Introduction
Administration of the cytotoxic agent 5-fluorouracil
(5-FU) is the predominant therapeutic approach for the treatment of
metastatic colorectal cancer (CRC) based on the capacity of its
three active metabolites [fluorodeoxyuridine triphosphate (FdUTP),
fluorouridine triphosphate (FUTP) and fluorodeoxyuridine
monophosphate (FdUMP)] to induce cytotoxicity and cell death, by
incorporation of FdUTP and FUTP into DNA and RNA, or by inhibition
of the enzyme thymidylate synthase by FdUMP (1). Nevertheless, its clinical applicability
is considerably limited by a number of disadvantages, particularly
its low bioavailability, the high rate of 5-FU degradation
(especially in the liver, at >80%) and the development of 5-FU
resistance mechanisms in CRC cells (1,2).
Furthermore, recent studies have demonstrated that the anticancer
efficiency of various chemotherapeutic agents (including
doxorubicin, docetaxel, cyclophosphamide, gemcitabine and 5-FU) can
be modulated by cancer cells and also by tumor-associated
macrophages (TAMs), via inducing chemoresistance or enhancing
chemosensitivity to these cytotoxic agents in different tumor
models, including leukemia, fibrosarcoma, pancreatic, breast and
colon cancer models (3).
It is well known that, among the immune cell
populations present in tumor microenvironment, TAMs are key
protagonists in promoting and coordinating tumor growth (4) through their ability to modulate all
processes involved in cancer progression, including the following:
i) Tumor angiogenesis and inflammation [secretion of vascular
endothelial growth factor (VEGF), platelet-derived growth factor,
transforming growth factor-β (TGF-β), fibroblast growth factor and
matrix metalloproteinases; and the cytokines and chemokines
interleukin (IL)-1β, IL-6, IL-8, IL-9, IL-10, chemokine (CC motif)
ligand (CCL)17, CCL22, CCL18 and tumor necrosis factor α (TNF-α)]
(5,6);
ii) metastasis (production of IL-1β and TNF-α) (7,8); iii)
immunosuppression [secretion of immunosuppressive cytokines (IL-10,
TGF-β) and prostaglandin E2] (9,10); and iv)
oxidative stress [generation of reactive oxygen species (ROS) which
are essential for the activation and expression of transcription
factors responsible for the maintenance of a malignant phenotype]
(11).
Therefore, the aim of the present study was to
provide greater insight into the effect of the tumor
microenvironment generated by the interaction between TAMs and C26
murine colon carcinoma cells on the response of these cancer cells
to 5-FU treatment. In this respect, the cytotoxic effects of
various concentrations of 5-FU were tested on C26 carcinoma cells
cultivated alone as well as on those co-cultured with murine
peritoneal macrophages. Furthermore, the role of TAMs in the
antitumor effects of 5-FU on key molecules involved in tumor
angiogenesis and inflammation, as well as in tumor oxidative
stress, was addressed. The results demonstrated that TAMs
orchestrate the response of C26 cells to 5-FU administration. Thus,
on one side, TAMs render C26 colon carcinoma cells more susceptible
to 5-FU treatment via inhibition of the production of inflammatory
and angiogenic factors in these cancer cells; however, on the other
side, they protect cancer cells from 5-FU-induced oxidative
stress.
Materials and methods
Cell line and culture conditions
C26 murine colon carcinoma cells (Cell Line Services
GmbH, Eppelheim, Germany) were cultured as a monolayer in complete
RPMI-1640 medium (Lonza Group AG, Basel, Switzerland) supplemented
with 10% heat-inactivated fetal bovine serum (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), at 37°C in a humidified
atmosphere of 5% CO2.
Co-culture of C26 tumor cells with
macrophages
Peritoneal macrophages were isolated from 6 male
BALB/c mice (Cantacuzino Institute, Bucharest, Romania).
Experiments were performed according to the national regulations
and were approved by the Babes-Bolyai University ethics committee
(Cluj-Napoca, Romania; registration no. 32652/01.07.2014). Mice
were previously injected intraperitoneally with 1 ml of 3%
thioglycollate (Fluka) (12). After 3
days, chemically elicited macrophages (with inflammatory and
antitumor action) were collected, and co-cultures were prepared by
seeding C26 tumor cell suspensions on macrophage monolayers. The
complex interaction of these macrophages with tumor cells in a
co-culture system has been shown to enable tumor cell-macrophage
crosstalk and to promote macrophage polarization into TAMs which
favor tumor progression (12,13). In our experiments, co-cultures of
macrophages and tumor cells were created at a cell density ratio of
1:4, as it has previously been demonstrated that this cell density
ratio ensures the optimal cytokine interplay between tumor cells
and macrophages, which provides an approximation of the
physiological conditions of colon carcinoma development in
vivo (13). In addition, the
presence of the TAM-specific phenotype in this co-culture was
verified by comparing angiogenic/inflammatory protein production in
co-culture cell lysates with the production of the same proteins in
cell lysates that resulted from the co-cultivation of IL-4-induced
TAMs with C26 cells at the same cell density ratio as described
above (data not shown). It was previously demonstrated that
incubation of peritoneal macrophages with 20 ng/ml of IL-4
(Sigma-Aldrich Chemie GmbH, Munich, Germany) for 24 h promotes the
complete polarization of macrophages into TAMs (14).
