Introduction
Osteosarcoma is the most common primary malignant
tumor of the bone and represents a significant therapeutic
challenge. This type of bone and soft tissue sarcoma predominantly
affects children and young adults (1). Rapid advancements have occurred in the
treatment of osteosarcoma, including the introduction of adjuvant
chemotherapy, conformal radiotherapy and improved surgical excision
(2–5),
and the 5-year survival rate in patients with localized disease is
50–70% (6). However, prognosis
remains poor in relapsed disease and in patients that are resistant
to the standard treatment modalities (7). These poor outcomes have resulted in the
search for novel treatments that are more effective and less toxic,
including dendritic cell (DC)-based immunotherapy for disease
management.
In contrast to recent studies that have described
successful immunotherapy for certain solid tumors (8–10), the
number of reports on immunotherapy for osteosarcoma is limited and
this topic has received less attention (11,12). DCs
are professional antigen-presenting cells (APCs) that are able to
activate naive T cells and to induce the generation and expansion
of certain cytotoxic T lymphocytes (CTLs) and T helper cells
(8). The ability of DCs to
internalize, process and present antigens, mediated by major
histocompatibility complex class I and class II molecules, provides
a basis for the development of cancer vaccines (8). Extensive studies with DC-based
vaccination for the treatment of various types of malignant tumor
have been conducted, and have produced evidence of immune and
clinical responses, particularly in patients with melanoma and
renal cell carcinoma (8,9,13).
However, there have been fewer investigations into DC-based cancer
vaccination for osteosarcoma (9,10). For
several reasons, osteosarcoma provides a rational target to
evaluate the potential to induce tumor-specific immunity by DC
vaccination. Firstly, a randomized phase III study previously
indicated that muramyl tripeptide, an immune modulatory agent, in
combination with chemotherapy had a positive effect on survival
(14). Secondly, the long and
indolent disease courses often observed in patients with pulmonary
metastases from osteosarcoma may be indicative of natural tumor
immunity; however, this remains to be confirmed histologically or
immunologically (15). Thirdly,
tissue for use in vaccine generation is commonly available, as
surgical resection is often performed to remove pulmonary
metastatic lesions in patients with disease relapse.
The purpose of the present study was to examine an
in vitro model for the generation and expansion of a
DC-mediated CTL response for the prospective treatment of
osteosarcoma. The generation of systemic antitumor immunity by LM8
murine osteosarcoma cell lysate-pulsed DCs was investigated in
mouse models with osteosarcoma, and the hallmarks of a potent CTL
response were also explored.
Materials and methods
Cell culture and generation of
DCs
LM8 cells, derived from Dunn osteosarcoma, were
obtained from the Japanese Collection of Research Bioresources Cell
Bank (Osaka, Japan). The cells were cultured in complete medium
consisting of RPMI-1640 (Lonza Group Ltd., Basel, Switzerland)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 µg/ml
streptomycin (Lonza Group Ltd.) and 100 U/ml penicillin (Lonza
Group Ltd.). Cells were cultured at 37°C in an atmosphere of 5%
CO2.
Bone marrow-derived DCs were generated according to
the protocol described by Inaba et al (16), with minor alterations incorporated.
Briefly, erythrocyte-depleted mouse bone marrow cells were
collected from flushed marrow cavities (1×106 cells/ml)
and were cultured in complete medium with 20 ng/ml recombinant
mouse granulocyte-macrophage colony-stimulating factor (GM-CSF)
(R&D Systems, Inc., Minneapolis, MN, USA) in 10-cm tissue
culture dishes at 37°C in an atmosphere containing 50 ml
CO2/l. LM8 cells (6×106) were lysed by
rapidly freezing 3-day cultured LM8 cells in liquid nitrogen and
thawing in a water bath at 37°C 3 times. The thawed LM8 cell lysate
was added to the DC cultures on day 6 at a ratio of 1:5, and
incubated at 37°C in an atmosphere containing 50 ml
CO2/l. Following 24 h of incubation, non-adherent cells
were harvested by gentle pipetting. The cells were washed and
resuspended in phosphate-buffered saline (PBS) for subcutaneous
immunizations.
Animal experiments
All experimental procedures involving animals were
conducted under a protocol reviewed and approved by the Ethics
Committee of Tai'an City Central Hospital (Tai'an, China). All
efforts were made to minimize the number of animals used and to
ameliorate suffering. For in vivo studies, C3H mice were
obtained from Sankyo Labo Service Corporation, Inc. (Toyama, Japan)
and maintained in pathogen-free conditions. A total of
5×106 LM8 cells were subcutaneously implanted into the
gluteal region of 18 C3H mice (female; age, 6–8 weeks) for the
development of tumors. Following the development of the tumors, two
groups consisting of 9 mice each were immunized with
1×106 DCs pulsed in LM8 cell lysate, or with
polyinosinic:polycytidylic acid (poly I:C, a Toll-like receptor 3
agonist that acts as a maturation stimulus)-matured DCs; the
freshly prepared vaccine was diluted to the appropriate
concentration and delivered intradermally with a needle in a total
volume of 200 µl into the animal's groin, twice per week for 3
weeks.
