Introduction
Liver cancer, particularly hepatocellular carcinoma
(HCC), is one of the most common human malignancies with poor
long-term survival rates (1–3). Although liver transplantation,
hepatectomy and local therapy are potentially curative therapies in
the early stages of HCC (4,5), the majority of patients, who are usually
diagnosed at an advanced stage, must rely mainly on traditional
chemotherapies (6,7). Currently, there is no proven effective
conventional systemic chemotherapy for patients with advanced HCC
(7). Therefore, novel therapeutic
agents with high efficacy are urgently required for the clinical
treatment of advanced HCC.
Trametes robiniophila Murr (Huaier) is a type
of fungus that exists in China, and previous chemical analyses
revealed that Huaier consists mainly of polysaccharide (8). Recent studies have noticed that Huaier
polysaccharide (HP) exerts a pro-apoptotic effect on the cells of a
variety of human cancers, including breast cancer (9,10),
hepatocarcinoma (11–14), lung adenocarcinoma (15) and ovarian cancer (16). In addition, Huaier and HP suppress
cancer cell metastasis and motility (12,16,17),
exhibit anti-angiogenic activity and enhance the host immune system
function (11,14,18).
Together, these data indicate that HP exhibits promising results
against cancer in pre-clinical trials.
The use of Huaier has been approved by the Chinese
Food and Drug Administration for the clinical treatment of patients
with malignant tumors (China Food and Drug Administration approval
number, Z20000109; http://app1.sfda.gov.cn/datasearch/face3/base.jsp).
Although several studies indicated that HP induces apoptosis in HCC
cells via different signaling pathways (13,19), the
detailed mechanism by which this drug inhibits HCC cell growth
remains to be explored.
Mitogen-activated protein kinase (MAPK) participate
in the regulation of cell proliferation, differentiation, cellular
stress responses and apoptosis (20,21). The
activation of the three major MAPK pathways [extracellular
signal-regulated kinase (ERK)1/2, c-Jun N-terminal kinase (JNK) and
p38 MAPK], has been implicated in the activity of numerous
chemotherapy and genotoxic drugs (22). Therefore, HP may participate and
regulate proliferation and apoptosis of HCC cells through the MAPK
signaling pathway.
The present study focused on the inhibitory effect
of HP on both HepG2 and Huh7 HCC cells, and explored the possible
mechanisms of its anticancer effect. Furthermore, the critical role
of MAPK in the regulation of these processes was investigated.
Materials and methods
Antibodies and reagents
Polyclonal rabbit caspase-3 (catalog no. 9662S),
monoclonal mouse caspase-8 (catalog no. 9746), polyclonal rabbit
caspase-9 (catalog no. 9502), monoclonal rabbit phosphorylated
(p)-p38 (catalog no. 9215S), polyclonal rabbit p-AKT (catalog no.
9271S), polyclonal rabbit total JNK (catalog no. 9252), polyclonal
rabbit total p38 (catalog no. 9212), polyclonal rabbit total AKT
(catalog no. 9272S), monoclonal rabbit B-cell lymphoma (Bcl)-2
(catalog no. 2870S), polyclonal rabbit Bcl-2-associated X protein
(Bax; catalog no. 2772S), polyclonal rabbit Bcl-extra large (xL)
(catalog no. 2762S), monoclonal rabbit myeloid cell leukemia-1
(Mcl-1; 5453S), monoclonal rabbit Bcl-2-like 11 (also known as Bim;
catalog no. 2933S), polyclonal rabbit p53 (catalog no. 9282) and
monoclonal mouse survivin (catalog no. 2802S) antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The dilution ratio of all of these antibodies was 1:1,000.
Polyclonal rabbit Anti-poly (ADP-ribose) polymerase
(PARP) p85 fragment (catalog no. G734A), anti-ERK (catalog no.
V114A), anti-p-JNK (V793B) and anti-active ERK1/2 (catalog no.
V803A) antibodies were obtained from Promega Corporation (Madison,
WI, USA). The dilution ratio of all of these antibodies was
1:4,000.
Polyclonal rabbit cyclin D1 (catalog no. sc753) and
monoclonal mouse cyclin-dependent kinase 2 (CDK2; catalog no.
sc6248) antibodies were obtained from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). The dilution ratio of these antibodies was
1:500.
Polyclonal rabbit glyceraldehyde 3-phosphate
dehydrogenase antibody (10494–1-AP; 1:8,000) was purchased from
Proteintech Group (Rosemont, IL, USA). Polyclonal rabbit p70S6
kinase antibody (catalog no. ABS431; 1:1,000 dilution) was
purchased from EMD Millipore.
Specific inhibitors of MAPK kinase (MEK) (PD98059)
(catalog no. 513000-5MGCN), JNK (SP600125) (catalog no.
420119-5MGCN) and p38 (SB203580) (catalog no. 559389-1MGCN) were
purchased from Calbiochem (EMD Millipore, Billerica, MA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Amresco, LLC (Solon, OH, USA). The pan-caspase
peptide inhibitor Z-VAD-FMK was purchased from Promega Corporation
and prepared in dimethyl sulfoxide (DMSO). HP was donated by Qidong
Gaitianli Pharmaceutical Co., Ltd. (Jiangsu, China).
Cell culture
The two types of HCC lines (HepG2 and Huh7) were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and were routinely maintained in Dulbecco's modified Eagle
medium supplemented with 10% fetal bovine serum, 100 U/ml
penicillin and 100 µg/ml streptomycin. The cells were incubated at
37°C in a 5% CO2 air incubator.
Measurements of cell viability by MTT
assay
Cells were seeded in 96-well plates. After
incubation overnight, the medium was replaced with different
concentrated solutions of HP and incubated for 24, 48 and 72 h.
Next, 10 µl MTT was added to each well and the cells were incubated
for 4 h. Subsequently, the solutions in each well were carefully
replaced by 100 µl DMSO for 10 min. The absorbance values were read
with a microplate reader (PerkinElmer, Inc., Waltham, MA, USA) at
490 nm wavelength. Each experiment was conducted in triplicate.
Effect of HP on cell morphology
Cells were treated with HP at a concentration of 100
µg/ml for 24, 48 and 72 h. The morphological changes of the treated
and untreated cells were observed at 24, 48 and 72 h under a light
microscope (Olympus Corporation, Tokyo, Japan), and
photomicrographs were captured with a digital camera (Olympus
Corporation).