Cell proliferation assay
The cytotoxicity of various concentrations of 5-FU
(0.125–16 µM) on C26 cells in standard cultures, as well as in
co-culture with macrophages, was assessed. The antiproliferative
effects of 5-FU at the aforementioned concentrations were
determined using the Cell Proliferation ELISA, BrdU (colorimetric)
immunoassay kit (#11647229001; Roche Applied Science, Mannheim,
Germany), according to the manufacturer instructions and as
described previously (15). This
method is based on the incorporation of the pyridine analog
bromodeoxyuridine (BrdU), instead of thymidine, into the DNA of
proliferating cells. C26 cells (1×104/well), cultured
alone or together with peritoneal macrophages at a density ratio of
1:4, were seeded into 96-well plates and incubated with different
concentrations of 5-FU for 72 h. Cells incubated only with medium
were used as controls. Subsequently, cells were incubated with BrdU
solution for 24 h and the culture medium was completely removed
from each well. Following this step, the cells were fixed and the
DNA was denatured. To detect the incorporated BrdU in the newly
synthesized cellular DNA, a monoclonal antibody conjugated with
peroxidase (anti-BrdU-POD, included in the kit) was added in each
well. The antibody was removed after 1 h of incubation and the
cells were washed three times with phosphate-buffered saline. A
peroxidase substrate, tetramethyl-benzidine, was added to each well
and the immune complexes were detected by measuring the absorbance
of the reaction product at 450 nm with a reference wavelength of
655 nm. The effects of administration of 5-FU at various
concentrations on C26 cells in the two culture conditions were
determined in triplicate.
Preparation of cell culture
lysates
To assess the effects of 5-FU on key molecules
involved in tumor oxidative stress, inflammation and angiogenesis,
as well as the role of TAMs in the antitumor effects of 5-FU,
lysates from 4 µM 5-FU-treated C26 colon carcinoma cells cultured
alone or in combination with peritoneal macrophages were obtained.
Cell cultures were lysed with lysis buffer containing 10 mM HEPES
(pH 7), 200 mM NaCl, 1% Triton X, 10 mM MgCl2, 1 mM
dithiothreitol and protease inhibitor cocktail tablets (Complete,
Roche Diagnostics GmbH, Mannheim, Germany). The homogenate was
incubated for 30 min on ice and then centrifuged for 10 min at
12,000 × g, at 4°C and the supernatant was collected and stored at
−80°C for further molecular measurements. The protein concentration
was determined through a Bradford assay (Bio-Rad Laboratories,
Inc., Hercules, CA) (16).
Assessment of nuclear factor κB
(NF-κB) production
To determine the effects of 4 µM 5-FU on the levels
of NF-κB (a key transcription factor involved in tumor inflammation
and angiogenesis) in the cell lysates obtained from standard C26
cell culture and from the mixed culture of C26 cells with
peritoneal macrophages, western blot analysis was performed. Thus,
20 µg of total protein from each lysate was loaded per lane onto a
10% polyacrylamide gel. Electrophoresis was performed at 95 mV and
then the protein fractions were electrotransferred onto a
nitrocellulose membrane at 100 mV for 40 min. The membranes were
blocked overnight at 4°C with 5% skimmed milk powder (Bio-Rad
Laboratories, Inc.) in Tris-buffered saline containing 0.1%
Tween-20 (TBS-T), under constant agitation. Subsequently, the
membranes were incubated for 2 h at room temperature with a
monoclonal mouse IgG anti-mouse NF-κB p65 antibody (SC-56735; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) diluted 1:500 in
TBS-T, with 5% skimmed milk powder (17). For the loading control, rabbit IgG
anti-mouse β-actin antibody (SC-130656; Santa Cruz Biotechnology,
Inc.) was used, diluted 1:500 with 5% skim milk powder in TBS-T.
Membranes were washed with TBS-T and incubated at room temperature
for 1 h with horseradish peroxidase (HRP)-conjugated goat IgG
anti-mouse IgG antibody (SC-2005; Santa Cruz Biotechnology, Inc.)
diluted 3,000-fold in TBS-T, with an additional washing step prior
to detection. For β-actin determination, HRP-conjugated goat IgG
anti-rabbit IgG antibody (SC-2004; Santa Cruz Biotechnology)
diluted 4,000-fold in TBS-T was used. Proteins were detected by
using Clarity™ Western ECL (Bio-Rad Laboratories, Inc.) and the
membranes were exposed to a Kodak X-ray film for 2 min. The films
were developed, photographed using a BioSpectrum Imaging System
(Ultra-Violet Products, Ltd., Cambridge, UK) and analyzed using
TotalLab Quant Software version 12 for Windows (TotalLab Limited,
Newcastle, UK). NF-κB expression levels in cell lysates following
5-FU treatment in the presence or absence of macrophages were
compared with the levels of NF-κB in untreated C26 cell lysates and
untreated co-cultures, respectively. The final results are
presented as the mean ± standard deviation (SD) of two independent
experiments.
Determination of inflammatory and
angiogenic protein production
The effects of treatment with 4 µM 5-FU on the
expression levels of inflammatory/angiogenic factors in cell
lysates obtained in standard or in co-culture conditions were
investigated by performing a screening for 24 proteins involved in
angiogenesis and inflammation using RayBio® Mouse
Angiogenesis Antibody Array 1 kit (AAM-ANG-1-8; RayBiotech, Inc.,
Norcross, GA, USA) as described previously (15). One array membrane containing 24 types
of primary antibodies against specific proteins was incubated with
200 µg of proteins of cell lysates for 2 h at room temperature.
Subsequently, a mixture of secondary biotin-conjugated antibodies
against the same angiogenic factors as those for primary
antibodies, was added on the membranes and incubated overnight at
4°C, followed by incubation with HRP-conjugated streptavidin for 2
h. Each incubation step was followed by five washing steps.
Thereafter, the membranes were incubated with a mixture of two
detection buffers for 1 min, exposed to an X-ray film (Kodak) for 2
min and then the films were developed. The protein expression level
was quantified by measuring the intensity of the color of each spot
on the membranes in comparison to the positive control spots
already bound to the membranes, using TotalLab Quant Software
version 12 for Windows. The expression of each angiogenic protein
in cell lysates from each cell culture condition was determined in
duplicate.