Immunophenotyping
The phenotype of immature and mature DCs (iDCs and
mDCs) was studied using single-color fluorescence analysis. Cells
(3×105) were resuspended in 50 µl of buffer (PBS, 2%
FBS, and 1% sodium azide) and incubated for 30 min at 4°C with 10
µl of the appropriate fluorescein isothiocyanate (FITC)-labeled
monoclonal antibodies. The following primary FITC-tagged antibodies
were used to characterize the cells: CD34 (#MCA1825GA; clone
MEC14.7; mouse IgG1; dilution, 1:1,000), CD80 (#IMCA2462F; clone
RM80; mouse IgG1; dilution, 1:1,000) from Beckman Coulter (Brea,
CA, USA), CD83 (#MCA2747F; clone 3D11; mouse IgG1; dilution,
1:2,000) and CD86 (#553691; clone RMMP-2; mouse IgG1; dilution,
1:1,000) from AbD Serotec (Raleigh, NC, USA); CD205 (#130 102 906;
clone NLDC 145; rat IgG2a anti-mouse; dilution, 1:1,000) from
Miltenyi Biotec, Inc. (Singapore); and CD209 (#11-2092-80; clone
LWC06; rat IgG2B anti-mouse; dilution, 1:1,000) from eBioscience,
Inc. (San Diego, CA, USA). FITC-conjugated F(ab')2 goat anti-rat
IgG (#IC108F; dilution, 1:1,000; R&D Systems, Inc.) was used as
secondary antibody. Following incubation, the cells were washed
twice and then were resuspended in 500 µl of BD Pharmingen™ Stain
Buffer (#554656; BD Biosciences). Fluorescence was studied using a
FACSCalibur™ flow cytometer and analyzed using BD CellQuest™
software (both from BD Biosciences). For each sample, 20,000 events
were recorded, and the percentage of positive cells was reported.
SigmaStat™ version 4.0 software (Systat Software, Inc., San Jose,
CA, USA) was used for statistical analysis.
Quantification of antigen uptake
In brief, 2×105 cells were equilibrated
to 37°C for 45 min and pulsed with FITC-conjugated dextran (40,000
molecular mass; Sigma-Aldrich, St. Louis, MO, USA) at a
concentration of 1 mg/ml. Cold staining buffer (BD Biosciences) was
added to stop the reaction. The cells were washed 3 times and
stained with mouse phycoerythrin-conjugated anti-CD11c antibody
(1:1,000; #ab210309; Abcam, Cambridge, UK), and then analyzed using
BD CellQuest software version 5.1 with the FACSCalibur™ flow
cytometer. To obtain a background level, the non-specific binding
of dextran to DCs was determined by the incubation of DCs with
FITC-conjugated dextran for 30 min at 37°C. The medium used in the
cultures with dextran stimulation was supplemented with GM-CSF,
which is required for DCs to capture the antigen.
Measurement of CTL activity and
cytokine profile
At the end of the study, blood was collected using a
heart puncture and mice were euthanized via an intraperitoneal
injection of 5 ml sodium pentobarbital (Jinan Haohua Industry Co.,
Ltd., Shandong, China). Murine interferon (IFN)-γ and interleukin
(IL)-4 release were measured by enzyme-linked immunosorbent assay
(ELISA) with IFN-γ ELISA kit (#EM10012; Thermo Fisher Scientific,
Inc.) and IL-4 ELISA kit (#EMIL4; Thermo Fisher Scientific, Inc.),
respectively, according to the manufacturer's protocol, using an
iMark Microplate Absorbance Reader (Bio-Rad, Laboratories, Inc.,
Hercules, CA, USA).
Pooled spleen cells from immunized mice were
stimulated by three parallel sets of cultures in the presence of 20
µl/ml mouse IL-2 (R&D Systems, Inc.). Equivalent spleen cells
were co-cultured with DCs pulsed with LM8 cell lysate, poly
I:C-matured DC or LM8 cell lysate alone (control). The stimulated T
cells were harvested at 48 h, separated by passing through nylon
wool and used as effector cells in the CTL assay. The LM8 cell
lysate-loaded DCs, poly I:C-matured DCs and LM8 cells were labelled
with 51Cr for 60 min at 37°C and plated in round-bottom
96-well plates at 2×104 cells/well. Effector cells were
added at various effector/target cell ratios in triplicate, in a
total volume of 200 µl. After 5 h incubation, 25 µl of cell-free
supernatants was collected from each well and assayed in a gamma
counter (Wizard 1470-005; Perkin Elmer, Inc., Waltham, MaA, USA)
for 51Cr release. The spontaneous release of
51Cr was assessed by incubation of the targets in the
absence of effectors. Maximum or total release of 51Cr
was determined by incubation of the targets in 0.1% Triton X-100 in
distilled water. The percentage of specific 51Cr release
was determined by the following calculation: Percentage of specific
release = [(experimental - spontaneous)/(maximum - spontaneous)] ×
100%.