Cell cycle analysis
A total of 3×105 cells/well were seeded
in 6-well plates and incubated overnight. After starvation with
serum-free medium for 12 h, the cells were incubated for 24 h in a
HP-containing solution (50 or 100 µg/ml). Next, the cells were
trypsinized and fixed in cold ethanol. Subsequently, the cells were
centrifuged at 168 × g for 5 min and washed twice with
phosphate-buffered saline (PBS). Next, RNase (KeyGen BioTech,
Corporation Ltd., Nanjing, China), 2% Triton X-100 and PBS were
added to the cells per Eppendorf tube, followed by the addition of
propidium iodide (PI) to stain the DNA of the cells. The DNA
contents were quantified using an Accuri C6 flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
PI-annexin V staining analysis
Cell apoptosis rates were determined by a flow
cytometric analysis. In brief, HepG2 and Huh7 cells were treated
with HP (100 µg/ml) for 0, 12, 24 and 48 h. Then, the cells were
harvested and resuspended in binding buffer (KeyGen BioTech,
Corporation Ltd.). Aliquots of 5 µl annexin V-fluorescein
isothiocyanate and 5 µl PI were added to the cells, and the samples
were then analyzed by flow cytometry on an Accuri C6. Cell
apoptosis rates were determined in three independent
experiments.
Hoechst 33258 staining
Apoptotic nuclear changes were observed using
Hoechst 33258 (Sigma-Aldrich, St. Louis, MO, USA) staining. Cells
were treated with HP (100 µg/ml) for different time periods. The
treated cells were fixed with 4% paraformaldehyde and stained with
Hoechst 33258 (5 µg/ml). The apoptotic nuclear changes of the cells
were examined with a fluorescence microscope equipped with a
digital camera (Olympus Corporation).
Immunoblot analysis
Cells were treated with HP at a concentration of 100
µg/ml at different time points (0, 4, 8, 12, 24 and 48 h). The
cells were lysed in a lysis buffer in the presence of protease
inhibitors. Equal amounts of protein samples were separated by
7.5–15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred onto nitrocellulose membranes. After blocking with
5% non-fat milk, the membranes were incubated overnight at 4°C with
the primary antibodies. On the following day, the membranes were
washed and incubated with the goat anti-mouse immunoglobulin
(Ig)G-horseradish peroxidase (HRP) (catalog no. sc-2005; 1:5,000
dilution; Santa Cruz Biotechnology Inc.) and goat anti-rabbit
immunoglobulin (Ig)G-HRP (catalog no. sc-2030; 1:5,000 dilution;
Santa Cruz Biotechnology, Inc.) secondary antibodies for 1 h at
room temperature. The protein bands were detected using enhanced
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc,. Waltham,
MA, USA) and analyzed by Gel Doc XR+ (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Inhibitors treatment
For the apoptosis assay and the study of the
detailed regulatory mechanism of apoptosis following HP treatment,
inhibitors of apoptosis and MAPK were used. HepG2 and Huh7 cells
were incubated with 50 µM Z-VAD-FMK or MAPK inhibitors (PD98059, 20
µM; SP600125, 10 µM; and SB203580, 10 µM) for 1 h prior to
treatment with HP. Control cultures were treated with DMSO, which
was used as a solvent for the peptide inhibitors. The treated cells
were monitored by MTT assay, flow cytometry or immunoblot analysis
for apoptosis.
Statistical analysis
All reported data are presented as the mean ±
standard error of the mean of three replicates. Student's t-test
was used for determining the statistical difference between two
groups, and one-way analysis of variance was performed for multiple
comparisons. Statistical analysis was performed using SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
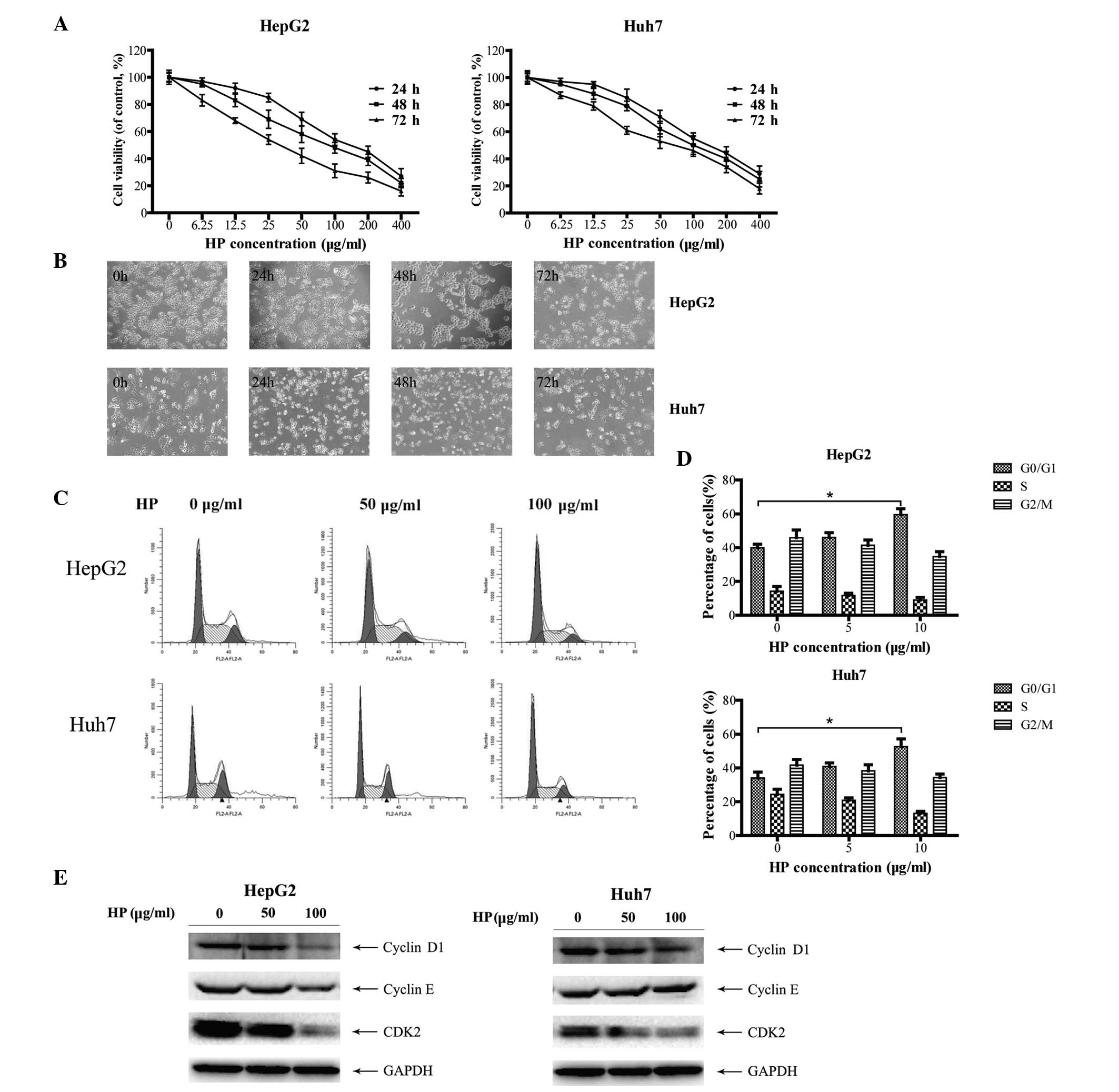
HP inhibits cell growth and induces
cell cycle arrest in HCC cells
HepG2 and Huh7 cells (23), were treated with HP for the indicated
time periods and concentrations, and subjected to cell growth
analysis. As shown in Fig. 1A, HP
significantly suppressed the proliferation of HepG2 and Huh7 cells
in a time- and dose-dependent manner. In addition, cell morphology
examination revealed that the majority of the HP-treated cells
presented the characteristics of shrinkage, irregularity, nuclear
condensation and fragmentation compared with mock-treated cells
(Fig. 1B), indicating cell damage
induced by the HP treatment.