Quantification of malondialdehyde
(MDA)
MDA is the main product of ROS-mediated lipid
peroxidation and is therefore a good indicator of overall oxidative
stress (18). MDA levels were
determined as previously described through high-performance liquid
chromatography (HPLC) (19).
Following deproteinization with HClO4, samples were
centrifuged at 4,500 × g for 5 min and 100 µl of each supernatant
was used for HPLC analysis. The column type was RP18 (5 µm)
(Supelco, Inc., Bellefonte, PA, USA) and the mobile phase consisted
of 30 mM KH2PO4/methanol in a volume ratio of
65:35. Flow rate was set at 0.5 ml/min and MDA was measured using a
UV detector (UV-2070/2075; Jasco, Tokyo, Japan) set at 254 nm. The
retention time of MDA was ~5.4 min. Data were expressed as µM MDA
and were normalized to the protein concentration from cell lysates.
Each sample was determined in duplicate.
Determination of nitric oxide (NO)
metabolites
The effect of 5-FU treatment on the production of NO
in standard culture and in co-culture was assessed by measuring
nitrites via colorimetric Griess assay, as previously described
(19). NO is a key signaling molecule
that becomes cytotoxic to cancer cells when produced in high
levels, whereas low levels of NO exert tumor promoting properties
(20). This assay relies on the
diazotization reaction in which nitrite reacts under acidic
conditions with sulfanilic acid (Sigma-Aldrich) to form a diazonium
salt, which subsequently couples with N-(1-naphthyl)
ethylenediamine dihydrochloride (Sigma-Aldrich) to form a stable
azo dye that can be measured spectrophotometrically at 548 nm.
Sample deproteinization with ZnSO4 was applied prior to
the assay and each sample was determined in duplicate. Sodium
nitrite (Sigma-Aldrich) was used as a standard. Data were expressed
as nM nitrites following normalization to the protein concentration
from cell lysates.
Statistical analysis
Data from the various experiments are expressed as
the mean ± SD. For statistical analysis of the effects of 5-FU on
C26 cells or co-culture of C26 cells and macrophages, an unpaired
t-test was used. The differences between the effects of 5-FU on the
production of each inflammatory/angiogenic factor in cells from
standard culture and co-culture were analyzed by two-way analysis
of variance (ANOVA) with Bonferroni correction for multiple
comparisons. All statistical analyses were performed by using
GraphPad Prism version 6 for Windows (GraphPad Software, San Diego,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
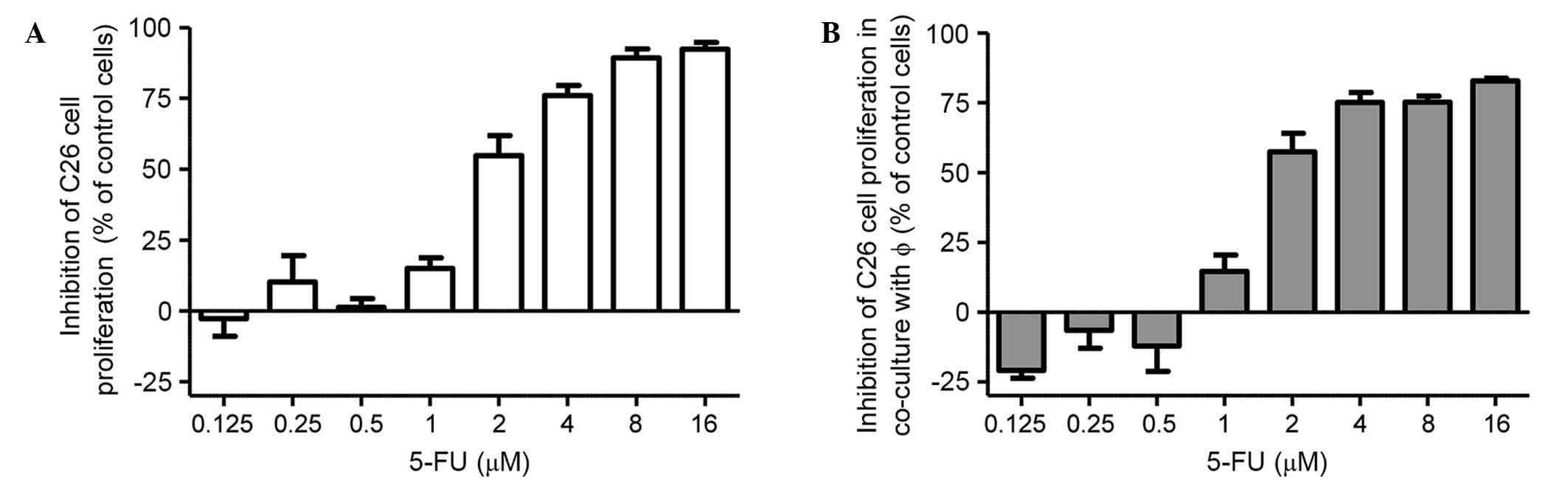
Effects of 5-FU on the C26 cell
proliferation
The effects of different concentrations of 5-FU on
the proliferation of C26 cells in standard culture and in the
presence of peritoneal macrophages were expressed as the percentage
of inhibition compared to the proliferation of the controls
(untreated cells) (Fig. 1A and B). In
the two culture conditions, C26 murine colon carcinoma cells were
incubated with increasing 5-FU concentrations ranging from 0.125 to
16 µM for 72 h. The results regarding the cytotoxic effects of 5-FU
on C26 cell proliferation indicated that 5-FU at concentrations of
≥4 µM strongly inhibited the growth of C26 cells (by 75% compared
to the proliferation of control cells) under standard culture
conditions (Fig. 1A) and after
co-cultivation with TAMs (Fig. 1B).
As 4 µM was the lowest concentration of 5-FU at which strong
cytotoxic effects were noted with regard to tumor cell
proliferation (Fig. 1A and B), this
concentration was used throughout the experiments performed for
testing the modulatory actions of TAMs on the response of C26 colon
carcinoma cells to 5-FU administration.
