Introduction
The first step of tumor metastasis is cell
detachment from a primary tumor (1).
Normally, adhesions between normal epithelial cells and the
cellular matrix are strong; cell detachment may be achieved via
downregulation of cell adhesion molecules (2). It has been reported that loss of
E-cadherin results in the detachment of adjacent cells (3,4). Focal
adhesion kinase (FAK) is a central regulator of focal adhesion,
promoting cell adhesion and metastasis (5). Previous studies have reported that
simultaneous inhibition of FAK promotes the detachment of colon
cancer cells and fibroblasts (6,7). However,
Wade et al (8) reported that
FAK tyrosine phosphorylation was present in detached fibroblast
cells. Therefore, the involvement of FAK in cell detachment remains
controversial.
Casitas B-lineage lymphoma-b (Cbl-b), a RING finger
E3 ubiquitin protein ligase, is composed of a tyrosine kinase
binding domain, an ubiquitin-associated domain, RING finger domains
and several tyrosine phosphorylation sites. It has been
hypothesized that Cbl-b exhibits a crucial function as a ubiquitin
ligase and multifunctional adaptor molecule (9). A previous study demonstrated that Cbl-b
is involved in the regulation of the cell cytoskeleton and adhesion
(10). Furthermore, it has been
reported that Cbl-b reduces cell adhesion via negative regulation
of integrin and receptor-mediated signaling (11). By contrast, a previous study reported
that Cbl-b enhances cell-to-cell adherens junctions and cell
adhesion (12). Schmidt and Dikic
(13) revealed that c-Cbl, a homolog
of Cbl-b, associates with FAK and affects cellular attachment to
the extracellular matrix (ECM). In addition, Rafiq et al
(14) demonstrated that c-Cbl
interacts with FAK, resulting in enhanced c-Cbl-mediated FAK
ubiquitination and subsequent downregulation of myocyte survival
signaling. However, whether Cbl-b targets FAK for degradation and
its involvement in the process of cell detachment requires further
investigation.
The aim of the present study was to evaluate the
effect of FAK and its function in the process of tumor cell
detachment. The present study demonstrated that FAK suppressed
trypsin-induced cell detachment, and lysosome inhibitor
NH4Cl suppressed cell detachment through
mono-ubiquitination of FAK, while Cbl-b promoted cell detachment
through ubiquitination of FAK. These results provide novel insights
into the role of Cbl-b and FAK in cell detachment.
Materials and methods
Reagents and antibodies
Antibodies against Cbl-b (mouse monoclonal catalog
no., sc-8006; dilution, 1:250) and actin (rabbit polyclonal;
catalog no., H-196; dilution, 1:1,000) were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Antibodies against Akt
(rabbit polyclonal; catalog no., 4691; dilution, 1:1,000),
phosphorylated (p)-Akt (rabbit polyclonal; catalog no., 4060;
dilution, 1:500), FAK (rabbit polyclonal; catalog no., 3285;
dilution, 1:500), anti-p-FAK (rabbit polyclonal; catalog no., 8556;
dilution, 1:500) and ubiquitin (rabbit polyclonal; catalog no.,
3936; dilution, 1:500) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Dimethyl sulfoxide was
obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The human gastric adenocarcinoma MGC803, lung
adenocarcinoma A549, colon carcinoma RKO, breast adenocarcinoma
MCF-7, breast carcinoma MDA-MB-231 and gastric epithelial cell
GES-1 cell lines were obtained from the Academy of Military Medical
Sciences (Beijing, China). The cells were cultured in RPMI-1640
medium or L-15 medium containing 10% fetal calf serum (all
Gibco®; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in a humidified incubator with an atmosphere of 5%
CO2.
MTT assay
The percentage of detached cells was analyzed using
MTT assay. The cells (2×105 cells/well) were treated
with 2.5 mg/l trypsin for various times (0, 1, 3 and 5 min) and
washed four times with ice-cold phosphate-buffered saline. The MTT
assay was performed as described in a previous study by the present
authors (15).