Mixed lymphocyte reaction (MLR)
assay
An allogeneic MLR assay was performed using mDCs
that were irradiated with 3,000 rad as a stimulator and allogeneic
T cells as responder cells in the ratios of 1:1, 1:10, 1:25 and
1:100. The positive and negative controls used were 2.5%
phytohemagglutinin-stimulated T cells (GlaxoSmithKline, Brentford,
UK), and the DCs or T cells alone, respectively. Cultures were made
in V bottom 96-well plates at a final volume of 200 µl of complete
medium supplemented with 10% human AB serum (Sigma-Aldrich) for 5
days and 3H-thymidine was added at a concentration of 1 µCurie/well
at 18 h prior to the end of the culture. Proliferative responses
were measured by a liquid scintillation counter (PerkinElmer, Inc.)
and expressed as the mean count/min obtained for triplicate
wells.
Statistical analysis
The differences between the two groups were
determined using analysis of variance and the Scheffe test. All
analyses were conducted using SigmaStat™ version 4.0 software.
Results were expressed as the mean ± standard deviation, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
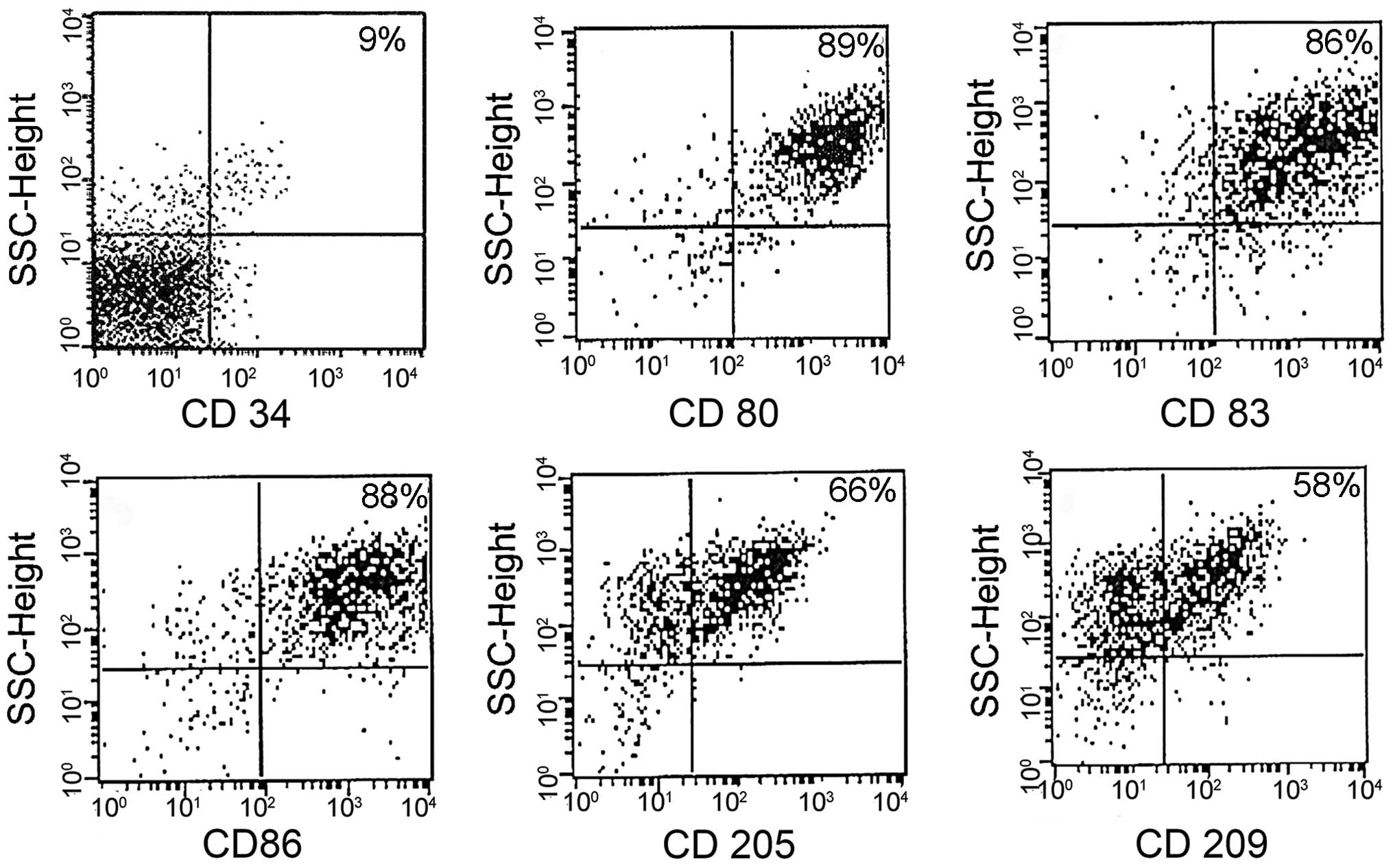