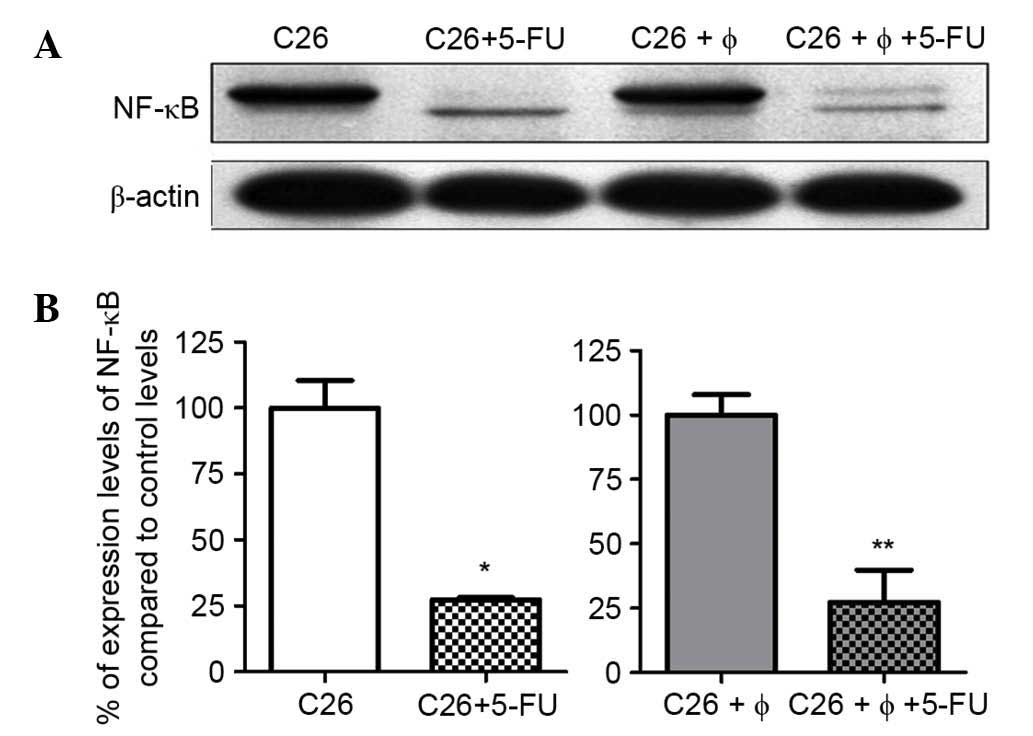
Influence of TAMs on the effects of
5-FU on NF-κB production
As several studies on colon carcinoma cell lines
have demonstrated the role of constitutively activated NF-κB on the
proliferative, antiapoptotic and angiogenic potential of these
tumor cell lines (21,22), the present study was conducted to
investigate whether cultivation of TAMs with C26 colon carcinoma
cells could modulate the effects of 4 µM 5-FU on the production of
this transcription factor. The results revealed that C26 cells
constitutively expressed NF-κB (Fig. 2A
and B). Notably, 5-FU exerted similar and strong inhibitory
effects on the expression of NF-κB (70% inhibition compared to
control levels) in lysates obtained under standard culture and
under co-culture conditions (Fig. 2A and
B).
Effects on the production of
inflammatory/angiogenic proteins in response to the action of TAMs
on 5-FU
To investigate whether TAMs modulate the action of
5-FU on the production of inflammatory and angiogenic proteins in
C26 tumor cells co-cultivated with peritoneal macrophages, we
performed a screening for 24 proteins involved in inflammation and
angiogenesis by using RayBio® Mouse Angiogenic Cytokine
Antibody Array kit. In addition, as a positive control for TAMs, we
compared the inflammatory/angiogenic protein profile in co-culture
lysates with the production of the same proteins in cell lysates
where activated macrophages received 20 ng/ml IL-4 pretreatment for
24 h prior to incubation with C26 cells. Two-way ANOVA with
Bonferroni correction for multiple comparisons was performed
between the two inflammatory/angiogenic protein profiles and the
statistical differences between each profile were not significant
(data not shown). Thus, it is confirmed that upon co-culture of
tumor cells with peritoneal macrophages, the latter were polarized
into TAMs.
In accordance with previous studies (4–6,19), the overall production of the majority
of the inflammatory and angiogenic factors in C26 cells
co-cultivated with peritoneal macrophages was 2-fold higher than
the production of the same factors in C26 cells cultivated alone
(Fig. 3). More specifically, the
co-cultivation of C26 cells with TAMs significantly stimulated the
production of IL-6, TNF-α, monocyte chemoattractant protein-1
(MCP-1), eotaxin, VEGF, platelet factor 4 (PF-4) and IL-12p70 by
50–150%; the production of IL-12p40, Fas ligand (FasL), basic
fibroblast growth factor (bFGF), interferon γ (IFN-γ), and monokine
induced by IFN-γ (MIG) by 150–300%; and the production of IL-1β by
~580%. In terms of inhibition, only the production of IL-9 was
significantly inhibited, by 50% compared to its control level
(Fig. 3).