Western blot analysis
The cells (2×105 cells/well) were seeded
in 6-well plates and incubated overnight. The cells were treated
with 2.5 mg/l trypsin for various times (0, 1, 3 and 5 min). The
medium was removed and the cells were added to a 1.5 ml Eppendorf
tube followed by transient centrifugalization. Western blot
analysis was performed as described in a previous study (15). Western blot images were analyzed using
National Institutes of Health image software (rsb.info.nih.gov/nih-image/) for further
statistical analysis.
Immunoprecipitation
The antibodies against FAK and Cbl-b, protein
G-agarose beads (Cell Signaling Technology, Inc.) and cell lysate
were incubated overnight at 4°C. Subsequently, immunoprecipitates
were washed four times with lysis buffer. Immunoprecipitation was
performed as described in a previous study (16).
Plasmid construction and
transfection
Plasmid construction was performed as described in a
previous study (15). Briefly, MGC803
and MDA-MB-231 cells were transfected with short hairpin (sh) RNA
targeting Cbl-b using Lipofectamine 2000 reagent (Invitrogen™;
Thermo Fisher Scientific, Inc), according to the manufacturer's
protocol. One set of synthetic oligonucleotides involved the sense
and antisense target sequences of human Cbl-b: Sense,
5′-GGATCCCGGATGTGTTTGGGACTAATTTGATATCCGATTAGTCCCAAACACATCCTTTTTTCCAAAAGCTT-3′
and antisense,
5′-AAGCTTTTGGAAAAAAGGATGTGTTTGGGACTAATCGGATATCAAATTAGTCCCAAACACATCCGGGATCC-3′
were annealed and ligated into the BamHI/HindIII-cleaved backbone
of pRNA-U6.1/Neo (Genescript, Piscataway, NJ, USA). Stably
transfected cell lines were selected according to the methods
described in a previous study (17).
The cDNA of Flag-tagged ubiquitin (Flag-Ub) was provided by Dr
Kiyonao Sada (Division of Genome Science and Microbiology,