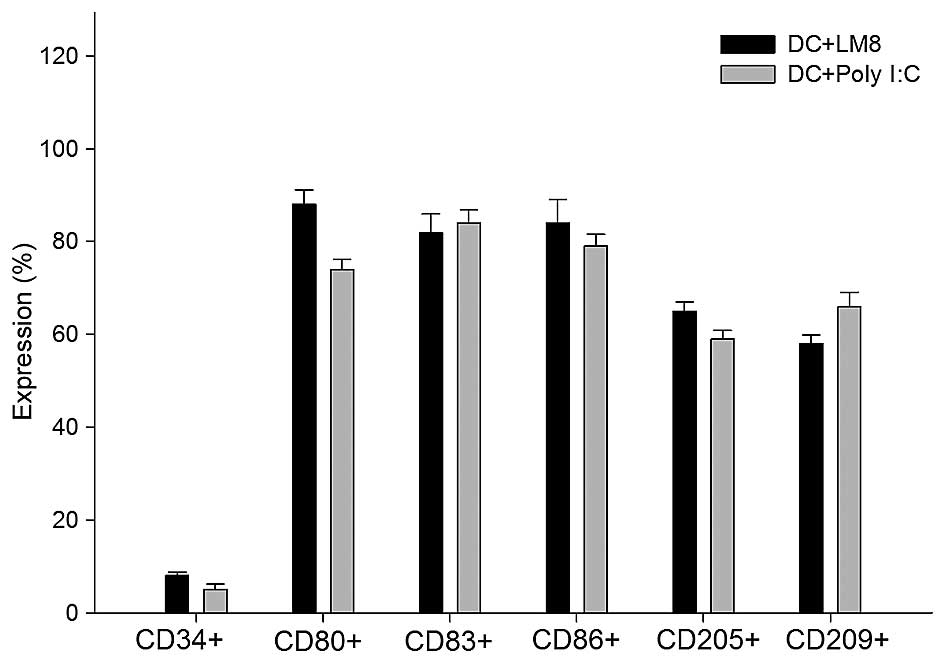
Immunophenotyping
DCs were assessed for cell surface phenotypes by
flow cytometry. A greater degree of maturation was observed in
LM8-pulsed DCs than in poly I:C-matured DCs. The results showed
that DCs expressed a high level of function of associated surface
antigens in the LM8-pulsed mDCs, including the following:
CD80+, 89%; CD83+, 86%; CD86+,
88%; CD205+, 66%; and CD209+, 58% (Fig. 1). The DCs matured with the poly I:C
antigen (control) showed similar surface antigen expression
(Fig. 2), and no significant
difference was indicated in the expression profile of the two
groups.
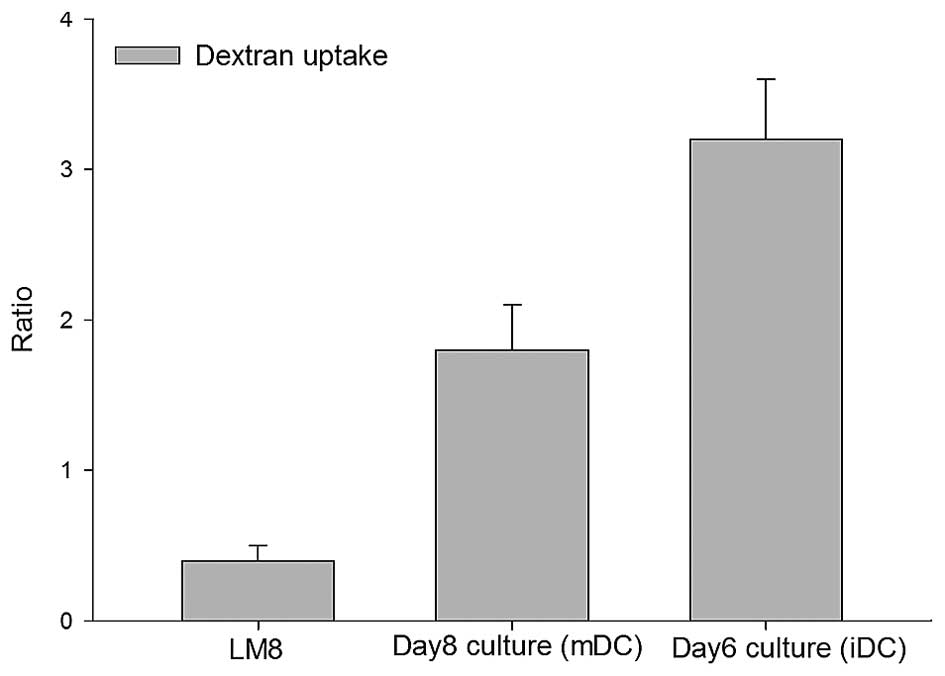
Quantitation of antigen uptake
Endocytosis is a functional feature of iDCs which
decreases with maturation (17). The
iDCs obtained on day 6 demonstrated high endocytotic capability. As
expected, an increased dextran uptake occurred by iDCs (day 6
culture) compared with the day 8 mDCs, but the increase was not
statistically significant (P=0.12) (Fig.
3). However, compared with the LM8 cells, a statistically
significant increase in dextran uptake was observed in the day 6
and 8 cells (P<0.026).
