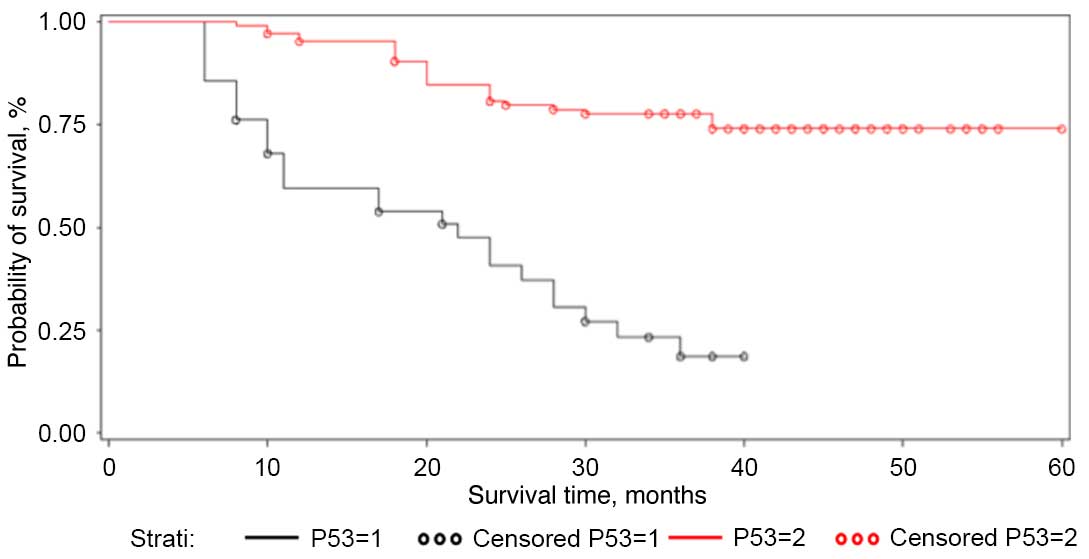
Tumors with p53 overexpression >50% showed a cumulative
probability of a 24-month survival time of 47.4%, while tumors with
p53 expression <50% showed a cumulative probability of 80.8%
(P<0.0001) (Table I; Figs. 1–3).
Table II shows the results of
multivariate analysis, including the variables considered at the
univariate analysis. Age (>65 years), advanced tumor stage
(stage IVa and IVb; RR=13.07 and 11.50, respectively), tumor
location (soft palate, floor and cheek; RR=8.94, 13.86 and 9.31,
respectively) and high p53 expression (>50%; RR=5.59) were
significantly associated with an increased risk of mortality.
 | Table I.Survival analysis for clinical
characteristics and p53 expression. |
Table I.
Survival analysis for clinical
characteristics and p53 expression.
| Variable | No. of patients
(n=96, 64%) | No. of mortalities
(n=54, 36%) | Cumulative
probability of 24 months of survival, % | Log-rank test
(P-value) |
|---|
| Age, years |
|
| ≤65 | 36 | 32 | 63.9 | 0.05 |
|
>65 | 60 | 22 | 76.2 |
|
| Gender |
|
| Male | 68 | 41 | 70.5 | 0.65 |
|
Female | 28 | 13 | 70.1 |
|
| Stage |
|
| I | 43 | 5 | 97.9 | <0.0001 |
| II | 23 | 4 | 96.3 |
|
| IVa | 23 | 35 | 40.2 |
|
| IVb | 7 | 10 | 45.2 |
|
| Site |
|
| Attached
gingiva | 32 | 19 | 72.5 | <0.0001 |
|
Cheek | 1 | 8 | 22.2 |
|
|
Floor | 3 | 6 | 25.9 |
|
| Soft
palate | 2 | 4 | 27.8 |
|
|
Tongue | 43 | 12 | 81.1 |
|
|
Retromolar trigone | 15 | 5 | 85.0 |
|
| p53 expression,
% |
|
|
>50 | 14 | 28 | 47.4 | <0.0001 |
| ≤50 | 82 | 26 | 80.8 |
|
 | Table II.Proportional hazards model according
to variables considered. |
Table II.