To investigate whether the HP-triggered inhibitory
effect on HCC cell growth is due to cell cycle arrest, the cell
cycle profiles were determined by flow cytometry. As illustrated in
Fig. 1C and D, HP treatment triggered
G0/G1 cell cycle arrest in both HepG2 and Huh7 cells. Cyclins D1
and E are the main proteins of cell cycle regulation in G1/S phase
(24,25). Fig. 1E
depicts the dose-dependent decrease of the expression of cyclin D1,
cyclin E and CDK2 in HepG2 cells following HP treatment, which was
in line with the fluorescence-activated cell sorting data. Similar
results were obtained in the HP-treated Huh7 cells, with the
exception of cyclin E.
HP treatment triggers cell apoptosis
in HCC cells
To investigate whether the HP-induced inhibitory
effects on HCC cell proliferation are due to apoptosis, an annexin
V/PI double staining assay was performed. As illustrated in
Fig. 2A and B, HP exposure resulted
in remarkable apoptosis in both HepG2 and Huh7 cells in a
time-dependent manner. Hoechst 33258 staining revealed that
apoptotic chromatin condensation in HepG2 and Huh7 cells was
readily observed at all time points subsequent to HP exposure
(Fig. 2C), whereas nearly no
chromatin condensation was observed in the cells subjected to
control treatment.
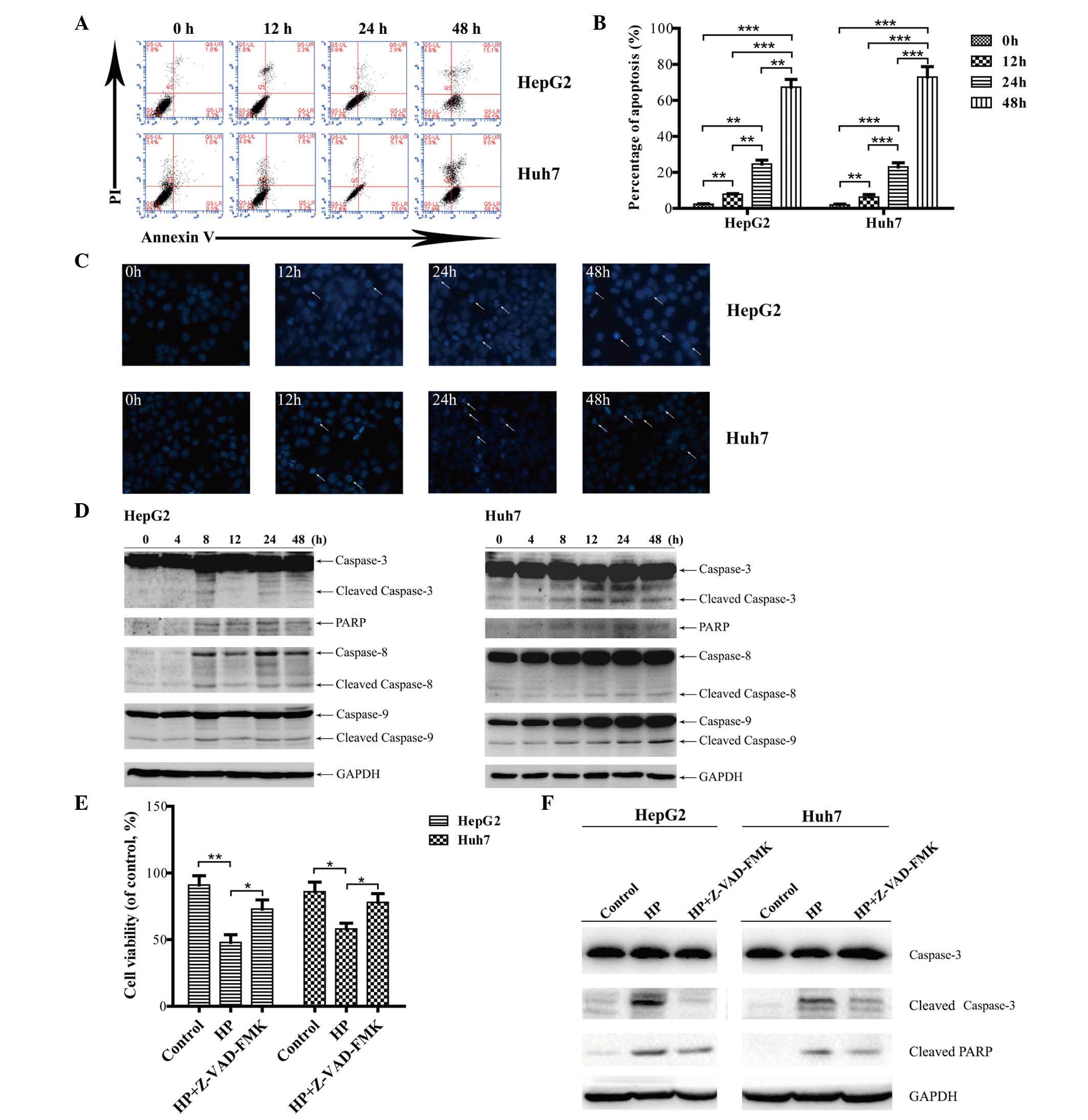 | Figure 2.HP induces apoptosis in HepG2 and
Huh7 cells in vitro. (A) Apoptosis was analyzed by flow
cytometry, and the dot plots of cells treated with 100 µg/ml HP for
0, 12, 24 and 48 h were obtained. (B) Comparison of apoptosis rates
at different times. The data are presented as the mean ± SEM from
three independent experiments. *P<0.05 indicates a significant
increase in the apoptosis rate in a time-dependent manner.