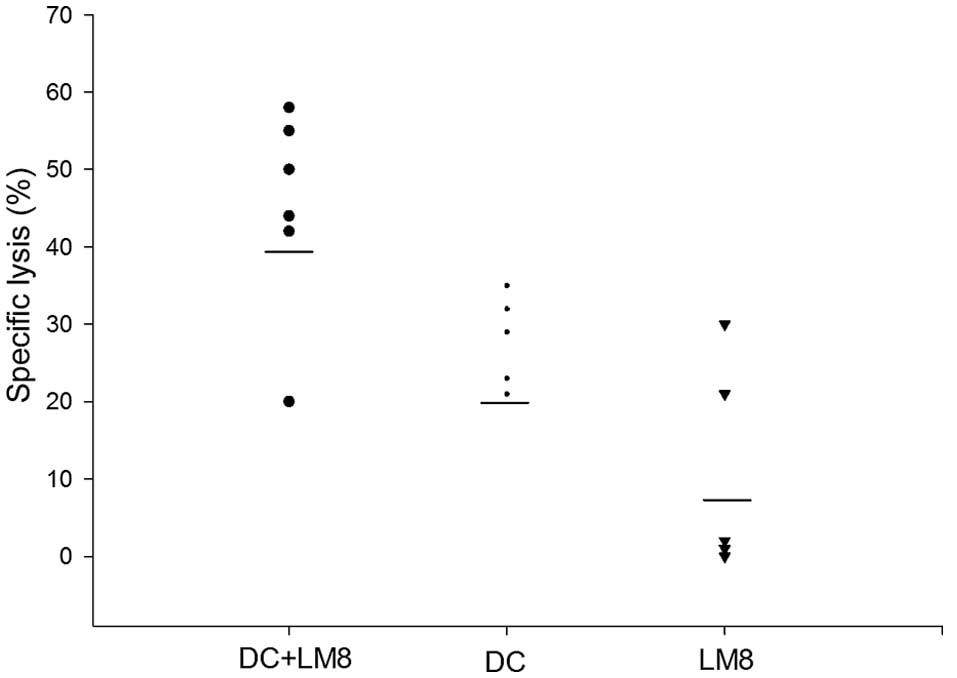
Measurement of CTL activity
To determine the ability of LM8 cell lysate-pulsed
DCs to generate LM8-specific CTLs against tumor cells, in
vitro CTL assays with splenocytes from C3H mice immunized with
the DC vaccine were performed. Mice that were previously injected
with LM8 cells to develop a tumor but were not administered DCs
acted as controls. As shown in Fig.
4, the highest CTL activity was noted in mice immunized with
LM8 cell lysate-pulsed DCs in vivo. Mice immunized with poly
I:C-matured DCs demonstrated a low level of CTL activity, whereas
mice injected with only LM8 cells demonstrated further decreased
LM8-specific lysis. Thus, LM8-specific CTL activity was
significantly enhanced in mice immunized with LM8 cell
lysate-matured DCs compared with poly I:C-matured DCs
(P<0.046).
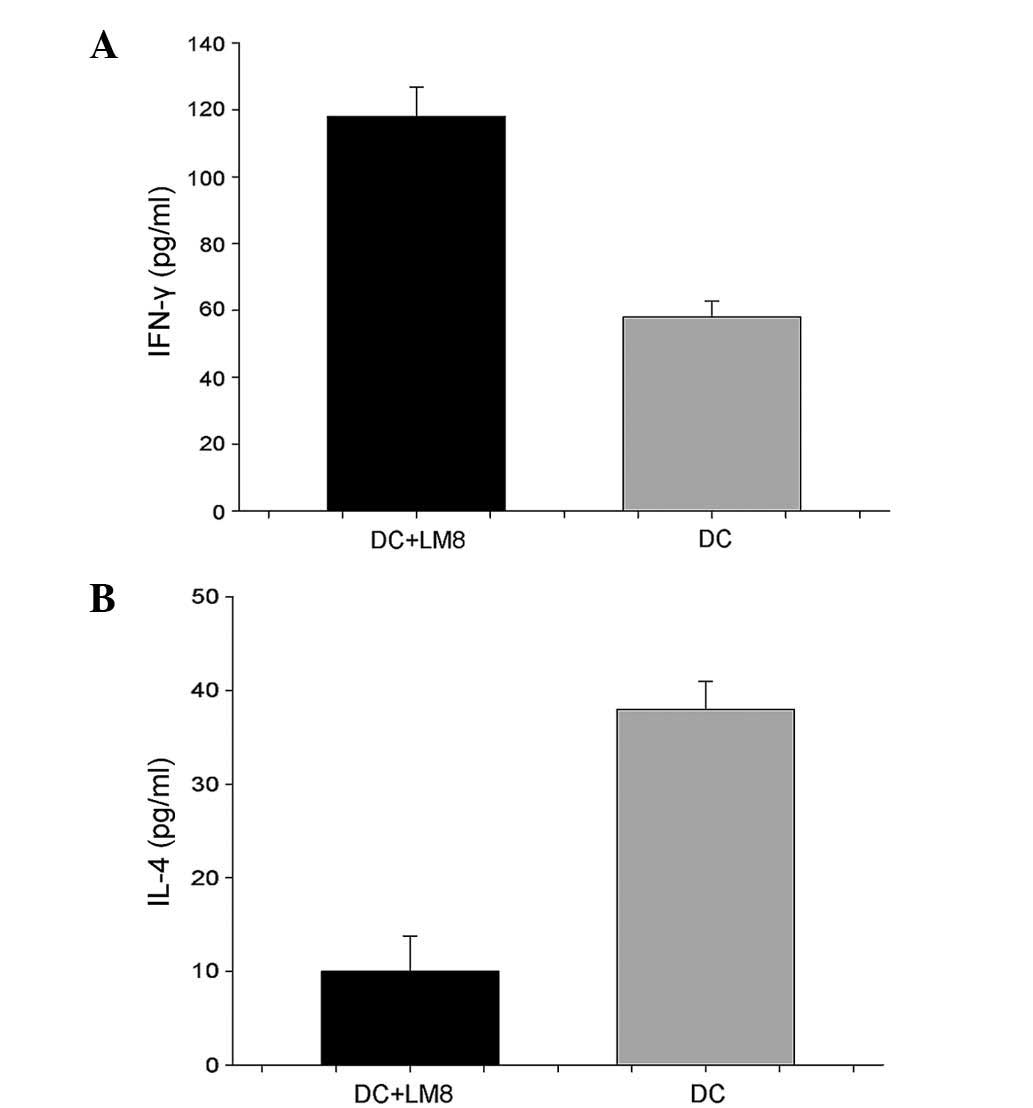
Serum IFN-γ levels were increased in mice that
received LM8 cell lysate-pulsed DCs (116.0±5.15 pg/ml) compared
with the poly I:C-matured DC group (57.33±2.31 pg/ml; P<0.05)
(Fig. 4). Serum IL-4 was decreased in
the mice that received DCs pulsed with LM8 cell lysate, at
12.23±4.75 pg/ml vs. 41.06±2.51 pg/ml in poly I:C mDCs (P<0.047)
(Fig. 5).
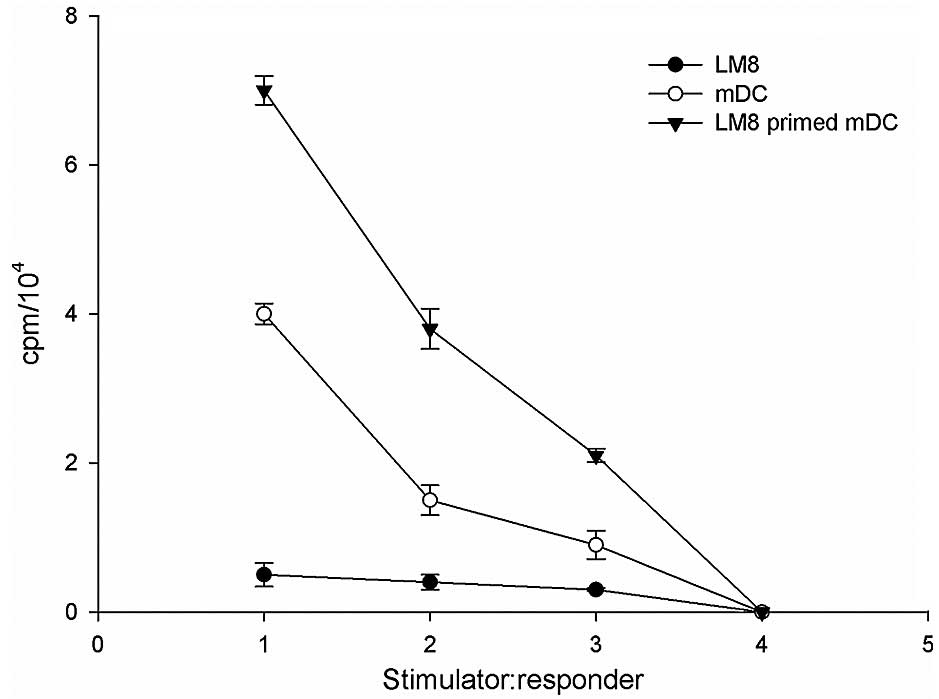
MLR assay
The effect of the two DC populations on the
allogeneic responder lymphocyte was assessed using an MLR assay.