Proportional hazards model according
to variables considered.
| Variable | No. of patients | No. of
mortalities | RR Cox model | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
<65 | 36 | 32 |
1.00a |
|
|
|
>65 | 60 | 22 | 0.57 | 0.33–0.98 | 0.04 |
| Gender |
|
|
|
|
|
|
Female | 68 | 41 |
1.00a |
|
|
| Male | 28 | 13 | 0.86 | 0.46–1.61 | 0.86 |
| Stage |
|
|
|
|
|
| I | 43 | 5 |
1.00a |
|
|
| II | 23 | 4 | 1.44 | 0.39–5.35 | 0.59 |
| IVa | 23 | 35 | 13.07 | 5.06–33.73 | <0.001 |
| IVb | 7 | 10 | 11.50 | 3.89–33.87 | <0.001 |
| Site |
|
|
|
|
|
|
Retromolar trigone | 32 | 19 |
1.00a |
|
|
|
Tongue | 1 | 8 |
1.42 | 0.46–4.36 | 0.54 |
|
Attached gingiva | 3 | 6 |
2.25 | 0.76–6.62 | 0.14 |
| Soft
palate | 2 | 4 |
8.94 | 2.18–36.74 | 0.002 |
|
Floor | 43 | 12 | 13.86 | 3.72–51.64 | <0.001 |
|
Cheek | 15 | 5 |
9.31 | 2.77–31.33 | 0.0003 |
| p53 expression,
% |
|
|
|
|
|
|
≤50 | 14 | 28 |
1.00a |
|
|
|
>50 | 82 | 26 |
5.59 | 3.23–9.67 | <0.001 |
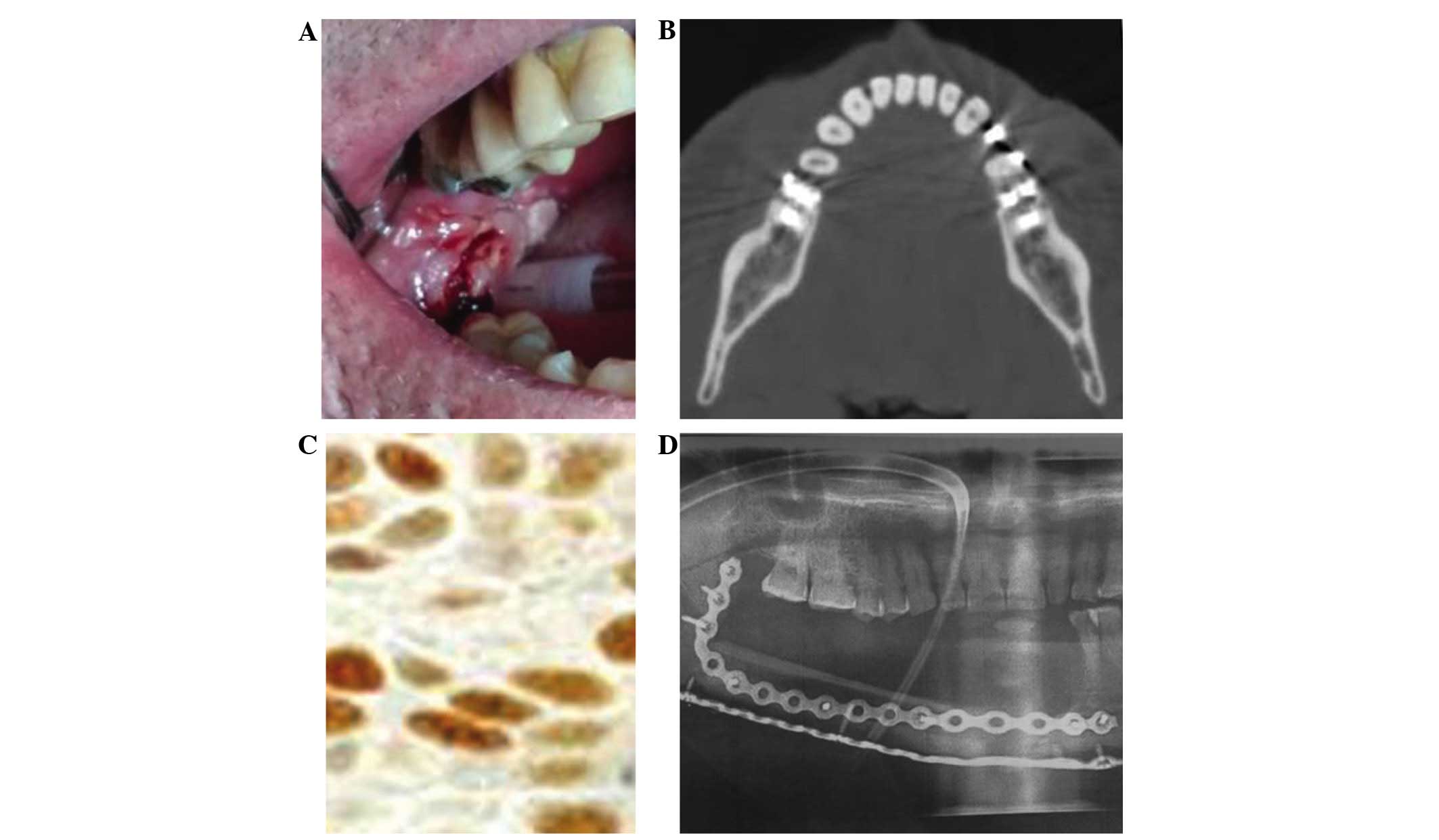
A representative case of OSCC of the retromolar
trigone is shown in Fig. 4.