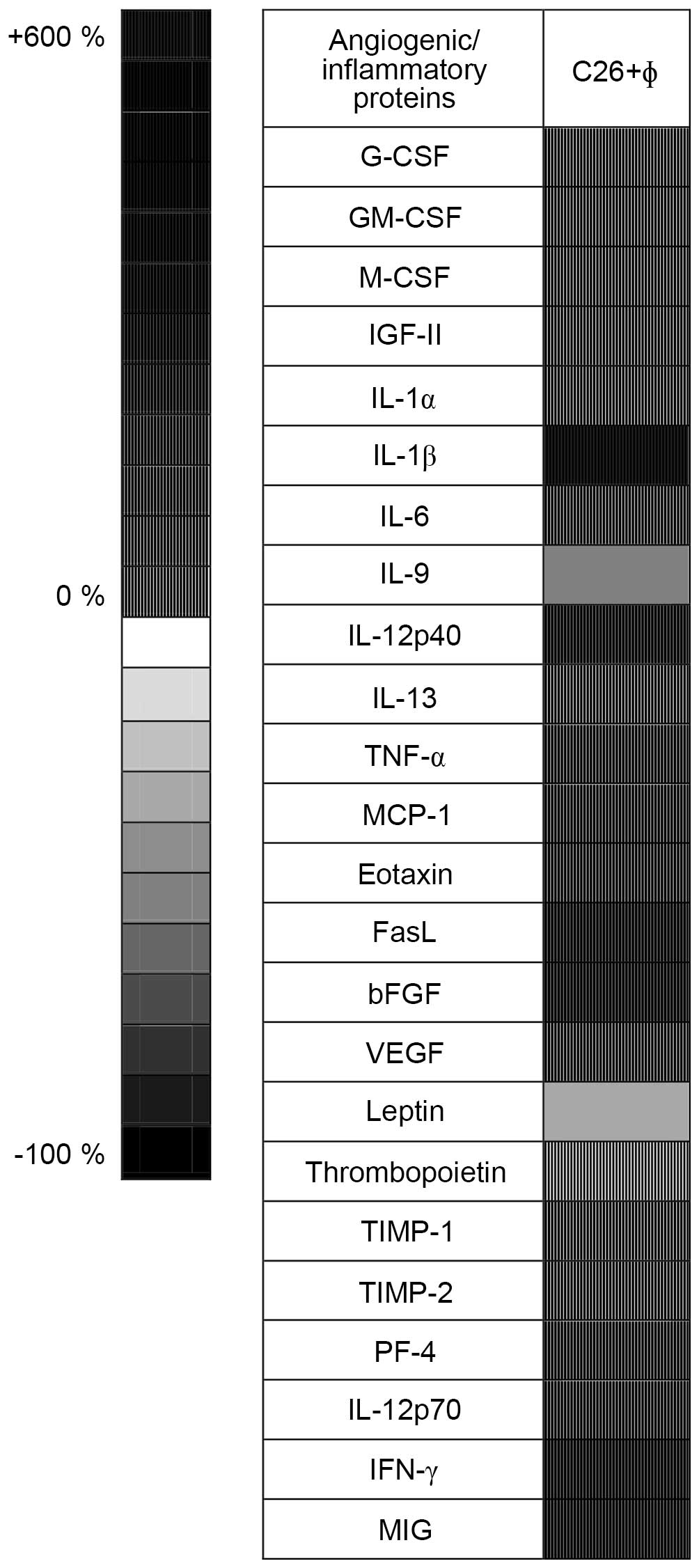 | Figure 3.Effects of tumor-associated
macrophages on the levels of angiogenic/inflammatory proteins in
the cell co-culture lysates. Results are presented either as
percentage (%) of reduction (−) of protein levels [ranging from 0%
(white) to 100% (black), shown as plain patterns] or as % of
stimulation (+) of production of proteins [ranging from 0% (white)
to 550% (black), shown as grid patterns] in cell lysates from
untreated cell co-culture lysates (C26+φ) compared to levels of the
same factors in lysates from untreated C26 cells. G-CSF,
granulocyte colony-stimulating factor; GM-CSF,
granulocyte-macrophage colony-stimulating factor; M-CSF, monocyte
colony-stimulating factor; IGF-II, insulin-like growth factor 2;
IL, interleukin; TNF-α, tumor necrosis factor α; MCP-1, monocyte
chemoattractant protein-1; FasL, Fas ligand; bFGF, basic fibroblast
growth factor; VEGF, vascular endothelial growth factor; TIMP,
tissue inhibitor of metalloproteinases; PF-4, platelet factor 4;
IFN-γ, interferon γ; MIG, monokine induced by IFN-γ. |
Notably, when C26 colon carcinoma cells cultivated
alone were incubated with 5-FU, the average production of the
inflammatory and angiogenic proteins was increased by 84% compared
to their levels in the untreated tumor cells (P<0.0001; Table I). Thus, the production of 19 out of
24 proteins studied was significantly stimulated. In particular,
compared with control levels, the production levels of
granulocyte-macrophage colony-stimulating factor (GM-CSF),
insulin-like growth factor 2 (IGF-II), IL-13, MCP-1 and IL-12p70
were stimulated by 100–200% and the production of FasL, bFGF and
IFN-γ was strongly stimulated by 200–500%. Only the levels of
production of VEGF, leptin and MIG were significantly reduced, by
40–60% (Table I).
 | Table I.Effects of 5-FU treatment on the
production of angiogenic/inflammatory proteins in standard culture
of C26 cells as well as in co-culture of C26 cells and
macrophages. |
Table I.
Effects of 5-FU treatment on the
production of angiogenic/inflammatory proteins in standard culture
of C26 cells as well as in co-culture of C26 cells and
macrophages.