The capacity of the DC subsets to stimulate allogeneic T cells was
associated with the levels of co-stimulatory molecules CD86, CD83,
CD205 and CD209 of the DC subpopulations (18). In order to investigate the critical
function of mDCs to activate T cell proliferation through antigen
presentation, the present study determined whether LM8 cell
lysate-pulsed DCs could induce antigen-specific CD4+ and
CD8+ T cell responses. The results revealed that priming
of DCs with LM8 effectively enhanced CD4+ and
CD8+ T cell proliferative responses at a DC:T cell ratio
of 1:1, 1:10 and 1:25, compared with LM8 cells alone (Fig. 6).
Discussion
DCs exposed to tumor antigens serve crucial roles in
antitumor immunity by inducing tumor-specific T cell responses. The
current study used lysate from LM8 osteosarcoma cells to pulse DCs
and elicit a CTL response in a mouse osteosarcoma model; this
strategy has also been studied in other solid tumors (18–20). LM8
is an osteosarcoma cell line, originally from the C3H/He mouse,
that has high metastatic potential and has been shown to cause
metastatic osteosarcoma in C3H mice (21). Unlike specific and known
tumor-associated peptide antigens, whole tumor cells or their
derivatives may be used to stimulate T cells in a variety of tumors
for which few or no defined tumor-specific antigens are available,
including colon cancer and osteosarcoma (10,19).