Discussion
Knowledge of the oncogenetic pattern of a tumor can
explain its natural history and behavior in single cases. Thus, a
number of oncogenetic studies of oral cancer have been performed to
identify prognostic and predictive markers (4–13).
The p53 tumor suppressor gene is correlated with
oral tumor induction and progression, and its significant role as a
prognostic marker was found in previous studies in advanced oral
tumors (21).
The aim of the present study was to quantify the
risk of mortality for all stages of OSCC. It is important to note
that the cohort of patients considered was not consecutive due to
the inclusion criteria used; thus, the distribution sites of the
oral neoplastic lesions were different from those found in
consecutive series.
Patients were selected on the basis of homogeneous
G2 histopathological grading. This allowed more significance to be
assigned to acquired data, as even more emphasis could be placed,
in the single case, on the oncogenetic pattern and the specific
biological features of neoplastic tissue, and the association with
the risk of mortality. Three prognostic factors were found to be
significantly associated with the prognosis.
These results fulfill the aim of the present study.
The study also shows that an age of >65 years is a variable that
has an effect on survival, significantly increasing the risk of
mortality compared with an age of <65 years. In addition, it is
important to highlight the fact that patients with a floor tumor
site exhibited a significantly higher risk of mortality than other
sites.
Surgery is the main treatment for OSCC. Advanced
oral tumors require wide resections and complex reconstruction
using multimodal protocols (25,26). Basic
cancer research results are useful for maxillofacial surgeons and
oncological teams in the management of the stage IV, but the
possibility of having prognostic markers is perhaps even more
important for the stages I and II.
Indeed the association found between p53
overexpression and the tumor site increases the indications for a
wide resection associated with a prophylactic selective neck
dissection in cases of T1-T2 N0 localized tumors of the tongue, the
floor of the mouth, the retromolar trigone and the attached
gingiva, but also in the cheek and soft palate, particularly when
p53 overexpression results are >50% (Fig. 4) (27).
A previous study verified that 50% linked p53 antibody in
histopathological specimens appears to be a significant cut-off
value. Percentages >50% of the expressed oncogene show a high
probability of genetic mutation, which compromises the genic
product and its related cellular function (21).
In the present study, survival analyses of the
examined cases of OSCC show that four variables have an effect on
survival, with a significantly increased risk of mortality: An age
>65 years; an advanced stage, IVa and IVb; the site of the
tumor; and p53 immunoexpression >50%.
These data indicated that, in oral cancer, every
variable that was considered, such as tumor site, showed a worse
prognosis, particularly when correlated with an altered p53
expression pattern. This can explain the natural history and
behavior of the tumor, and the prognosis in single cases, while the
classic clinical and radiological parameters, humoral markers and
histopathological grading are not reflective.
The results of the present study allowed the
quantification of the risk of mortality for OSSC with regard to the
stage, the site and the p53 expression pattern of the tumor. The
results also emphasized the importance of preliminary knowledge of
the pattern of p53 as a prognostic marker of survival in order to
obtain the best chance for a cure for these tumors in terms of the
best surgical and therapeutic options.
References
|
1
|
Durmus K, Apuhan T and Ozer E: Transoral
robotic surgery for retromolar trigone tumours. Acta
Otorhinolaryngol Ital. 33:425–427. 2013.PubMed/NCBI
|
|
2
|
Freudlsperger C, Bodem JP, Engel E and
Hoffmann J: Mandibular reconstruction with a prefabricated free
vascularized fibula and implant-supported prosthesis based on fully
three-dimensional virtual planning. J Craniofac Surg. 25:980–982.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bànkfalvi A and Piffkò J: Prognostic and
predictive factors in oral cancer: The role of the invasive tumor
front. J Oral Pahol Med. 29:291–298. 2000. View Article : Google Scholar
|
|
4
|
Jan JC, Hsu WH, Liu SA, Wong YK, Poon CK,
Jiang RS, Jan JS and Chen IF: Prognostic factors in patients with
buccal squamous cell carcinoma: 10-year experience. J Oral
Maxillofac Surg. 69:396–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao CT, Huang SF, Chen IH, Kang CJ, Lin
CY, Fan KH, Wang HM, Ng SH, Hsueh C, Lee LY, et al: Outcome
analysis of patients with pN2 oral cavity cancer. Ann Surg Oncol.
17:1118–1126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Holmes JD, Martin RA and Gutta R:
Characteristics of head and neck cancer patients referred to an
oral and maxillofacial surgeon in the United States for management.