University of Fukui, Fukui, Japan). All cDNAs were then subcloned
into the pSVL expression vector (GE Healthcare, Piscataway, NJ,
USA). MGC803/NC and MGC803/shCbl-b, MDA-MB-231/NC and
MDA-MB-231/shCbl-b cells transfected with shRNA-Cbl-b were used for
the following experiments.
Small interfering (si)RNA
transfections
FAK siRNA was obtained from Shanghai GeneChem Co.
Ltd., (Shanghai, China). MGC803 cells were transfected with siRNAs
using Lipofectamine 2000, according to the manufacturer's protocol.
FAK siRNA sequences were synthesized as follows: FAK siRNA
sequences were synthesized as follows: siFAK-1, CAGGUGAAGAGCGAUUAU
Att; siFAK-2, CUCCAGUCUACAGAUUUGAtt; siFAK-3,
CCCAGGUUUACUGAACUUAtt. After 48 h of transient transfection, the
cells were analyzed by western blotting to determine the effect of
FAK siRNA on FAK expression.
Statistical analysis
All experiments were performed in triplicate. Data
were expressed as the mean ± standard deviation. Statistical
significance was determined using the Student's t-test. Statistical
analysis was performed using SPSS version 18.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
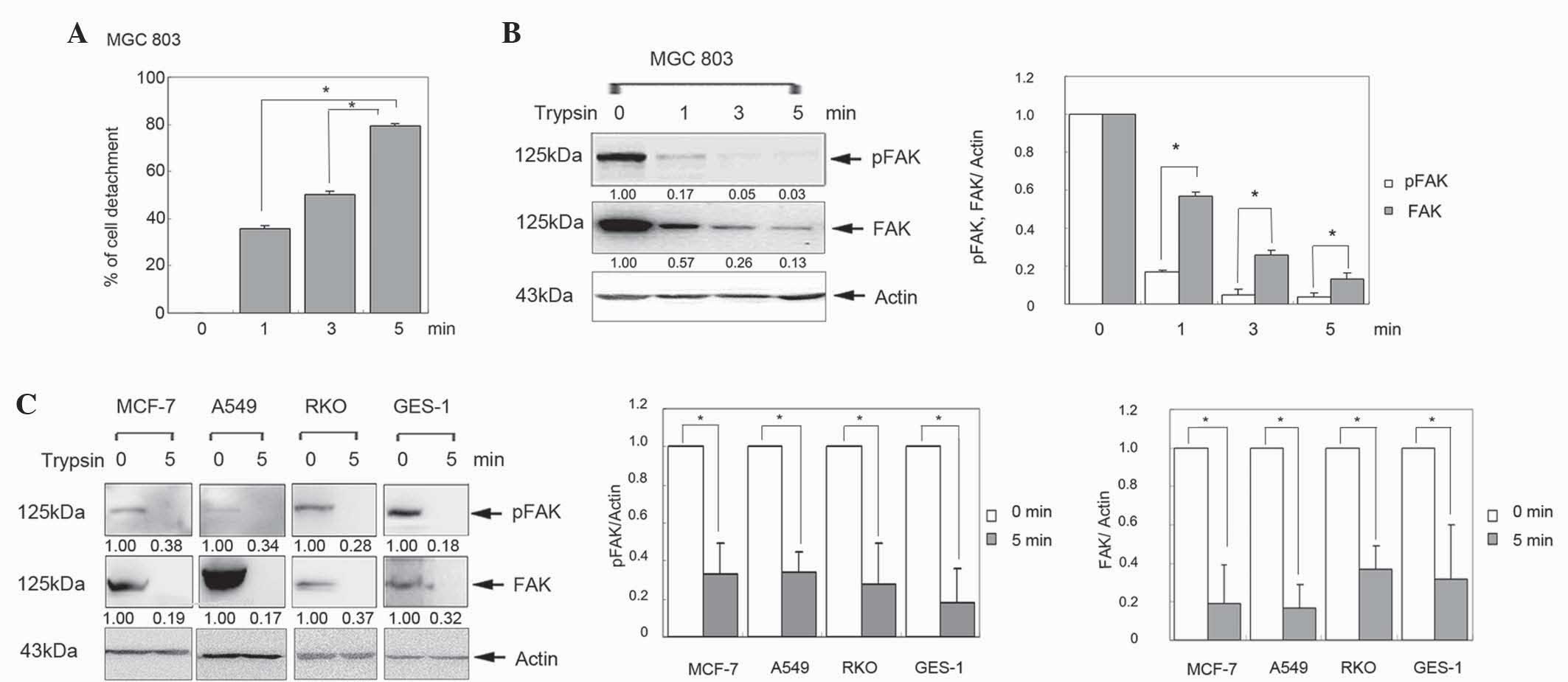
Decreased FAK expression is associated
with trypsin-induced cell detachment
To investigate the effect of FAK in cell detachment,
the human gastric cancer MGC803 cell line was treated with trypsin
for 0, 1, 3 and 5 min. The percentage of detached cells was
significantly increased in a time-dependent manner (Fig. 1A; P<0.05). Furthermore, western
blot analysis revealed that FAK was gradually reduced in a
time-dependent manner (Fig. 1B).
Similar results were observed in human breast cancer MCF-7, lung
cancer A549, colon cancer RKO and gastric epithelial GES-1 cells
(Fig. 1C; P<0.05). These results
indicate that FAK is downregulated in the process of cell
detachment.
