Introduction
Chronic inflammation is associated with the
development of multiple types of cancer, but there are
discrepancies between different studies concerning prostate cancer
(1). Two previous meta-analyses
provided significant evidence of the association between
prostatitis and prostate cancer (1,2). There
are, however, several studies showing no association or a negative
correlation between prostatitis and prostate cancer (3–7). It has
been proposed that chronic inflammatory infiltrate-positive
prostatitis may protect against prostate cancer (8). The majority of previous studies included
patients with diagnosis of prostate cancer and controls from
patients records or interviews (1,2,9). By contrast, the present study used a
randomly selected sample of Finnish men surveyed in the 1990s to
evaluate the effect of prostatitis on prostate cancer
incidence.
Materials and methods
Patients
From April 1996 to February 1997, a survey was
conducted in the northern region of Finland to evaluate the
prevalence of prostatitis. A total of 2,500 men aged 20–59 years
were randomly selected from the population registry (Population
Register Centre, Helsinki, Finland) to receive a questionnaire, and
1,832 of them responded. A database review was then performed
focusing on prostate cancer diagnoses in the cohort. Respondents
were identified via a personal identification number from the Oulu
University Hospital registry (Oulu, Finland), based on the name and
address information available at the time of the original survey.
When present, prostate cancer diagnoses and diagnosis years for the
surveyed men were obtained from the Finnish Cancer Registry
(Helsinki, Finland). Clinical characteristics [Gleason score,
T-class, prostate-specific antigen (PSA) levels and primary
therapy] of prostate cancer cases were reviewed from the patients'
medical charts.
Ethics statement
The local ethics council of Oulu University Hospital
(Oulu, Finland) approved the present study, which was conducted
according to the Declaration of Helsinki (10). Written consent from the patients was
not obtained, but the volunteers responding to the original survey
(11) was considered as consent to
participate in the present study. The National Institute for Health
and Welfare (Helsinki, Finland), approved the present study and the
use of registry data following local ethical approval, according to
the Finnish law. Patient data was anonymized and de-identified
prior to statistical analysis.
Statistics
Summary statistics included the mean and standard
deviation (SD), or the median with the 25th-75th percentile if
biased, unless otherwise stated. Comparisons for categorical data
were performed using the χ2 test or the Fisher's exact
test. Continuous variables were analyzed using the Mann-Whitney U
non-parametric test. Prostate cancer incidence and standardized
incidence ratio (SIR) with 95% confidence interval (CI) were
calculated for the study population and for the whole population of
the survey area, which included men aged 41–80 years living at the
study area (obtained from the Statistics Finland database)
(12) and the number of newly
diagnosed prostate cancer cases (obtained from the Finnish Cancer
Registry). Data were analyzed using SPSS statistical software
version 22.0 (IBM SPSS, Armonk, NY, USA). Two-tailed P-values are
reported and P<0.05 were considered to indicate a statistically
significant difference.
Results
Of the 1,832 men responding to the original survey,
261 had prostatitis symptoms, leading to a lifetime prevalence of
14.2% (13). In the present study,
detailed data were available for 251 and 1,772 out of 261 and 1,832
men with prostatitis symptoms and the total number of men
responding to the original survey, respectively. Missing cases
(n=60) were due to incomplete identification data recorded
following the original survey. According to the Finnish Cancer
Registry, there were a total of 40 prostate cancer cases diagnosed
among men in the cohort in 2012. The incidence of prostate cancer
was more than double among men reporting prostatitis symptoms in
the original survey (Table I).
 | Table I.Incidence of prostate cancer among
Finnish men with and without self-reported prostatitis
symptoms. |
Table I.
Incidence of prostate cancer among
Finnish men with and without self-reported prostatitis
symptoms.
|
| Prostatitis
symptoms |
|
|---|
|
|
|
|
|---|
| Patients | No, n (%) | Yes, n (%) | Total, n |
|---|
| Prostate cancer
diagnosis |
| No | 1,494 (98.2) | 238 (94.8) | 1,732 |
| Yes | 27
(1.8) | 13
(5.2)a |
40 |
| Total | 1,521 | 251 | 1,772 |
There was no significant difference in the ages of
men at the time of prostate cancer diagnosis between the groups.