|
| Percentage of
inhibition and stimulation of angiogenic/inflammatory protein
production compared to controls |
|---|
|
|
|
|---|
|
Angiogenic/inflammatory factors | C26+5-FU | C26+φ+5-FU |
|---|
| Granulocyte
CSF |
+7.07±0.37 |
−46.80±0.73c |
|
Granulocyte-macrophage CSF |
+148.58±0.21c |
−48.13±2.69c |
| Monocyte CSF |
−2.30±10.67 |
−60.21±2.29c |
| Insulin-like growth
factor 2 |
+138.56±1.95c |
+11.09±5
37 |
| IL-1α |
+117.25±1.33c |
−58.22±0.73c |
| IL-1β |
+59.80±18.98c |
−70.84±0.44c |
| IL-6 |
+1.96±5.15 |
−43.79±1.61c |
| IL-9 |
+4.12±0.60 |
+15.27±1.33c |
| IL-12p40 |
+53.29±11.95c |
−73.02±0.15c |
| IL-13 |
+101.89±4.79c |
+10.82±1.80 |
| Tumor necrosis
factor α |
+39.59±11.20a |
+19.53±14.65c |
| Monocyte
chemoattractant protein-1 |
+118.83±10.46c |
−54.80±1.68c |
| Eotaxin |
+10.11±9.18 |
−58.02±0.90c |
| Fas ligand |
+498.13±4.91c |
−44.23±1.18c |
| Basic fibroblast
growth factor |
+315.23±12.42c |
−45.08±1.84c |
| Vascular
endothelial growth factor |
−56.69±8.27c |
−75.84±6.96c |
| Leptin |
−45.53±3.11b |
−33.05±10.34 |
| Thrombopoietin |
−5.51±3.99 |
−52.67±1.77c |
| TIMP-1 |
+75.99±0.03c |
−49.62±1.28c |
| TIMP-2 |
+93.76±14.77c |
−48.97±0.11c |
| Platelet factor
4 |
+85.30±35.92c |
−66.08±2.36c |
| IL-12p70 |
+101.51±2.46c |
−56.46±0.45c |
| Interferon γ |
+216.83±4.55c |
−48.25±1.94c |
| Monokine induced by
interferon-γ |
−59.64±10.89c |
−87.02±2.56c |
When macrophages were cultivated with C26 cells,
5-FU significantly reduced the production of inflammatory and
angiogenic factors with an overall inhibitory effect of 44%
(P<0.0001) compared to their control levels. Specifically, the
levels of VEGF and MIG were strongly inhibited by 75–100%, the
levels of monocyte colony-stimulating factor (M-CSF), IL-1α, IL-1β,
IL-12p40, MCP-1, eotaxin, thrombopoietin, IL-12p70 and PF-4 were
reduced by 50–75% and the levels of granulocyte colony-stimulating
factor (G-CSF), GM-CSF, IL-6, FasL, bFGF, tissue inhibitor of
metalloproteinases (TIMP)-1, TIMP-2 and IFN-γ by 25–50% (Table I). The production levels of IL-9 and
TNF-α were stimulated marginally, by 15–20% (P<0.001), following
incubation of the cells in the co-culture with 5-FU.
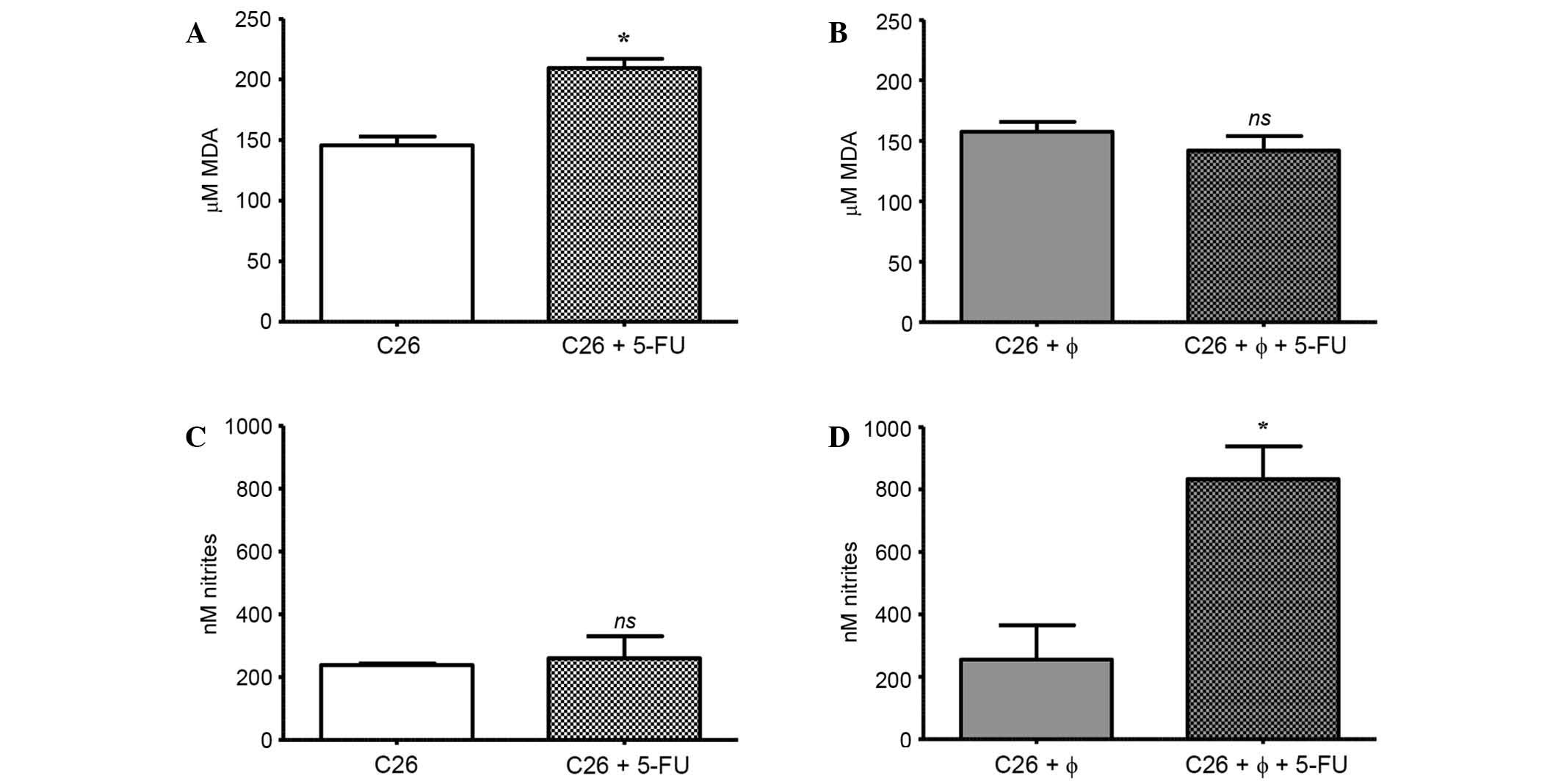
Influence of TAMs on the effects of
5-FU on oxidative stress
To determine whether TAMs are able to modulate the
effects exerted by 4 µM of 5-FU on oxidative stress in C26 cancer
cells, important oxidative stress markers in cell lysates, such as
MDA and nitrite levels (18,23), were quantified. The results are shown
in Fig. 4A-D. Notably, the treatment
with 5-FU significantly increased the level of MDA in C26 cells
cultivated alone (by 45% compared to its control levels; P<0.05)
(Fig. 4A). No significant differences
between MDA levels in untreated cells under either culture
condition were noted (P=0.273) (Fig. 4A
and B). Furthermore, when C26 cells were exposed to 5-FU
treatment in the presence of TAMs, there was no significant
increase in the MDA level compared to the control level (Fig. 4B). This finding may suggest that TAMs
counteracted the pro-oxidant action of 5-FU on C26 cells, as this
effect could be noted only when C26 cells were cultivated alone
(Fig. 4A and B).