**P<0.01 and ***P<0.001. (C) The cells were treated with HP
for 0, 12, 24 and 48 h, stained with Hoechst 33258, and the cell
morphology was analyzed by fluorescence microscopy (magnification,
×200). (D) Detection of the activated forms of caspases-3, −8 and
−9 and cleaved PARP. The protein levels of glyceraldehyde
3-phosphate dehydrogenase served as a loading control. The proteins
were analyzed at the indicated time points, and all immunoblotting
experiments were performed twice. (E) Measurements by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
of cell viability upon treatment with 100 µg/ml HP, with or without
Z-VAD-FMK for 24 h. Data are presented as the mean ± SEM from three
independent experiments. *P<0.05 indicates a significant
increase in the cell viability upon treatment with Z-VAD-FMK. (F)
HepG2 and Huh7 cells were treated with HP (100 µg/ml), with or
without 50 µM Z-VAD-FMK for 24 h. The cell lysates were prepared
for immunoblotting to examine the expression of caspase-3 and PARP.
UL, upper left; UR, upper right; LL, lower left; LR, lower right;
PI, propidium iodide; PARP, poly (ADP-ribose) polymerase; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; HP, Huaier
polysaccharide; SEM, standard error of the mean. |
The activation of the death receptors (extrinsic)
and mitochondria (intrinsic) apoptotic pathways was next examined.
As shown in Fig. 2D, cleavage of
caspase-8 or caspase-9 was observed in HepG2 and Huh7 cells,
indicating that both the extrinsic and intrinsic apoptotic pathways
were involved in the HP-induced apoptosis in HCC cells. Caspase-3
is the major effector of apoptosis, and PAPR is cleaved during the
induction of apoptosis (26).
Fig. 2D depicts the activated forms
of caspase-3 and PARP cleavage that were detected in the HP-treated
HCC cells.
To further confirm that HP-induced apoptosis in
HepG2 and Huh7 cells is caspase-dependent, cells were pretreated
with a broad-specificity caspase inhibitor (Z-VAD-FMK), and cell
viability was analyzed by MTT assay and immunoblotting which
revealed the expression of caspase-3 and PARP. As shown in Fig. 2E, cell viability was markedly
recovered in cells treated with 50 µM Z-VAD-FMK for 24 h compared
with those treated with HP alone. The expression of cleaved
caspase-3 and PARP was markedly inhibited compared with the
HP-treated group (Fig. 2F).
Therefore, these results indicated that caspase activity is
required for HP-triggered apoptosis in HepG2 and Huh7 cells.
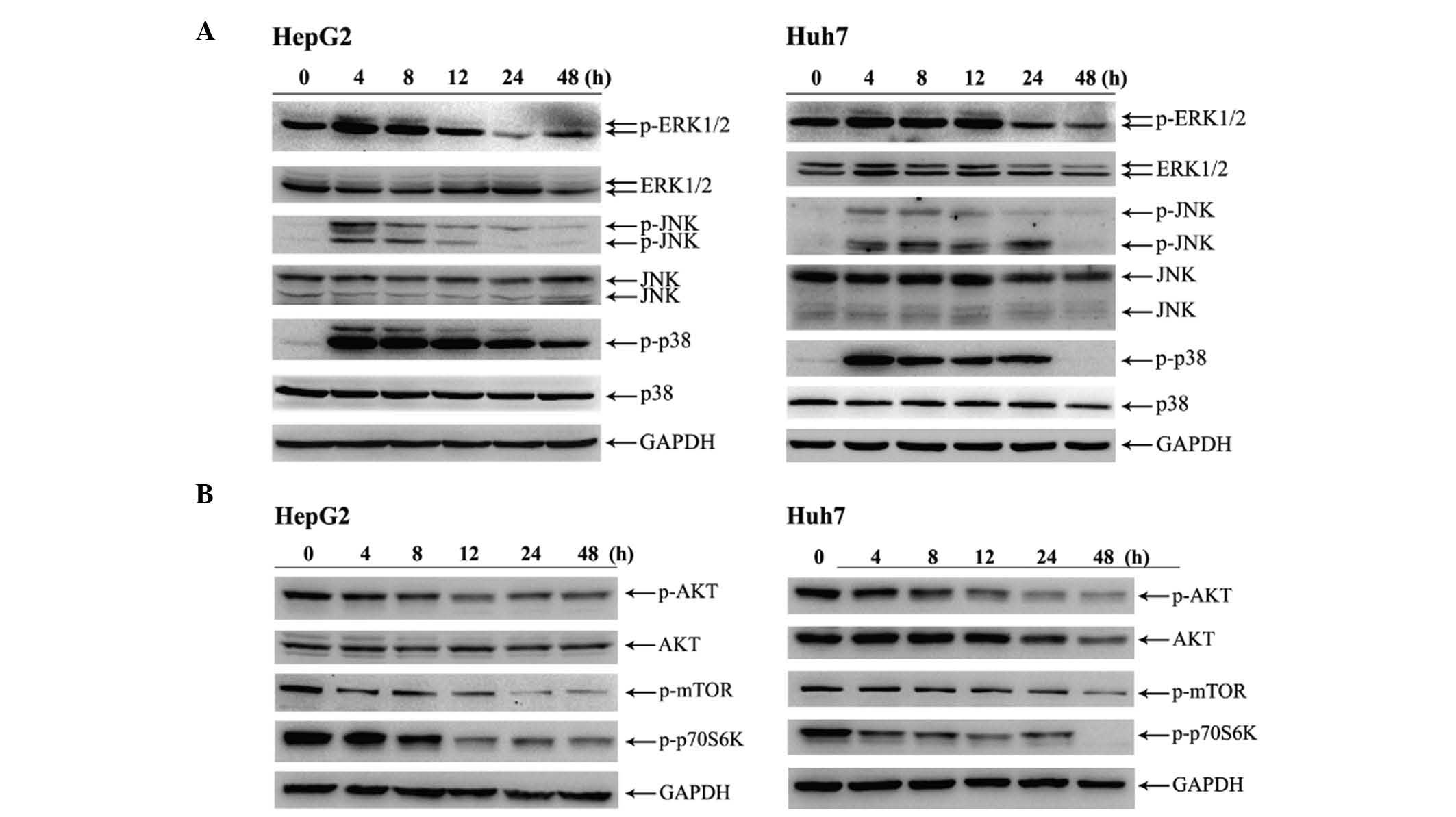
HP exposure activates MAPK pathways
and downregulates AKT phosphorylation in HCC cells
To gain a further insight into the mechanism by
which HP induces apoptosis, the phosphorylation levels of MAPK and
AKT were investigated. As presented in Fig. 3A, 4 h after HP treatment, the
phosphorylation levels of ERK1/2, JNK and p38 MAPK in HepG2 and
Huh7 cells were markedly increased. Notably, p38 MAPK was robustly
activated in the time interval from 4 to 24 h in both cell lines
(Fig. 3A). On the contrary, the HP
treatment attenuated the phosphorylation levels of both AKT and
mechanistic target of rapamycin (mTOR) in HepG2 and Huh7 cells
compared with their initial levels (Fig.