Mean ages (SD, range) were 64.8 years (3.7, 58–73 years) and 62.7
years (6.2, 51–73 years) for subjects with and without prostatitis
symptoms, respectively (P=0.26). PSA values at prostate cancer
diagnosis were available for 13 and 24 subjects with and without
prostatitis symptoms, respectively. Median PSA values (25th-75th
percentile, range) were 8.1 µg/l (5.2–13.2, 0.4–26.0 µg/l) and 9.2
µg/l (5.9–18.9, 0.9–1,759.0 µg/l) for subjects with and without
prostatitis symptoms, respectively (P=0.33). The Gleason score at
diagnosis was available for 13 and 25 subjects with and without
prostatitis symptoms, respectively. The median Gleason scores
(25th-75th percentile, range) were 6 (6–7, 4–8) and 6 (6–8, 4–9)
for subjects with and without prostatitis symptoms, respectively
(P=0.55).
Table II contains the
distribution of clinical T-class (tumor-node-metastasis
classification) (14) among men with
prostate cancer. Although there was a tendency for an increased
number of subjects with locally advanced disease (T3-T4) among men
with prostatitis symptoms, the difference was not significant
(P=0.63). There were no data available for one subject. The
distribution of different primary treatment modalities did not
differ between the groups (P=0.61). There were no data available
for one subject. Furthermore, there was no significant difference
between the groups in the time lag between the survey and the
diagnosis of prostate cancer (P=0.79).
 | Table II.Clinical T-class, primary prostate
cancer therapy and year of prostate cancer diagnosis among Finnish
men with prostate cancer with or without a history of self-reported
prostatitis. |
Table II.
Clinical T-class, primary prostate
cancer therapy and year of prostate cancer diagnosis among Finnish
men with prostate cancer with or without a history of self-reported
prostatitis.
|