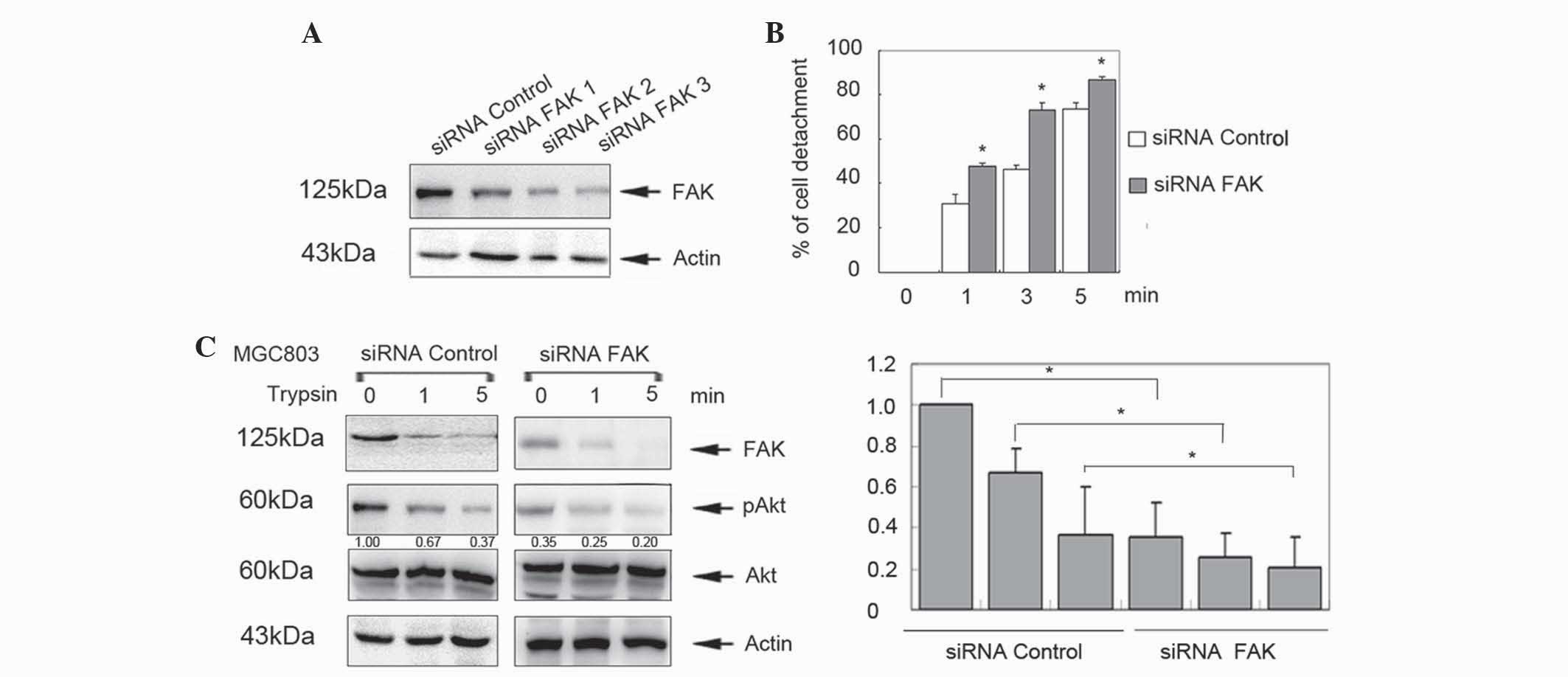
FAK inhibits trypsin-induced cell
detachment
To further clarify the function of FAK in the
process of cell detachment, MGC803 cells were transfected with
FAK-specific siRNAs for 48 h. Knockdown of FAK expression was
detected by western blotting (Fig.
2A). siRNA 3 was selected for further analysis, which revealed
that knockdown of FAK significantly increased cell detachment
compared with controls in a time-dependent manner (P<0.05;
Fig. 2B). In addition, p-Akt levels
were decreased in the siRNA/FAK group (Fig. 2C). These data indicate that FAK
inhibits cell detachment in cancer cells and human gastric
epithelial cells.