3B).
Pharmacological inhibition of p38 MAPK
attenuates the HP-induced apoptosis in both HepG2 and Huh7
cells
The specific inhibitors PD98059, SP600125 and
SB203580, which target MEK, JNK and p38 MAPK, respectively, were
used to treat the cells prior to HP exposure. The effective
concentrations of these inhibitors were tested in a dose-response
assay for each inhibitor to prevent cytotoxicity (data not shown).
As illustrated in Fig. 4A, the
HP-activated levels of ERK1/2, JNK and p38 in HepG2 and Huh7 cells
were blocked markedly in the presence of the corresponding
inhibitor at its specific concentration (20 µM for PD98059, 10 µM
for SP600125 and 10 µM for SB203580). Fig. 4B displays the decreased HP-induced
growth inhibition in HepG2 and Huh7 cells by SB203580 treatment,
while the other inhibitors had no significant effects. Flow
cytometry analysis demonstrated that the HP-triggered apoptosis in
HepG2 and Huh7 cells was attenuated by SB203580 (Fig. 4C). Notably, the application of
SP600125 led to decreased apoptosis in HP-treated HepG2 cells but
not in Huh7 cells.
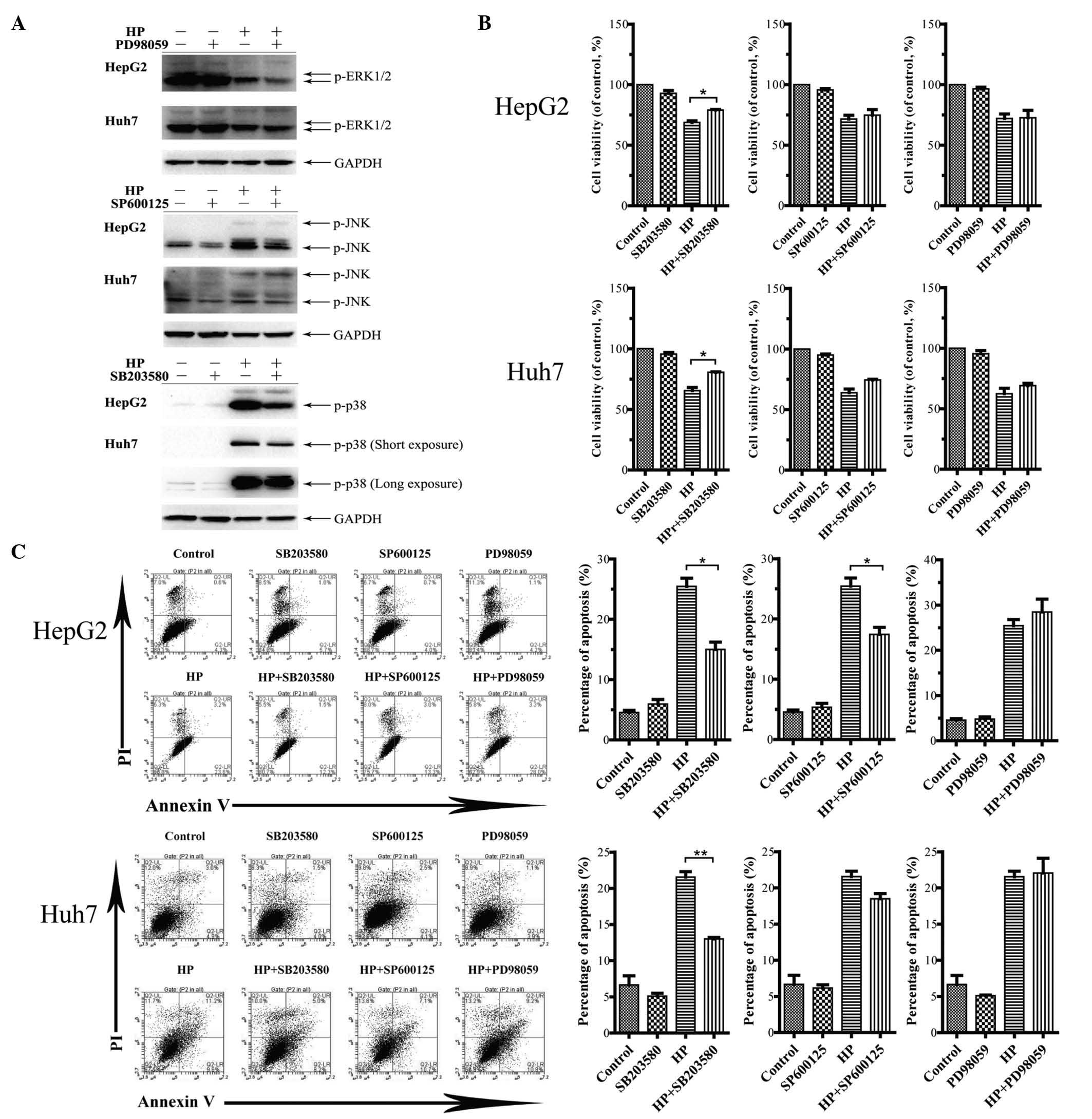 | Figure 4.Effects of inhibiting the MAPK
signaling pathway on cell viability and apoptosis. (A) HepG2 and
Huh7 cells were pretreated with PD98059 (20 µM), SP600125 (10 µM)
and SB203580 (10 µM) for 24 h. The cell lysates were prepared for
immunoblotting to examine the inactivation of the target pathways.
(B) Measurements by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
of cell viability upon treatment with 100 µg/ml HP, with or without
MAPK inhibitors for 24 h. The data are presented as the mean ± SEM
from three independent experiments. *P<0.05 indicates a
significant increase in cell viability following treatment with
MAPK inhibitors. (C) Influence of the MAPK signaling pathway on
apoptosis. Cell apoptosis was analyzed by flow cytometry upon
treatment with 100 µg/ml HP, with or without MAPK inhibitors for 24
h. The statistical results are presented in a bar chart. The data
correspond to the mean ± SEM from three independent experiments.
*P<0.05 and **P<0.01 indicate a significant decrease in the
apoptosis rate following treatment with MAPK inhibitors. UL, upper
left; UR, upper right; LL, lower left; LR, lower right; PI,
propidium iodide; HP, Huaier polysaccharide; p, phosphorylated;
ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MAPK,
mitogen-activated protein kinase; SEM, standard error of the
mean. |
Inactivation of p38 MAPK modulates the
effect of HP on the expression of apoptosis-related proteins
To explore the underlying mechanism by which the
inactivation of p38 MAPK pathway attenuates the apoptosis induced
by HP, the expression levels of a variety of apoptosis-related
proteins were investigated. As shown in Fig. 5A, the treatment with HP increased the
expression of the pro-apoptotic proteins Bim, Bax and p53 in HepG2
and Huh7 cells in a time-dependent manner, while the expression of
the pro-survival proteins Bcl-2, Bcl-xL, Mcl-1 and survivin was
downregulated. Next, HepG2 and Huh7 cells were treated with HP for
24 h in the presence or absence of the aforementioned MAPK
inhibitors. Fig. 5B indicates that
SB203580 treatment significantly increased the expression of Bcl-2
and survivin in HP-treated HepG2 cells compared with cells
subjected to treatment with HP alone, whereas the expression of Bax
was substantially downregulated by the combination treatment.