| Prostatitis
symptoms |
|
|---|
|
|
|
|
|---|
| Characteristics | No, n (%) | Yes, n (%) | Total |
|---|
| Clinical T-class |
|
|
|
|
T1a-T1c | 6
(22.2) | 3 (25.0) | 9 |
| T2 | 10 (37.0) | 6 (50.0) | 16 |
|
T3-T4 | 11 (40.7) | 3 (25.0) | 14 |
| Primary prostate
cancer therapy |
|
|
|
| Active
surveillance | 1 (3.7) | 2 (16.7) | 3 |
| Permanent
seed implantation radiation therapy | 5
(18.5) | 1
(8.3) | 6 |
| Androgen
deprivation therapy | 5
(18.5) | 3 (25.0) | 8 |
| Radical
prostatectomy | 6
(22.2) | 2 (16.7) | 8 |
| External
beam radiation therapy | 10 (37.0) | 4 (33.3) | 14 |
| Year of prostate
cancer diagnosis |
|
|
|
| 1997 | 1 (3.7) | 0 (0.0) | 1 |
| 1998 | 0 (0.0) | 1 (7.7) | 1 |
| 2000 | 1 (3.7) | 0 (0.0) | 1 |
| 2001 | 1 (3.7) | 0 (0.0) | 1 |
| 2002 | 2 (7.4) | 1 (7.7) | 3 |
| 2003 | 1 (3.7) | 1 (7.7) | 2 |
| 2004 | 2 (7.4) | 0 (0.0) | 2 |
| 2005 | 3
(11.1) | 1 (7.7) | 4 |
| 2006 | 4
(14.8) | 3
(23.1) | 7 |
| 2007 | 1 (3.7) | 0 (0.0) | 1 |
| 2008 | 0 (0.0) | 1 (7.7) | 1 |
| 2009 | 4
(14.8) | 3
(23.1) | 7 |
| 2010 | 2 (7.4) | 1 (7.7) | 3 |
| 2011 | 3
(11.1) | 0 (0.0) | 3 |
| 2012 | 2 (7.4) | 1 (7.7) | 3 |
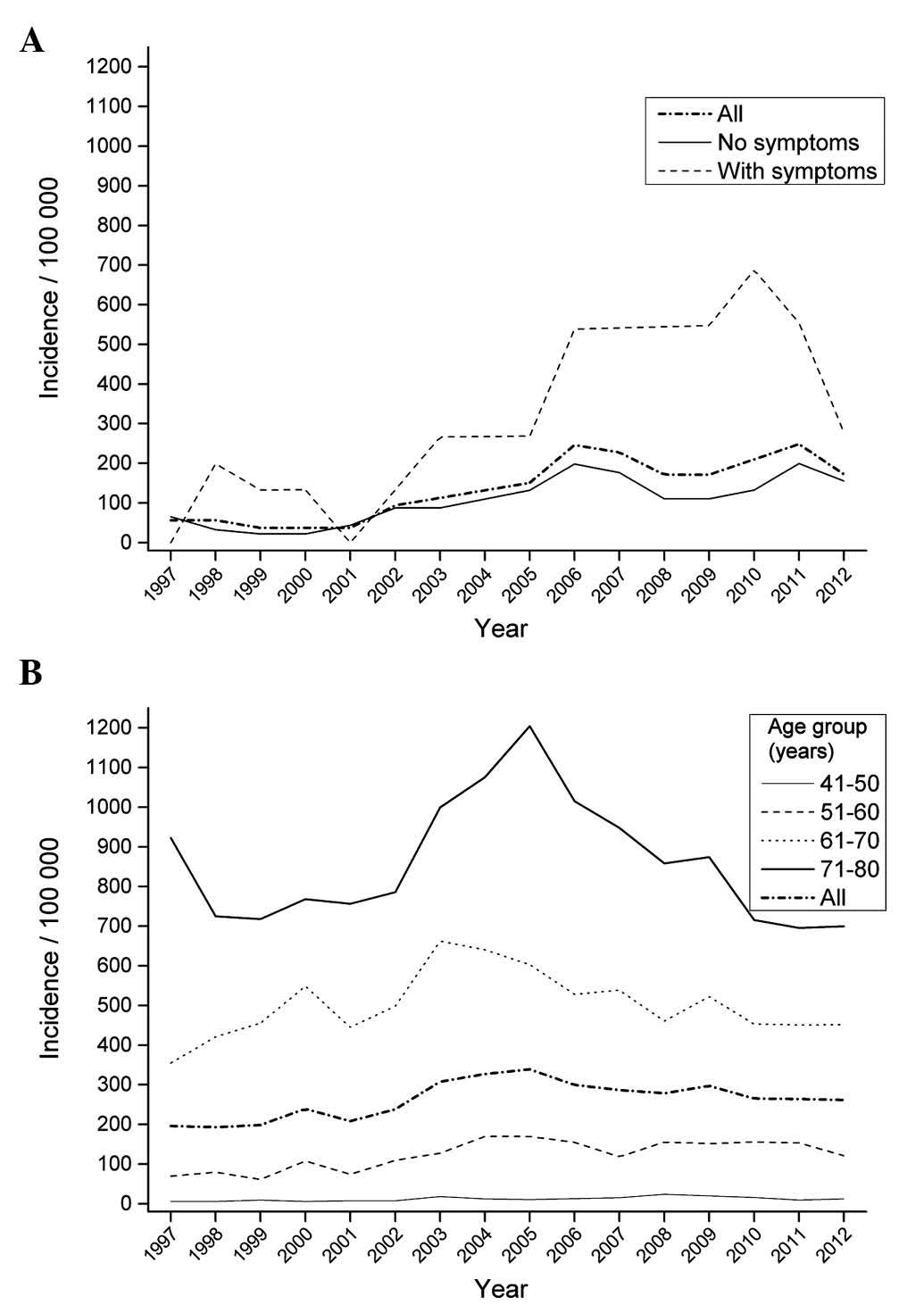
The present study further evaluated the incidence of
prostate cancer in the present cohort compared with that observed
during 15 years in the geographical area where the original survey
was conducted. Despite the seemingly high incidence of prostate
cancer among men with prostatitis symptoms, the incidence was not
higher than that reported among men in the age groups of 61–70 and
71–80 years in the aforementioned geographical area (Fig. 1). Furthermore, the analysis of SIR of
prostate cancer revealed that the SIR was slightly increased among
men with prostatitis symptoms, but the 95% CI covered 1.0,
indicating no significant difference compared with the population.