To assess whether TAMs modulate the effects of 5-FU
on NO production in C26 cells, the nitrite levels from cell lysates
were determined, since nitrites are the major final metabolites of
NO (23). The results are shown in
Fig. 4C and D. The treatment with
5-FU did not affect the nitrite production in C26 cells under
standard culture conditions (Fig.
4C). In the absence of 5-FU treatment, there was no difference
between nitrite levels in cell lysates obtained under standard
culture and those obtained under co-culture conditions (P=0.8499;
Fig. 4C and D). Notably, when 5-FU
was administered in co-culture, there was a significant 4-fold
increase in nitrite levels compared to their production in control
lysates (lysates from co-culture without treatment; P=0.0329)
(Fig. 4D).
Discussion
The controversial role of TAMs in colon carcinoma
development directed our studies to further investigate whether
this cell type influences the effects of 5-FU on the expression of
the transcription factor NF-κB, a constitutively activated protein
in 67% of CRC cell lines (21). The
results confirmed that C26 cells constitutively expressed NF-κB
(Fig. 2A) and this production was
maintained in cells from the co-culture of C26 cells and TAMs
(Fig. 2B). Since the intratumor
constitutive expression and activation of NF-κB are predominantly
associated with tumor proliferation as well as with rescue of the
cancer cells from cell death (21,24,25),
inhibition of this transcription factor has been demonstrated to be
a potent therapeutic strategy in CRC (26).
Notably, in the present study, 5-FU administration
strongly reduced the levels of NF-κB (by 70% compared to control
levels) in cell lysates obtained under the two culture conditions
(Fig. 2A and B). The suppression of
NF-κB production exerted by 5-FU may be linked to the high
cytotoxicity of this drug on C26 cells (Figs. 1A and 2). This finding is also supported by
previous reports of an association between the high cytotoxicity of
several cytotoxic drugs (5-FU, doxorubicin, paclitaxel and
bortezomib) on different cancer cell lines (human stomach cancer
cells, human myeloid leukemia cells, human salivary gland cancer
cells and C26 colon carcinoma cells) and the inhibition of NF-κB
activation and, finally, induction of apoptosis in these tumor
cells (22,24,27).
Since NF-κB also participates in the induction of
several genes encoding for proteins that support tumor angiogenesis
and inflammation (21,28), we assessed the expression levels of 24
proteins involved in these two pro-tumor processes via protein
array analysis. In line with previous studies (4), our data confirmed that TAMs serve a
crucial role in supporting tumor angiogenesis and inflammation, as
co-cultivation of C26 cells with macrophages led to a doubling of
the average production of the majority of the inflammatory and
angiogenic factors compared to their production in C26 cells
cultivated alone (Fig. 3). Notably,
TNF-α and IL-1β cytokines, which constitutively activate NF-κB and
finally emphasize the angiogenic and metastatic capacity of tumor
cells (7,8,29–31), are overexpressed under co-culture
conditions (Fig. 3). However, in the
presence of macrophages, 5-FU treatment moderately to very strongly
reduced the production of the majority of the pro-angiogenic (VEGF,
eotaxin, thrombopoietin, bFGF) and pro-inflammatory (M-CSF, IL-1α,
IL-1β, IL-12p40, MCP-1, G-CSF, GM-CSF, IL-6, FasL) proteins
compared to their production in the untreated cell co-culture
(Table I). The significant inhibition
of these proteins could account for the high 5-FU cytotoxicity
induced through the suppression of NF-κB since the aforementioned
proteins have also been associated with the proliferation, survival
and metastasis of cancer cells (7,8,10,31,32).
Furthermore, NF-κB signaling has been described as the main
mechanism for maintaining the pro-tumor phenotype of TAMs (33,34).
Interestingly, in the absence of macrophages, 5-FU considerably
stimulated the C26 cell production of 19 out of 24 angiogenic and
inflammatory proteins (Table I).