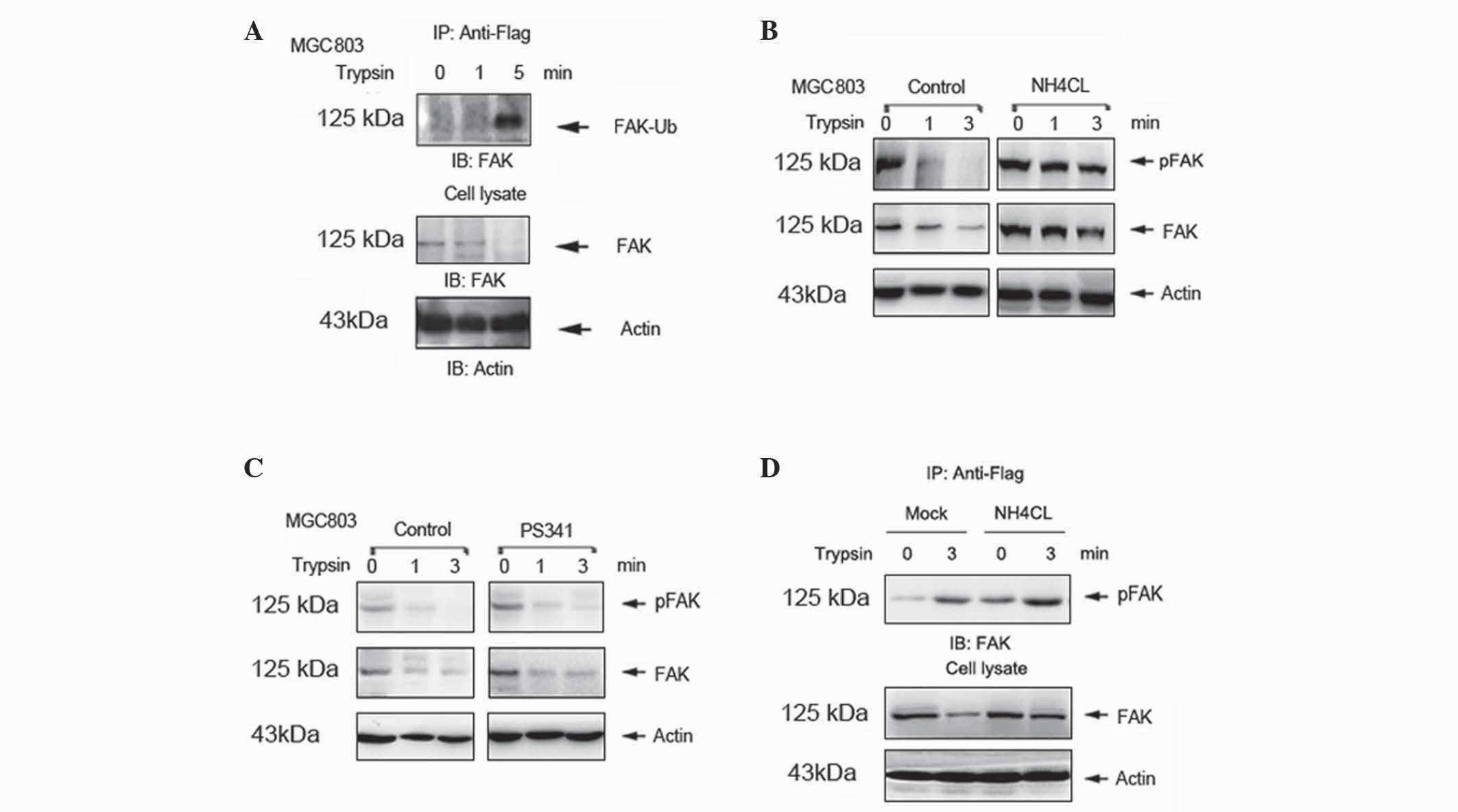
Ubiquitin-lysosome degradation
mediates trypsin-induced degradation of FAK
A previous study demonstrated that
ubiquitin-dependent protein degradation is critical in the
regulation of FAK (14). However,
whether ubiquitination degradation mediates trypsin-induced
degradation of FAK is currently unknown. In the present study,
MGC803 cells were transfected with plasmids expressing Flag-Ub.
Mono-ubiquitination of FAK was increased in a time-dependent manner
following trypsin treatment of the transfected cells (Fig. 3A). In the NH4Cl-pretreated
group, FAK degradation was suppressed compared with the control
(Fig. 3B). However, similar results
were not observed in cells pretreated with the proteasome inhibitor
PS-341 (Fig. 3C). To further
investigate whether the degradation of FAK occurs via the
ubiquitin-lysosome system, MGC803 cells were transfected with
plasmid expressing Flag-Ub for 48 h, in the absence or presence of
50 nM NH4Cl for 12 h. Mono-ubiquitination of FAK was
enhanced in the NH4Cl pretreated group (Fig. 3D). These results indicate that the
ubiquitin-lysosome system mediates trypsin-induced degradation of
FAK.