The SIR of prostate cancer among men with no prostatitis symptoms
was lower than expected (Table
III).
 | Table III.SIRs of men with and without
prostatitis symptoms in different age groups in the geographical
area of the study. |
Table III.
SIRs of men with and without
prostatitis symptoms in different age groups in the geographical
area of the study.
|
| Prostate cancer cases
in the study population, n | Expected cases in the
study population, n | SIRs |
|---|
|
|
|
|
|
|---|
|
|
|
|
|
| Prostatitis
symptoms | No symptoms |
|---|
|
|
|
|
|
|
|
|
|---|
| Age group, years | Prostatitis
symptoms | No symptoms | Prostatitis
symptoms | No symptoms | 1/100,000 | 95% CI | 1/100,000 | 95% CI |
|---|
| 41–50 | 0 | 0 | 0.08 | 1.03 | 0.00 | 0.00–46.40 | 0.00 | 0.00–3.57 |
| 51–60 | 1 | 10 | 1.84 | 14.20 | 0.55 | 0.01–3.04 | 0.70 | 0.34–1.29 |
| 61–70 | 11 | 13 | 6.87 | 35.30 | 1.60 | 0.80–2.87 | 0.37 | 0.20–0.63 |
| 71–80 | 1 | 4 |
2.40 | 11.30 | 0.42 | 0.01–2.32 | 0.35 | 0.10–0.91 |
| Total, n | 13 | 27 | 11.20 | 61.90 | 1.16 | 0.62–1.99 | 0.44 | 0.29–0.64 |
Discussion
To the best of our knowledge, the present study is
the first to evaluate a large cohort of randomly selected men for
several years following the report of prostatitis symptoms in order
to measure the risk of developing prostate cancer. The prevalence
of self-reported prostatitis in the present cohort was 14.2%
(11), which is consistent with a
previous survey conducted by health professionals in the USA, where
the prevalence of prostatitis was 16% (13), thus supporting the validity of the
present cohort. Previously, an association between self-reported
prostatitis and self-reported prostate cancer was documented
(15); however, that study was not
longitudinal, in contrast to the current study. Additionally, a
previous retrospective study among men with prostate cancer
revealed an elevated incidence of history of any type of
prostatitis compared with matched control men (9). On the contrary, histological prostatitis
has been reported to be significantly more prevalent in benign
prostatic hyperplasia than in prostate cancer (16). However, it is well known that
histological prostatitis does not correlate with clinical symptoms
(17).
In the present study, the time lag between the
original survey and the diagnosis of prostate cancer was long
(Table I), which further challenges
the connection between prostatitis symptoms and prostate cancer.
However, in another study, the mean time from the most recent
episode of acute prostatitis and the diagnosis of prostate cancer
was 12.2 years among a cohort of prostate cancer patients (9). Based on the present data, it is possible
to suggest that the increased incidence of prostate cancer among
men with prostatitis symptoms compared with that among men with no
symptoms may be due to a larger number of prostate cancer
diagnostic examinations based on the patients symptoms. Data
supporting this hypothesis have been published recently (18). In that study, the increased lower
urinary tract symptoms were not associated with the intensity of
prostate cancer diagnosis, but the diagnostic intensity increased
when symptoms were brought to the attention of physicians (18). However, the present data do not enable
the reliable evaluation of this aspect. The current results
demonstrated that prostatitis symptoms did not lead to a higher
incidence of prostate cancer in the geographical area evaluated
after 15 years of follow-up, compared with that in the general
population. Furthermore, despite the seemingly higher prostate
cancer incidence among men with prostatitis symptoms, the 95% CIs
of SIRs revealed that the differences were not significant, which
may be due to the low amount of prostate cancer cases and the
limited number of men with prostatitis symptoms in the present
study. The current cohort was obtained by random sampling from a
population registry. Therefore, the low SIR of prostate cancer
among men with no symptoms is likely to be coincidental.