Furthermore, the application of whole tumor cell immunizations may
result in the polyvalent stimulation of CD8+ CTLs and
CD4+ T cells against a broad range of antigens.
Experiments in vitro and in mouse models support the notion
that tumor-specific T cells may be activated and expanded in
vitro, and that they may inhibit tumor growth (18).
In the current study, LM8 lysate-pulsed DCs and poly
I:C-matured DCs derived from bone marrow were found to express
CD80, CD83, CD86, CD205 and CD209, which is consistent with the
properties of APCs and earlier studies (22). Although the poly I:C-based protocol
has been proposed as a potentially superior alternative to the
conventional method proposed by Inaba et al (16), the present study has, for the first
time, shown that minor modifications to the whole tumor cell lysate
strategy may elicit a better CTL response.
DCs without sufficient tumor-associated antigens
lack the ability to target tumor cells and present the antigen to T
lymphocytes. Therefore, the present study evaluated endocytosis,
using FITC-labelled dextran, as a functional feature of DC. As
expected, an increased dextran uptake by iDCs (day 6 culture) was
observed compared with the day 8 mDCs; however, the increase was
not statistically significant. Various studies have indicated that
the uptake of dextran may be a measure of immune response (23–25). The
present results also support earlier studies that suggest that
increased antigen uptake is associated with IL-12 and IFN-γ
secretion, and CTL response (17,26,27).
LM8-specific CTL activity was significantly enhanced in mice
immunized with LM8 cell lysate-pulsed DCs compared with poly
I:C-matured DCs (P<0.026).
The capacity of the DC subsets to stimulate
allogeneic T cells was associated with the levels of the
co-stimulatory molecules CD86, CD83, CD205 and CD209 in the DC
subpopulations (18). Pulsing of DCs
with LM8 cell lysate effectively enhanced CD4+ and
CD8+ T cell proliferative responses compared with LM8
cells alone. The group treated with the combination of LM8 cell
lysate-pulsed DCs showed increased expression of serum IFN-γ levels
compared with the poly I:C-matured DC group. Serum IL-4 was
decreased in the mice that received DCs pulsed with LM8 cell lysate
vs. the poly I:C-matured DCs.
The present study was subject to certain limitations
as the clinical setting used in humans was not completely
replicated using the mouse model, and synergistic effects with
chemotherapy were not assessed.
In summary, the present study expands the validation
of the whole tumor cell lysate-based strategy in the active
immunotherapy of patients with osteosarcoma. Additional validation
in a clinical setting may support the use of its approach in
conjunction with chemotherapy.
References
|
1
|
Renard AJ, Veth RP, Schreuder HW,
Pruszczynski M, Bökkerink JP, van Hoesel QG and van Der Staak FJ:
Osteosarcoma: Oncologic and functional results. A single
institutional report covering 22 years. J Surg Oncol. 72:124–129.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrari S, Smeland S, Mercuri M, Bertoni
F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini
G, et al: Neoadjuvant chemotherapy with high-dose Ifosfamide,
high-dose methotrexate, cisplatin and doxorubicin for patients with
localized osteosarcoma of the extremity: A joint study by the
Italian and Scandinavian Sarcoma Groups. J Clin Oncol.
23:8845–8852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kager L, Zoubek A, Dominkus M, Lang S,
Bodmer N, Jundt G, Klingebiel T, Jürgens H, Gadner H and Bielack S:
COSS Study Group: Osteosarcoma in very young children: Experience
of the cooperative osteosarcoma study group. Cancer. 116:5316–5324.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewis VO: What's new in musculoskeletal
oncology. J Bone Joint Surg Am. 89:1399–1407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stiller CA, Bielack SS, Jundt G and
Steliarova-Foucher E: Bone tumours in European children and
adolescents, 1978–1997. Report from the automated childhood cancer
information system project. Eur J Cancer. 42:2124–2135. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bacci G, Briccoli A, Ferrari S, Longhi A,
Mercuri M, Capanna R, Donati D, Lari S, Forni C and DePaolis M:
Neoadjuvant chemotherapy for osteosarcoma of the extremity:
Long-term results of the Rizzoli's 4th protocol. Eur J Cancer.
37:2030–2039. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bacci G, Briccoli A, Rocca M, Ferrari S,
Donati D, Longhi A, Bertoni F, Bacchini P, Giacomini S, Forni C, et
al: Neoadjuvant chemotherapy for osteosarcoma of the extremities
with metastases at presentation: Recent experience at the Rizzoli
Institute in 57 patients treated with cisplatin, doxorubicin and a
high dose of methotrexate and ifosfamide. Ann Oncol. 14:1126–1134.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nestle FO, Alijagic S, Gilliet M, Sun Y,
Grabbe S, Dummer R, Burg G and Schadendorf D: Vaccination of
melanoma patients with peptide- or tumor lysate-pulsed dendritic
cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berntsen A, Geertsen PF and Svane IM:
Therapeutic dendritic cell vaccination of patients with renal cell
carcinoma. Eur Urol. 50:34–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suminoe A, Matsuzaki A, Hattori H, Koga Y
and Hara T: Immunotherapy with autologous dendritic cells and tumor
antigens for children with refractory malignant solid tumors.