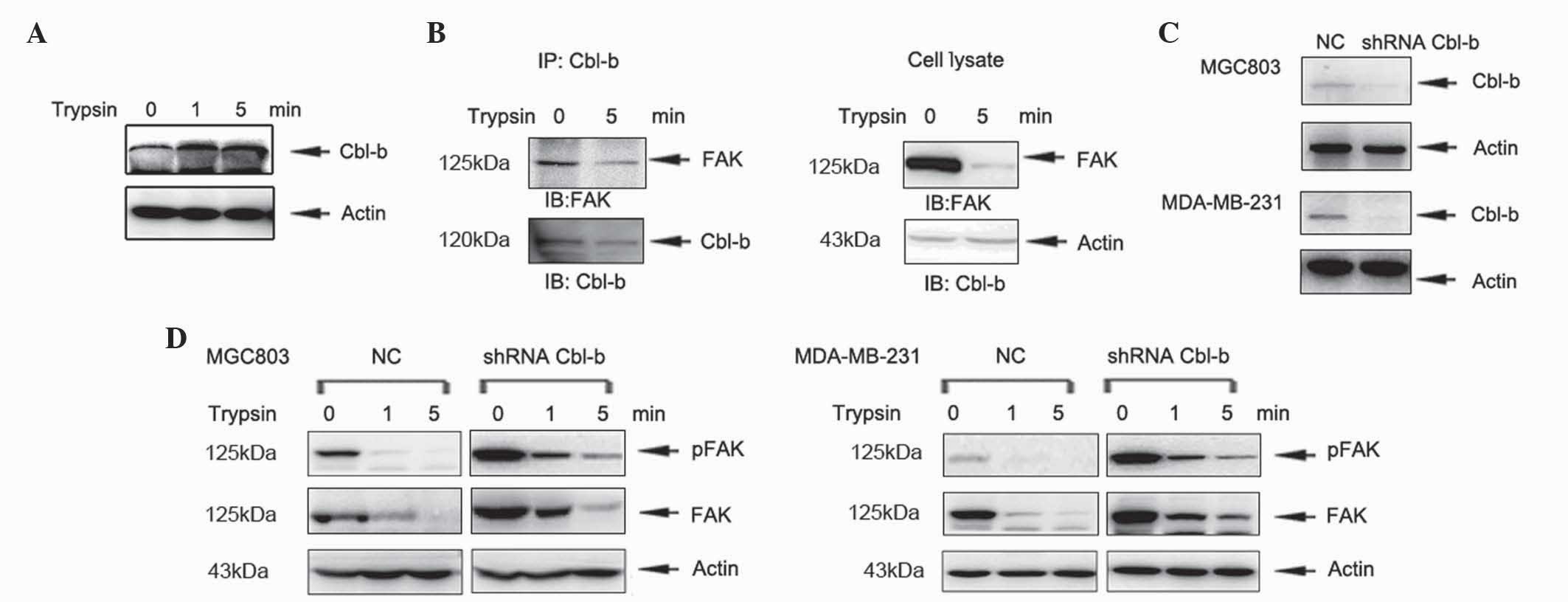
Cbl-b accelerates trypsin-induced cell
detachment
A previous study demonstrated that the E3 ubiquitin
protein ligase c-Cbl is involved in the ubiquitination of FAK
(14). Therefore, the involvement of
Cbl-b in ubiquitination and degradation of FAK requires further
investigation. In the present study, MGC803 cells were treated with
trypsin for 1 and 5 min and the level of Cbl-b protein was
analyzed. The expression of Cbl-b increased in a time-dependent
manner (Fig. 4A). Furthermore, Cbl-b
interacted with FAK in the absence and presence of trypsin
treatment, as demonstrated by co-immunoprecipitation (Fig. 4B). In order to further investigate the
function of Cbl-b in cell detachment, previously established shRNA
plasmids targeting Cbl-b, which were stably transfected into MGC803
cells (16), were used for further
experiments. The shRNA plasmids targeting Cbl-b were also stably
transfected into MDA-MB-231 cells and Cbl-b expression was
evaluated by western blotting (Fig.
4C). Knockdown of Cbl-b increased the expression of FAK
(Fig. 4D). These results indicate
that Cbl-b accelerates trypsin-induced cell detachment via
degradation of FAK.