Although there was a significant difference in the
number of cancer cases between men with and without a history of
prostatitis, the limited number of cancer cases included in the
present study prevents any firm conclusions. In the present cohort,
a remarkable amount of men were young at the time of the original
survey, and were not in the highest risk group for prostate cancer,
as estimated by age 15 years later, despite the fact that ~1/3 of
the men were 50–59 years old at the time of the original survey
(11).
There are several limitations in the present study.
Firstly, there was no differentiation between the various types of
prostatitis. Therefore, it could not be concluded whether the risk
of cancer is different in patients with chronic prostatitis
compared with that in men with 1–2 acute episodes of prostatitis.
Secondly, the diagnosis of prostatitis was based on a
questionnaire, and it has been reported that self-reported
genitourinary diseases such as benign prostatic hyperplasia and
prostatitis are poorly concordant with data from medical records
(19). However, the respondents
provided the details of the health care professional (general
practitioner or hospital doctor/urologist) who established the
diagnosis of prostatitis; thus, the diagnosis was not based solely
on patient self-evaluation (11).
Possible symptoms at the time of prostate cancer diagnosis were not
collected from the patients charts, as the retrospective evaluation
of symptoms is likely to be misleading, due to the lack of
systematic recording of the presence or absence of symptoms.
The incidence of prostate cancer in the present
study was based on data from the Finnish Cancer Registry, which
automatically receives notification of each suspected or diagnosed
cancer directly from every pathology laboratory (20). However, despite the estimated high
consistency of the Finnish Cancer Registry (diagnosed and
registered prostate cancer cases, 99%), a number of prostate cancer
diagnoses may be missed (21).
It is challenging to draw conclusive deductions
regarding the connection between prostatitis and prostate cancer.
Chronic prostatitis is a symptom with no objective diagnostic test.
In certain men, chronic pelvic pain may mimic prostatitis with no
inflammation of the prostate (22).
Thus, including these men will produce bias in similar studies.
To conclude, after 15 years of follow-up subsequent
to self-reported prostatitis, no evidently increased incidence of
prostate cancer was detected in the present cohort of Finnish men.
Despite the higher percentage of prostate cancer among men with
prostatitis symptoms compared with that among men with no symptoms,
the SIR of prostate cancer among men with prostatitis symptoms was
within the expected range of values. It may be suggested that the
higher percentage of prostate cancer among men with prostatitis
symptoms compared with that among men without symptoms is due to
the low SIR of prostate cancer cases among men without prostatitis
symptoms, and it may also be due to more frequent prostate cancer
diagnostic examinations based on symptoms. The present results do
not support extensive diagnostic interventions in order to detect
possible prostate cancer among men with prostatitis symptoms,
considering that the clinical characteristics of prostate cancer
did not differ between men with and without prostatitis
symptoms.
Acknowledgements
The present authors would like to thank Mrs. Leena
Heikkilä (Oulu University Hospital, Oulu, Finland) for her
technical assistance and Dr Maarit Leinonen (Finnish Cancer
Registry, Helsinki, Finland) for her assistance with the Finnish
Cancer Registry data.
References
|
1
|
Dennis LK, Lynch CF and Torner JC:
Epidemiologic association between prostatitis and prostate cancer.
Urology. 60:78–83. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang J, Li J, Yunxia Z, Zhu H, Liu J and
Pumill C: The role of prostatitis in prostate cancer:
Meta-analysis. PLoS One. 8:e851792013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gerstenbluth RE, Seftel AD, MacLennan GT,
Rao RN, Corty EW, Ferguson K and Resnick MI: Distribution of
chronic prostatitis in radical prostatectomy specimens with
up-regulation of bcl-2 in areas of inflammation. J Urol.
167:2267–2270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Irani J, Goujon JM, Ragni E, Peyrat L,
Hubert J, Saint F and Mottet N: Pathologist Multi Center Study
Group: High-grade inflammation in prostate cancer as a prognostic
factor for biochemical recurrence after radical prostatectomy.
Urology. 54:467–472. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Engelhardt PF, Brustmann H, Seklehner S
and Riedl CR: Chronic asymptomatic inflammation of the prostate
type IV and carcinoma of the prostate: Is there a correlation?