Similar results were obtained in Huh7 cells (Fig. 5C). However, the expression levels of
Bax, Bcl-2 and survivin were all increased in HepG2 and Huh7 cells
following the combination treatment with SP600125 and HP, compared
with the application of HP alone (Fig. 5B
and C). Notably, treatment with PD98059 considerably augmented
the expression of Bax in HP-treated HepG2 cells in comparison with
its expression levels when only HP was utilized (Fig. 5B), while the expression levels of
survivin were decreased and those of Bcl-2 remained unchanged. In
the HP-treated Huh7 cells, the expression of survivin was
significantly reduced in the presence of PD98059, which was
accompanied by a decline in the expression levels of Bcl-2 and an
unchanged level exhibited by Bax (Fig.
5C).
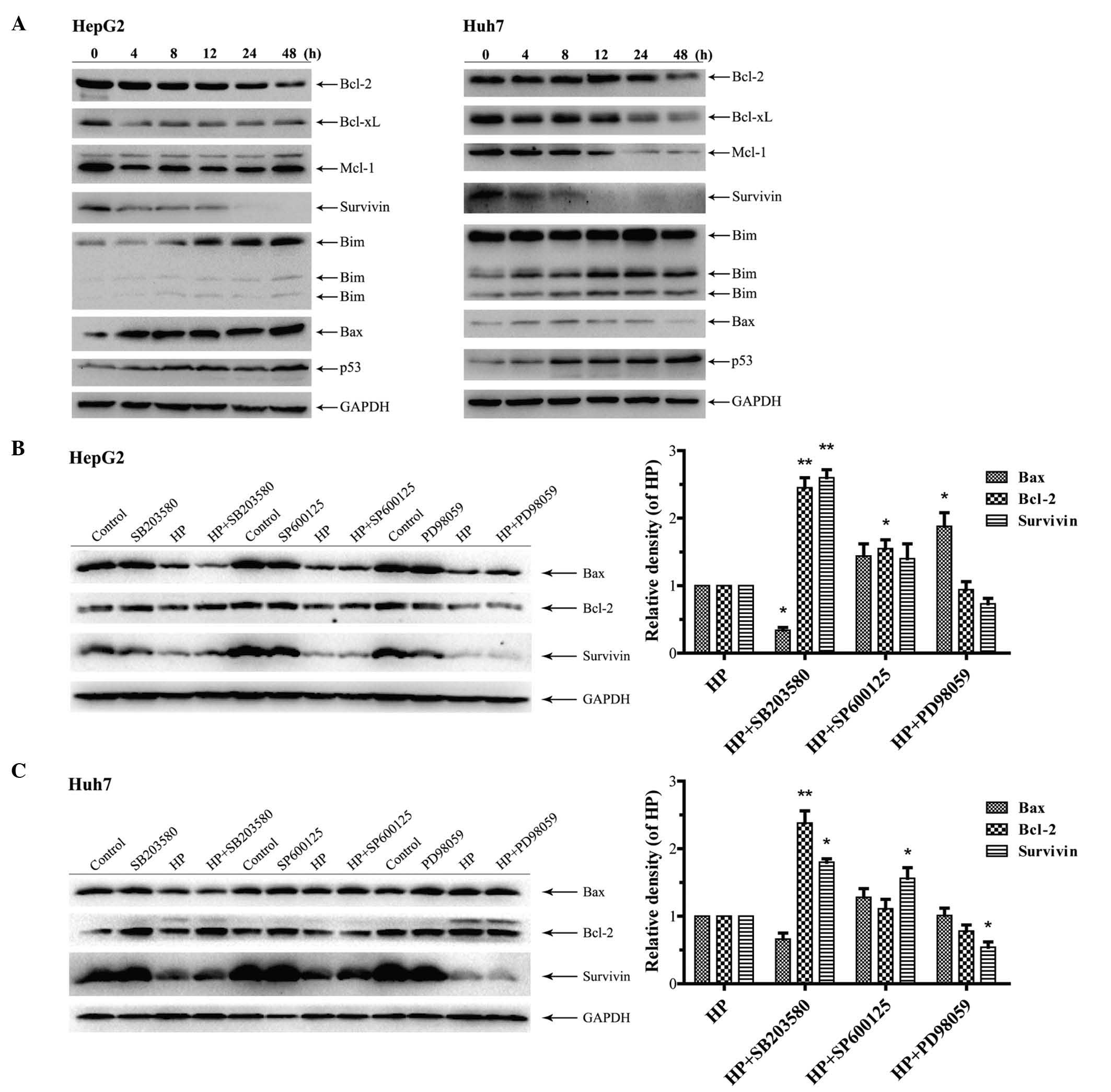 | Figure 5.Effects of HP treatment and
inhibition of the MAPK signaling pathway on the expression of AKT
and mitochondrial pathway proteins. (A) Cells were treated with HP
(100 µg/ml) for 0, 4, 8, 12, 24 and 48 h. The different expression
of the B-cell lymphoma-2 family proteins indicated that the
mitochondrial pathway was affected after the treatment with HP. The
expression of GAPDH was used as an internal control. All
immunoblotting experiments were performed twice. (B and C) Effects
of inhibiting the MAPK signaling pathway on the mitochondrial
pathway proteins in (B) HepG2 and (C) Huh7 cells. HP treatment at a
concentration of 100 µg/ml for 24 h, with or without MAPK
inhibitors (PD98059, 20 µM; SP600125, 10 µM; and SB203580, 10 µM).
The statistical results are presented in a column diagram. Data are
represented as the mean ± standard error of the mean from three
independent experiments. *P<0.05 and **P<0.01 indicate a
significant alteration in the expression levels of proteins after
the treatment with MAPK inhibitors. The expression of GAPDH was
used as an internal control. All immunoblotting experiments were
performed in duplicates. Bcl, B-cell lymphoma; Bax,
Bcl-2-associated X protein; xL, extra large; Mcl-1, myeloid cell
leukemia-1; Bim, Bcl-2-like 11; HP, Huaier polysaccharide; p,
phosphorylated; GAPDH, glyceraldehyde 3-phosphate dehydrogenase;
MAPK, mitogen-activated protein kinase. |
Discussion
In the present study, the role of the MAPK signaling
pathways in HP-induced apoptosis in HCC cells was investigated. It
was observed that HP activates the three major MAPK signaling
pathways, namely ERK, JNK and p38 MAPK, in HepG2 and Huh7 HCC
cells. Furthermore, the present findings provide evidence that the
pharmacological inhibition of p38 MAPK attenuates HP-induced
apoptosis in both cell lines. The present data demonstrate for the
first time a role of p38 MAPK in the HP-mediated effect on HCC
cells.