Discussion
It has been reported that FAK not only promotes cell
adhesion, but is also involved in cell detachment (6–8,17). Kim et al (18) reported that okadaic acid induces cell
detachment, which is accompanied by FAK dephosphorylation. Zouq
et al (7) reported that
fibroblasts and epithelial cells exhibit rapid dephosphorylation of
FAK in response to detachment from the ECM. Conversely,
overexpression of the FAK N-terminus induces rounding, detachment
and apoptosis in breast carcinoma cells (17). Thus, the effect of FAK in cell
detachment remains controversial. In the present study, expression
and phosphorylation of FAK were decreased following trypsin-induced
detachment in cancer cell lines. Knockdown of FAK further enhanced
trypsin-induced cell detachment. These data indicate that FAK
inhibits trypsin-induced cell detachment.
The ubiquitin-dependent protein degradation pathway
is important for the regulation of FAK (14,19). A
previous study has demonstrated that Cat.G, a neutrophil-derived
serine protease, induces ubiquitin-proteasome-dependent degradation
of FAK (14). Inhibition of
proteasome activity markedly attenuates FAK degradation in T-,
myofibril and ovarian carcinoma cells (14,20,21). In
the present study, trypsin induced the mono-ubiquitination of FAK
in a time-dependent manner. Additionally, the downregulation of FAK
was suppressed by pretreatment with the lysosome inhibitor
NH4Cl. However, similar results were not observed
following pretreatment with the proteasome inhibitor PS-341.
Furthermore, ubiquitination of FAK was increased in cells
pretreated with NH4Cl. These results indicate that the
ubiquitin-lysosome system mediates trypsin-induced degradation of
FAK.
Results from previous studies have revealed that
MG53, an E3 ubiquitin ligase, mediates FAK ubiquitination during
skeletal myogenesis (21), and that
c-Cbl is involved in FAK degradation thereby suppressing cell
adhesion and myocyte survival (14).
Cbl-b, a negative regulator of non-receptor tyrosine kinases, may
cause FAK ubiquitination and degradation. In the present study,
Cbl-b interacted with FAK in the absence and presence of trypsin.
Knockdown of Cbl-b increased FAK expression and decreased
trypsin-induced degradation of FAK. These findings further indicate
that Cbl-b accelerates cell detachment via mono-ubiquitination of
FAK.
In conclusion, the results of the present study
indicate that cell detachment is mediated via ubiquitin-lysosome
degradation of FAK. Furthermore, the E3 ubiquitin ligase Cbl-b
contributes to cell detachment by facilitating FAK degradation.
These findings provide novel insights with regard to the functions
of Cbl-b and FAK in the process of cell detachment.
Acknowledgements
This study was supported by the Chinese National
Foundation of National Sciences (grant nos. 81172369, 81302128,
81472193 and 81372547), the General Project of Liaoning Province
Department of Education (grant no. L2014296), the National Science
and Technology Major Project (grant no. 2013ZX09303002) and the
Science and Technology Plan Project of Liaoning Province (grant no.
2014225013).
References
|
1
|
Finger EC and Giaccia AJ: Hypoxia,
inflammation, and the tumor microenvironment in metastatic disease.
Cancer Metastasis Rev. 29:285–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paschos KA, Canovas D and Bird NC: The
role of cell adhesion molecules in the progression of colorectal
cancer and the development of liver metastasis. Cell Signal.
21:665–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hwang S, Gwon SY, Kim MS, Lee S and Rhee
KJ: Bacteroides fragilis toxin induces IL-8 secretion in HT29/C1
cells through disruption of E-cadherin junctions. Immune Netw.
13:213–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang M, Alsaigh T, Kistler EB and
Schmid-Schönbein GW: Breakdown of mucin as barrier to digestive
enzymes in the ischemic rat small intestine. PLoS One.
7:e400872012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei L, Yang Y, Zhang X and Yu Q: Altered
regulation of Src upon cell detachment protects human lung
adenocarcinoma cells from anoikis. Oncogene. 23:9052–9061. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Golubovskaya VM, Gross S, Kaur AS, Wilson
RI, Xu LH, Yang XH and Cance WG: Simultaneous inhibition of focal
adhesion kinase and SRC enhances detachment and apoptosis in colon
cancer cell lines. Mol Cancer Res. 1:755–764. 2003.PubMed/NCBI
|
|
7
|
Zouq NK, Keeble JA, Lindsay J, Valentijn
AJ, Zhang L, Mills D, Turner CE, Streuli CH and Gilmore AP: FAK
engages multiple pathways to maintain survival of fibroblasts and
epithelia: Differential roles for paxillin and p130Cas. J Cell Sci.