Scand J Urol. 47:230–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karakiewicz PI, Benayoun S, Bégin LR,
Duclos A, Valiquette L, McCormack M, Bénard F, Saad F and Perrotte
P: Chronic inflammation is negatively associated with prostate
cancer and high-grade prostatic intraepithelial neoplasia on needle
biopsy. Int J Clin Pract. 61:425–430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moreira DM, Nickel JC, Gerber L, Muller
RL, Andriole GL, Castro-Santamaria R and Freedland SJ: Baseline
prostate inflammation is associated with a reduced risk of prostate
cancer in men undergoing repeat prostate biopsy: Results from the
REDUCE study. Cancer. 120:190–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Porcaro AB, Rubilotta E, Petrozziello A,
Ghimenton C, Migliorini F, Antoniolli Zecchini S, Lacola V, Monaco
C, Curti P, Cavalleri S, et al: Chronic inflammation of the
prostate type IV with respect to risk of prostate cancer. Arch Ital
Urol Androl. 86:208–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roberts RO, Bergstralh EJ, Bass SE, Lieber
MM and Jacobsen SJ: Prostatitis as a risk factor for prostate
cancer. Epidemiology. 15:93–99. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
WMA Declaration of Helsinki - Ethical
Principles for Medical Research Involving Human Subjects.
https://www.wma.net/en/30publications/10policies/b3/Accessed.
Apr 15–2016
|
|
11
|
Mehik A, Hellström P, Lukkarinen O,
Sarpola A and Järvelin M: Epidemiology of prostatitis in Finnish
men: A population-based cross-sectional study. BJU Int. 86:443–448.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Statistics Finland. https://www.tilastokeskus.fi/index_en.htmlAccessed. Apr
15–2016
|
|
13
|
Collins MM, Meigs JB, Barry MJ, Corkery
Walker E, Giovannucci E and Kawachi I: Prevalence and correlates of
prostatitis in the health professionals follow-up study cohort. J
Urol. 167:1363–1366. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sobin L and Wittekind C: Union for
International Cancer Control: TNM Classification of Malignant
Tumours (6th). Wiley. New York: 2002.
|
|
15
|
Daniels NA, Ewing SK, Zmuda JM, Wilt TJ
and Bauer DC: Osteoporotic Fractures in Men (MrOS) Research Group:
Correlates and prevalence of prostatitis in a large community-based
cohort of older men. Urology. 66:964–970. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edlin RS, Heyns CF, Van Vuuren SP and
Zarrabi AD: Prevalence of histological prostatitis in men with
benign prostatic hyperplasia or adenocarcinoma of the prostate
presenting without urinary retention. S Afr J Surg. 50:127–130.
2012.PubMed/NCBI
|
|
17
|
Nickel JC, Roehrborn CG, O'leary MP,
Bostwick DG, Somerville MC and Rittmaster RS: Examination of the
relationship between symptoms of prostatitis and histological
inflammation: Baseline data from the REDUCE chemoprevention trial.
J Urol. 178:896–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weight CJ, Kim SP, Jacobson DJ, McGree ME,
Boorjian SA, Thompson RH, Leibovich BC, Karnes RJ and St Sauver J:
The effect of benign lower urinary tract symptoms on subsequent
prostate cancer testing and diagnosis. Eur Urol. 63:1021–1027.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu K, McKnight B, Stergachis A, Daling JR
and Levine RS: Comparison of self-report data and medical records
data: Results from a case-control study on prostate cancer. Int J
Epidemiol. 28:409–417. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Finnish Cancer Registry - Legislation.
https://www.cancer.fi/syoparekisteri/en/general/legislation/Accessed.
Apr 15–2016
|
|
21
|
Teppo L, Pukkala E and Lehtonen M: Data
quality and quality control of a population-based cancer registry.
Experience in Finland. Acta Oncol. 33:365–369. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krieger JN, Egan KJ, Ross SO, Jacobs R and
Berger RE: Chronic pelvic pains represent the most prominent
urogenital symptoms of ‘chronic prostatitis’. Urology. 48:715–722.
1996. View Article : Google Scholar : PubMed/NCBI
|