J Oral Maxillofac Surg. 68:555–561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pérez-Sayáns M, Somoza-Martín JM,
Barros-Angueira F, Reboiras-López MD, Gándara Rey JM and
García-García A: Genetic and molecular alterations associated with
oral squamous cell cancer (Review). Oncol Rep. 22:1277–1282. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwartz JL: Biomarkers and molecular
epidemiology and chemoprevention of oral carcinogenesis. Crit Rev
Oral Biol Med. 11:92–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shah NG, Trivedi TI, Tankshali RA, Goswami
JA, Shah JS, Jetly DH, Kobawala TP, Patel KC, Shukla SN, Shah PM
and Verma RJ: Molecular alterations in oral carcinogenesis:
Significant risk predictors in malignant transformation and tumor
progression. Int J Biol Markers. 22:132–143. 2007.PubMed/NCBI
|
|
10
|
Shah NG, Trivedi TI, Tankshali RA, Goswami
JV, Jetly DH, Shukla SN, Shah PM and Verma RJ: Prognostic
significance of molecular markers in oral squamous cell carcinoma:
A multivariate analysis. Head Neck. 31:1544–1556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shillitoe EJ, May M, Tale V, Lethanakul C,
Enseley JF, Strausberg RL and Gutkind JS: Genome-wide analysis of
oral cancer-early results from the cancer genome anaromy project.
Oral Oncol. 36:8–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murugan AK, Munirajan AK and Tsuchida N:
Ras oncogenes in oral cancer: The past 20 years. Oral Oncol.
48:383–392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito T, Nakajima T and Mogi K:
Immunohistochemical analysis of cell cycle-associated proteins p16,
pRb, p53, p27 and Ki-67 in oral cancer and precancer with special
reference to verrucous carcinoma. J Oral Pathol Med. 28:226–232.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sidransky D, Boyle J and Koch W: Molecular
screening. Prospects for a new approach. Arch Otolaryngol Head Neck
Surg. 119:1187–1190. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boyle JO, Hakim J, Koch W, van der Riet P,
Hruban RH, Roa RA, Correo R, Eby YJ, Ruppert JM and Sidransky D:
The incidence of p53 mutations increases with progression of head
and neck cancer. Cancer Res. 53:4477–4480. 1993.PubMed/NCBI
|
|
16
|
Jones A: A general review of the p53 gene
and oral squamous cell carcinoma. Ann R Australas Coll Dent Surg.
14:66–69. 1998.PubMed/NCBI
|
|
17
|
Nylander K, Dabelsteen E and Hall PA: The
p53 role molecule and its prognostic role in squamous cell
carcimonas of the head and neck. J Oral Pathol Med. 29:413–425.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tandon S, Tudur-Smith C, Riley RD, Boyd MT
and Jones TM: A systematic review of p53 as a prognostic factor of
survival in squamous cell carcinoma of the four main anatomical
subsites of the head and neck. Cancer Epidemiol Biomarkers Prev.
19:574–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cutilli T, Papola F and Corbacelli A: p53
overexpression and mutation, chemoresistance and patient survival
in oral and maxillofacial squamous carcinoma. J Chemother.
9:123–124. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cutilli T, Papola F, Di Emidio P and
Corbacelli A: p53 tumor suppressor protein and H-RAS oncogene in
maxillofacial tumors: Immunohistochemical and genetic investigaion,
induction chemotherapy response and prognosis evaluation. J
Chemother. 10:411–417. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cutilli T, Leocata P, Dolo V and Altobelli
E: Evaluation of p53 protein as a prognostic factor for oral cancer
surgery. Br J Oral Maxillofac Surg. 51:922–927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Green FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: AJCC cancer staging manual. 6th.
Springer; New York: 2002, View Article : Google Scholar
|
|
23
|
World Health Organization = IARC
monographs on the evaluation of carcinogenic risks to humans:
Biological Agents. 100B. A review of human carcinogens.
International Agency for Research on Cancer; Lyon: 2012
|
|
24
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: AJCC Cancer Staging Manual. 6th.
Springer-Verlag; New York, NY: 2002, View Article : Google Scholar
|
|
25
|
Specenier P and Vermorken JB: Advances in
the systemic treatment of head and neck cancers. Curr Opin Oncol.
22:200–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jenwitheesuk K, Surakunprapha P, Chowchuen
B, Tangvoraphongchai V, Pesee M, Krusun S and Supaadirek C: Results
of multidisciplinary therapy of squamous cell carcinoma of the
buccal mucosa at Srinagarind Hospital, Thailand. J Med Assoc Thai.
93:1262–1267. 2010.PubMed/NCBI
|
|
27
|
Hakeem AH, Pradhan SA, Tubachi J and
Kannan R: Outcome of per oral wide excision of T1-2 N0 localized
squamous cell cancer of the buccal mucosa-analysis of 156 cases.
Laryngoscope. 123:177–180. 2013. View Article : Google Scholar : PubMed/NCBI
|