122:357–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wade R, Brimer N, Lyons C and Vande Pol S:
Paxillin enables attachment-independent tyrosine phosphorylation of
focal adhesion kinase and transformation by RAS. J Biol Chem.
286:37932–37944. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang G, Liu J, Zhang Y, Qu J, Xu L, Zheng
H, Liu Y and Qu X: Cbl-b-dependent degradation of FLIP (L) is
involved in ATO-induced autophagy in leukemic K562 and gastric
cancer cells. FEBS Lett. 586:3104–3110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan L, Raja SM, Chen G, Virmani S,
Williams SH, Clubb RJ, Mukhopadhyay C, Rainey MA, Ying G, Dimri M,
et al: Negative regulation of EGFR-Vav2 signaling axis by Cbl
ubiquitin ligase controls EGF receptor-mediated epithelial cell
adherens junction dynamics and cell migration. J Biol Chem.
286:620–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scharner D, Rössig L, Carmona G, Chavakis
E, Urbich C, Fischer A, Kang TB, Wallach D, Chiang YJ, Deribe YL,
et al: Caspase-8 is involved in neovascularization-promoting
progenitor cell functions. Arterioscler Thromb Vasc Biol.
29:571–578. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qu X, Liu Y, Ma Y, Zhang Y, Li Y and Hou
K: Up-regulation of the Cbl family of ubiquitin ligases is involved
in ATRA and bufalin-induced cell adhesion but not cell
differentiation. Biochem Biophys Res Commun. 367:183–189. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt MH and Dikic I: The Cbl
interactome and its functions. Nat Rev Mol Cell Biol. 6:907–918.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rafiq K, Guo J, Vlasenko L, Guo X,
Kolpakov MA, Sanjay A, Houser SR and Sabri A: C-Cbl ubiquitin
ligase regulates focal adhesion protein turnover and myofibril
degeneration induced by neutrophil protease cathepsin G. J Biol
Chem. 287:5327–5339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qu X, Zhang Y, Li Y, Hu X, Xu Y, Xu L, Hou
K, Sada K and Liu Y: Ubiquitin ligase Cbl-b sensitizes leukemia and
gastric cancer cells to anthracyclines by activating the
mitochondrial pathway and modulating Akt and ERK survival signals.
FEBS Lett. 583:2255–2262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu L, Zhang Y, Liu J, Qu J, Hu X, Zhang F,
Zheng H, Qu X and Liu Y: TRAIL-activated EGFR by Cbl-b-regulated
EGFR redistribution in lipid rafts antagonises TRAIL-induced
apoptosis in gastric cancer cells. Eur J Cancer. 48:3288–3299.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beviglia L, Golubovskaya V, Xu L, Yang X,
Craven RJ and Cance WG: Focal adhesion kinase N-terminus in breast
carcinoma cells induces rounding, detachment and apoptosis. Biochem
J. 373:201–210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim B, van Golen CM and Feldman EL:
Degradation and dephosphorylation of focal adhesion kinase during
okadaic acid-induced apoptosis in human neuroblastoma cells.
Neoplasia. 5:405–416. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sekine Y, Tsuji S, Ikeda O, Sugiyma K,
Oritani K, Shimoda K, Muromoto R, Ohbayashi N, Yoshimura A and
Matsuda T: Signal-transducing adaptor protein-2 regulates
integrin-mediated T cell adhesion through protein degradation of
focal adhesion kinase. J Immunol. 179:2397–2407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Selvendiran K, Ahmed S, Dayton A, Ravi Y,
Kuppusamy ML, Bratasz A, Rivera BK, Kálai T, Hideg K and Kuppusamy
P: HO-3867, a synthetic compound, inhibits the migration and
invasion of ovarian carcinoma cells through downregulation of fatty
acid synthase and focal adhesion kinase. Mol Cancer Res.
8:1188–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nguyen N, Yi JS, Park H, Lee JS and Ko YG:
Mitsugumin 53 (MG53) ligase ubiquitinates focal adhesion kinase
during skeletal myogenesis. J Biol Chem. 289:3209–3216. 2014.
View Article : Google Scholar : PubMed/NCBI
|