Recent studies have established that Huaier or HP
manifest antitumor effects in a variety of cancer cells in
vitro and in vivo, and various signaling pathways are
involved in the Huaier or HP-triggered antitumor activities in
different types of cancer cells. For instance, Huaier suppressed
the growth of colorectal cancer stem cells partially via
downregulation of the Wnt/β-catenin signaling pathway (26), whereas the upregulation of
microRNA-26b-5p contributed to the Huaier-induced apoptosis in
human pulmonary cancer cells (15).
In addition, Huaier inhibited ovarian cancer cell motility via the
AKT/glycogen synthase kinase 3β/β-catenin signaling pathway
(16). HP exhibited prominent
antitumor activities in vivo via enhancement of the host
immune system function in HCC tumor-bearing mice (17). Additionally, HP was able to suppress
human HCC MHCC97-H cell metastasis via inactivation of the
epithelial-mesenchymal transition and the astrocyte elevated gene-1
signaling pathway (12).
The MAPK signaling pathways are generally subdivided
into three separate pathways: ERK, JNK and p38 MAPK (20,27).
Normally, p38 MAPK activities are significantly lower in HCC, while
the activation of p38 MAPK may cause apoptosis of HCC cells
(28). Previous studies have
demonstrated that certain toxic agents induce cell apoptosis via
activation of p38 MAPK (29). The
present findings revealed that HP induced cell cycle arrest and
apoptosis, and activated the three major MAPK pathways in HCC
cells, although the expression of p38 MAPK significantly increased
compared with that of JNK and ERK. However, upon treatment with the
specific MAPK inhibitors PD98059, SP600125 and SB203580 (targeting
MEK, JNK and p38 MAPK, respectively), it was observed that the
inactivation of p38 MAPK markedly antagonized the effect of
HP-induced apoptosis and inhibition of proliferation in both HepG2
and Huh7 cells, indicating that p38 MAPK may play a role in the
process of apoptosis. The present results also revealed that HP
diminished the phosphorylation levels of AKT and mTOR, which is
consistent with previous findings reported for HP-treated ovarian
cancer cells and MHCC97-H cells (13,16).
The present study further unveiled the potential
molecular mechanisms by which p38 MAPK contributes to HP-induced
apoptosis in HCC cells. It has been reported that p38 MAPK acts as
a tumour suppressor in various cancer cells (30), and activated p38 MAPK is able to
induce apoptosis by regulating several proteins, including p53 and
Bcl-2 (31–33). Several studies have indicated that
Huaier and HP modulate the expression of apoptosis-related
proteins, including p53, Bcl-2, Bcl-xL and Bax (11,19,34), which
may be a critical mechanism employed by HP to mediate its effect on
cancer cell death (35). The present
data support the above observations, since upon treatment with HP,
the expression of the pro-survival proteins Bcl-2 and Bcl-xL
decreased, whereas that of the pro-apoptotic proteins Bax and p53
increased, thereby promoting the HP-induced apoptosis. In addition,
the present study is the first to report that HP downregulates the
expression of survivin in HCC cells, which is an inhibitor of
apoptotic proteins (36,37). Importantly, it was observed that the
inactivation of p38 MAPK, but not that of JNK or ERK,
simultaneously antagonized the effect of HP on the expression of
the apoptosis-related proteins Bcl-2, Bax and survivin, thereby
suggesting a potential mechanism by which p38 MAPK contributes to
the HP-induced apoptosis.
Previous in vivo data demonstrated that p53
expression was downregulated in HCC mouse models (11,19).
However, the present findings are contradictory to these
observations, as increased expression of p53 was observed in HepG2
cells expressing wild-type and functional p53, and in Huh7 cells
carrying the mutated and inactivated p53 gene (23). The results of the current study
suggested that HP could induce apoptosis in HCC cells regardless of
the p53 status. Based on the activity of p53 as a tumor suppressor
in cancer, its role in HP-induced apoptosis requires to be further
investigated.
In summary, the present study provides evidence that
HP induces apoptosis in HCC cells expressing either the wild-type
or the mutated p53 gene. Furthermore, it demonstrates the role of
p38 MAPK in HP-triggered cancer cell death. Thus, the present
findings enhance the understanding and provide novel insights into
the underlying mechanism by which HP exerts its antitumor
effect.
Acknowledgements
The authors would like to thank Dr Songshu Meng
(Institute of Cancer Stem Cell, Dalian Medical University Cancer
Center, Dalian, China), for providing guidance in the scientific
research and critical reading of the manuscript. The present study
was partly supported by the Specialized Research Fund for the
Doctoral Program of Higher Education (Beijing, China; grant no.
20122105110009), the National Natural Science Foundation of China
(Beijing, China; grant no. 81473504), the Program for Excellent
Talents in Universities of Liaoning province (Liaoning, China;
grant no. LR201518), the Hundred, Thousand, and Ten Thousand Talent
Project of Liaoning province (Liaoning, China; grant no. 2015–234)
and the Distinguished Professor Award of Liaoning Province
(Liaoning, China; grant no. 2013-204).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosch FX, Ribes J, Diaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127(5 Suppl 1): S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferenci P, Fried M, Labrecque D, Bruix J,
Sherman M, Omata M, Heathcote J, Piratsivuth T, Kew M, Otegbayo JA,
et al: World gastroenterology organisation guideline.
Hepatocellular carcinoma (HCC): A global perspective. J
Gastrointestin Liver Dis. 19:311–317. 2010.PubMed/NCBI
|
|
4
|
Thomas MB and Abbruzzese JL: Opportunities
for targeted therapies in hepatocellular carcinoma. J Clin Oncol.
23:8093–8108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Signoriello S, Annunziata A, Lama N,
Signoriello G, Chiodini P, De Sio I, Daniele B, Di Costanzo GG,
Calise F, Olivieri G, et al: Survival after locoregional treatments
for hepatocellular carcinoma: A cohort study in real-world
patients. Scientific World Journal. 2012:5647062012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan SL and Yeo W: Targeted therapy of
hepatocellular carcinoma: Present and future. J Gastroenterol
Hepatol. 27:862–872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu SC: Where are we in the chemoprevention
of hepatocellular carcinoma? Hepatology. 51:734–736.