Pediatr Transplant. 13:746–753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Campbell CJ, Cohen J and Enneking WF:
Editorial: New therapies for osteogenic sarcoma. J Bone Joint Surg
Am. 57:143–144. 1975.PubMed/NCBI
|
|
12
|
Kawaguchi S, Wada T, Tsukahara T, Ida K,
Torigoe T, Sato N and Yamashita T: A quest for therapeutic antigens
in bone and soft tissue sarcoma. J Transl Med. 3:312005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thurner B, Haendle I, Röder C, Dieckmann
D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von
den Driesch P, et al: Vaccination with mage-3A1 peptide-pulsed
mature, monocyte-derived dendritic cells expands specific cytotoxic
T cells and induces regression of some metastases in advanced stage
IV melanoma. J Exp Med. 190:1669–1678. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meyers PA, Schwartz CL, Krailo MD, Healey
JH, Bernstein ML, Betcher D, Ferguson WS, Gebhardt MC, Goorin AM,
Harris M, et al: Osteosarcoma: The addition of muramyl tripeptide
to chemotherapy improves overall survival-a report from the
Children's Oncology Group. J Clin Oncol. 26:633–638. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palmerini E, Staals EL, Ferrari S, Rinaldi
R, Alberghini M, Mercuri M and Bacci G: Nonresectable multiple lung
metastases of high-grade osteosarcoma of the humerus: Stable after
twelve years. A case report. J Bone Joint Surg Am. 90:2240–2244.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inaba K, Inaba M, Romani N, Aya H, Deguchi
M, Ikehara S, Muramatsu S and Steinman RM: Generation of large
numbers of dendritic cells from mouse bone marrow cultures
supplemented with granulocyte/macrophage colony-stimulating factor.
J Exp Med. 176:1693–1702. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiang CL, Hagemann AR, Leskowitz R, Mick
R, Garrabrant T, Czerniecki BJ, Kandalaft LE, Powell DJ Jr and
Coukos G: Day-4 myeloid dendritic cells pulsed with whole tumor
lysate are highly immunogenic and elicit potent anti-tumor
responses. PLoS One. 6:e287322011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hatfield P, Merrick AE, West E, O'Donnell
D, Selby P, Vile R and Melcher AA: Optimization of dendritic cell
loading with tumor cell lysates for cancer immunotherapy. J
Immunother. 31:620–632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alaniz L, Rizzo MM and Mazzolini G:
Pulsing dendritic cells with whole tumor cell lysates. Methods Mol
Biol. 1139:27–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kandalaft LE, Powell DJ Jr, Chiang CL,
Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA,
et al: Autologous lysate-pulsed dendritic cell vaccination followed
by adoptive transfer of vaccine-primed ex vivo co-stimulated T
cells in recurrent ovarian cancer. Oncoimmunology. 2:e226642013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozawa E, Sugiura H, Wasa J, Kohyama K,
Yamada K, Nishioka A, Nishida Y, Ishiguro N and Taguchi O:
Suppression of tumour metastasis in a murine osteosarcoma model
with anti-CD25 monoclonal antibody treatment. Anticancer Res.
30:5019–5022. 2010.PubMed/NCBI
|
|
22
|
Thomas-Kaskel AK, Zeiser R, Jochim R,
Robbel C, Schultze-Seemann W, Waller CF and Veelken H: Vaccination
of advanced prostate cancer patients with PSCA and PSA
peptide-loaded dendritic cells induces DTH responses that correlate
with superior overall survival. Int J Cancer. 119:2428–2434. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Napoletano C, Pinto D, Bellati F, Taurino
F, Rahimi H, Tomao F, Panici PB, Rughetti A, Frati L and Nuti M: A
comparative analysis of serum and serum-free media for generation
of clinical grade DCs. J Immunother. 30:567–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coester C, Nayyar P and Samuel J: In
vitro uptake of gelatin nanoparticles by murine dendritic cells
and their intracellular localisation. Eur J Pharm Biopharm.
62:306–314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Araki H, Katayama N, Mitani H, Suzuki H,
Nishikawa H, Masuya M, Ikuta Y, Hoshino N, Miyashita H, Nishii K,
et al: Efficient ex vivo generation of dendritic cells from CD14+
blood monocytes in the presence of human serum albumin for use in
clinical vaccine trials. Br J Haematol. 114:681–689. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mahdian R, Kokhaei P, Najar HM, Derkow K,
Choudhury A and Mellstedt H: Dendritic cells, pulsed with lysate of
allogeneic tumor cells, are capable of stimulating MHC-restricted
antigen-specific antitumor T cells. Med Oncol. 23:273–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vegh Z and Mazumder A: Generation of tumor
cell lysate-loaded dendritic cells preprogrammed for IL-12
production and augmented T cell response. Cancer Immunol
Immunother. 52:67–79. 2003.PubMed/NCBI
|