2010.PubMed/NCBI
|
|
8
|
Chen XP, He SQ, Zhao X, Huang ZY and Li
CH: Chinese medicine Extractum trametes robiniophila murr augment
tumor necrosis factor related apoptosis-inducing ligand induced
apoptosis in human hepatic cancer cell lines. Zhonghua Wai Ke Za
Zhi. 43:1524–1527. 2005.(In Chinese). PubMed/NCBI
|
|
9
|
Zhang N, Kong X, Yan S, Yuan C and Yang Q:
Huaier aqueous extract inhibits proliferation of breast cancer
cells by inducing apoptosis. Cancer Sci. 101:2375–2383. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Zhang N, Huo Q, Sun M, Dong L,
Zhang Y, Xu G and Yang Q: Huaier aqueous extract inhibits stem-like
characteristics of MCF7 breast cancer cells via inactivation of
hedgehog pathway. Tumour Biol. 35:10805–10813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren J, Zheng C, Feng G, Liang H, Xia X,
Fang J, Duan X and Zhao H: Inhibitory effect of extract of fungi of
Huaier on hepatocellular carcinoma cells. J Huazhong Univ Sci
Technolog Med Sci. 29:198–201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng J, Li C, Wu X, Liu M, Sun X, Yang Y,
Hao M, Sheng S, Sun Y, Zhang H, et al: Huaier polysaccharides
suppresses hepatocarcinoma MHCC97-H cell metastasis via
inactivation of EMT and AEG-1 pathway. Int J Biol Macromol.
64:106–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng J, Li C, Wu X, Liu M, Sun X, Yang Y,
Hao M, Sheng S, Sun Y, Zhang H, et al: Astrocyte elevated gene-1
(AEG-1) shRNA sensitizes Huaier polysaccharide (HP)-induced
anti-metastatic potency via inactivating downstream P13K/Akt
pathway as well as augmenting cell-mediated immune response. Tumour
Biol. 35:4219–4224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Wu X, Zhang H, Yang G, Hao M, Sheng
S, Sun Y, Long J, Hu C, Sun X, et al: A Huaier polysaccharide
inhibits hepatocellular carcinoma growth and metastasis. Tumour
Biol. 36:1739–1745. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu T, Chen W, Liu S, Lu H, Wang H, Kong D,
Huang X, Kong Q, Ning Y and Lu Z: Huaier suppresses proliferation
and induces apoptosis in human pulmonary cancer cells via
upregulation of miR-26b-5p. FEBS Lett. 588:2107–2114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan X, Lyu T, Jia N, Yu Y, Hua K and Feng
W: Huaier aqueous extract inhibits ovarian cancer cell motility via
the AKT/GSK3β/β-catenin pathway. PLoS One. 8:e637312013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Wu X, Zhang H, Yang G, Hao M, Sheng
S, Sun Y, Long J, Hu C, Sun X, et al: A Huaier polysaccharide
restrains hepatocellular carcinoma growth and metastasis by
suppression angiogenesis. Int J Biol Macromol. 75:115–120. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Zhang N, Huo Q and Yang Q:
Anti-angiogenic and antitumor activities of Huaier aqueous extract.
Oncol Rep. 28:1167–1175. 2012.PubMed/NCBI
|
|
19
|
Xu X, Wei Q, Wang K, Ling Q, Xie H, Zhou L
and Zheng S: Anticancer effects of Huaier are associated with
down-regulation of P53. Asian Pac J Cancer Prev. 12:2251–2254.
2011.PubMed/NCBI
|
|
20
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haakenson J, Wu JY, Xiang S, Williams KA,
Bai W and Zhang X: HDAC6-Dependent functions in tumor cells:
Crossroad with the MAPK Pathways. Crit Rev Oncog. 20:65–81. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo Z, Yu G, Lee HW, Li L, Wang L, Yang D,
Pan Y, Ding C, Qian J, Wu L, et al: The Nedd8-activating enzyme
inhibitor MLN4924 induces autophagy and apoptosis to suppress liver
cancer cell growth. Cancer Res. 72:3360–3371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casimiro MC, Velasco-Velázquez M,
Aguirre-Alvarado C and Pestell RG: Overview of cyclins D1 function
in cancer and the CDK inhibitor landscape: Past and present. Expert
Opin Investig Drugs. 23:295–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pestell RG: New roles of cyclin D1. Am J
Pathol. 183:3–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rathmell JC and Thompson CB: The central
effectors of cell death in the immune system. Annu Rev Immunol.
17:781–828. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsieh SC, Huang MH, Cheng CW, Hung JH,
Yang SF and Hsieh YH: α-Mangostin induces mitochondrial dependent
apoptosis in human hepatoma SK-Hep-1 cells through inhibition of
p38 MAPK pathway. Apoptosis. 18:1548–1560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lamy E, Herz C, Lutz-Bonengel S, Hertrampf
A, Márton MR and Mersch-Sundermann V: The MAPK pathway signals
telomerase modulation in response to isothiocyanate-induced DNA
damage of human liver cancer cells. PLoS One. 8:e532402013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chiba T, Suzuki E, Yuki K, Zen Y, Oshima
M, Miyagi S, Saraya A, Koide S, Motoyama T, Ogasawara S, et al:
Disulfiram eradicates tumor-initiating hepatocellular carcinoma
cells in ROS-p38 MAPK pathway-dependent and -independent manners.
PLoS One. 9:e848072014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang Q, Li F, Shi K, Wu P, An J, Yang Y
and Xu C: ATF4 activation by the p38MAPK-eIF4E axis mediates
apoptosis and autophagy induced by selenite in Jurkat cells. FEBS
Lett. 587:2420–2429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taylor CA, Zheng Q, Liu Z and Thompson JE:
Role of p38 and JNK MAPK signaling pathways and tumor suppressor
p53 on induction of apoptosis in response to Ad-eIF5A1 in A549 lung
cancer cells. Mol Cancer. 12:352013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hui K, Yang Y, Shi K, Luo H, Duan J, An J,
Wu P, Ci Y, Shi L and Xu C: The p38 MAPK-regulated PKD1/CREB/Bcl-2
pathway contributes to selenite-induced colorectal cancer cell
apoptosis in vitro and in vivo. Cancer Lett. 354:189–199. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Bi L, Ye Y and Chen J:
Formononetin induces apoptosis in PC-3 prostate cancer cells
through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt
pathway. Nutr Cancer. 66:656–661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mayo LD and Donner DB: The PTEN, Mdm2, p53
tumor suppressor-oncoprotein network. Trends Biochem Sci.
27:462–467. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fernández JG, Rodriguez DA, Valenzuela M,
Calderon C, Urzúa U, Munroe D, Rosas C, Lemus D, Díaz N, Wright MC,
et al: Survivin expression promotes VEGF-induced tumor angiogenesis
via PI3K/Akt enhanced β-catenin/Tcf-Lef dependent transcription.
Mol Cancer. 13:2092014. View Article : Google Scholar : PubMed/NCBI
|