Although 5-FU treatment strongly reduced VEGF expression (>50%),
the levels of bFGF and FasL were significantly increased (by 315%
for bFGF and ~500% for FasL) compared to their production in the
untreated C26 cells cultivated alone (Table I). These data are supported by
previous findings related to the upregulation of the angiogenic
factor bFGF as a result of suppression of VEGF-regulated signaling
pathways (35). Furthermore, the
overexpression of bFGF and FasL was previously associated with the
increase of the aggressiveness and metastatic potential of cancer
cells (35,36). In conclusion, these data may suggest
the limitation of the 5-FU cytotoxicity on C26 cells, probably due
to the presence of efficient scavenger mechanisms in these cancer
cells via enhancing their angiogenic and inflammatory capacity
(Table I). It is noteworthy that the
cultivation of macrophages with C26 cells counteracted the escape
mechanisms of the carcinoma cells from cytotoxic drug effects,
since the suppression of most of the angiogenic and inflammatory
protein levels was noted after 5-FU administration.
Additionally, as many studies have demonstrated the
role of TAMs in maintaining the physiological range of oxidative
stress necessary for tumor cell proliferation and metastasis
(37–39), we assessed the levels of two important
oxidative stress markers in cell lysates (MDA and nitrites, as
stable final products of NO metabolism) (18,23) after
incubation of C26 cells in standard culture and co-culture with
5-FU. As previously shown in other colon carcinoma cell lines
(40–42), 5-FU exerted pro-oxidant effects on C26
cells cultivated alone. In the present study, the significantly
increased MDA levels following 5-FU treatment (+45%; Fig. 4A) compared to the levels in untreated
C26 cells, could account for 5-FU cytotoxicity on C26 cells, as
several studies already demonstrated that an increased oxidative
stress over the physiological range induced inhibition of cell
proliferation and finally cell death via ROS-induced apoptosis
(40,42,43).
Nevertheless, this pro-oxidant effect of 5-FU may be
responsible for stimulating the production of the angiogenic and
inflammatory proteins in the remaining C26 cancer cells mentioned
earlier (Table I). This finding is
also supported by several studies that suggest that an essential
feature of the aggressive phenotype, acquired by the undestroyed
cancer cells exposed to high ROS levels, is the ability to enhance
the production of angiogenic and inflammatory proteins (44). However, when 5-FU was administered in
the co-culture, there was no effect on MDA levels in cell lysates
(Fig. 4B). This finding suggested
that TAMs may protect cancer cells against 5-FU-induced oxidative
stress and, subsequently, from ROS-induced cytotoxicity.
Furthermore, our data regarding the NO amount in cell lysates
revealed significant increases in nitrite levels (4 times higher
than in controls or 5-FU-treated C26 cells alone) only after 5-FU
administration in C26 cells cultivated with TAMs (Fig. 4C and D). Nevertheless, the increase of
NO production noted following co-cultivation of C26 cells with TAMs
did not exceed the physiological range of NO production in tumors
(nM range) described previously (20). This result is likely related to the
cytoprotective effects of TAMs against pro-oxidant effects of 5-FU
on C26 cells (Fig. 4A and D).
As other studies previously suggested, the increased
production of NO could be a phenomenon strictly related to the
tumor cell-macrophage interaction (12). Previous studies demonstrated that
nanomolar concentrations of NO produced by inducible/endothelial
nitric oxide synthases in human colon and ovarian carcinoma cells
as a result of administration of cytotoxic drugs other than 5-FU,
such as doxorubicin or cisplatin, ensure protection against
ROS-induced apoptosis in these cancer cells (39,45,46).
Although TAMs protect C26 cells against the pro-oxidant effect of
5-FU, this action may avoid the acquisition of the aggressive
phenotype of the remaining C26 cells, since the production of the
majority of the angiogenic and inflammatory proteins was reduced
notably in cells under co-culture conditions (Table I).
Taken together, the results suggest that TAMs have a
dual role in the modulation of the response of C26 cells to 5-FU
treatment. On one side, TAMs increase chemosensitivity of these
cancer cells to 5-FU treatment via mediating an overall strong
reduction of inflammatory and angiogenic factors; however, on the
other side, TAMs protect cancer cells against the pro-oxidant
effect of 5-FU by maintaining ROS levels in the physiological range
of C26 cell oxidative stress.
Finally, the current results suggest that
therapeutic strategies of CRC should further exploit the intrinsic
oxidative stress of cancer cells by combining the administration of
5-FU with pharmacological agents that prevent TAMs to maintain the
physiological range of tumor oxidative stress.
Acknowledgements
This paper is a result of doctoral research made
possible by the financial support of the Executive Agency for
Higher Education, Research, Development and Innovation Funding,
grant-PN-II-PT-PCCA-2011-3.2-1060 (contract number 95/2012), and
the Sectorial Operational Program for Human Resources Development
2007–2013, co-financed by the European Social Fund [projects
POSDRU/159/1.5/S/133391 (Doctoral and Postdoctoral Excellence
Programs for Training Highly Qualified Human Resources for Research
in the Fields of Life Sciences, Environment and Earth; granted to
Mrs. Laura Patras and Dr Alina Sesarman) and
POSDRU/187/1.5/S/155383 (Quality, Excellence, and Transnational
Mobility in Doctoral Research; granted to Mrs. Lavinia Luca)].
References
|
1
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang N, Yin Y, Xu SJ and Chen WS:
5-fluorouracil: Mechanisms of resistance and reversal strategies.
Molecules. 13:1551–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Palma M and Lewis CE: Macrophage
regulation of tumor responses to anticancer therapies. Cancer Cell.
23:277–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Banciu M, Metselaar JM, Schiffelers RM and
Storm G: Antitumor activity of liposomal prednisolone phosphate
depends on the presence of functional tumor-associated macrophages
in tumor tissue. Neoplasia. 10:108–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Solinas G, Germano G, Mantovani A and
Allavena P: Tumor-associated macrophages (TAM) as major players of
the cancer-related inflammation. J Leukoc Biol. 86:1065–1073. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crowther M, Brown NJ, Bishop ET and Lewis
CE: Microenvironmental influence on macrophage regulation of
angiogenesis in wounds and malignant tumors. J Leukoc Biol.
70:478–490. 2001.PubMed/NCBI
|
|
7
|
Hagemann T, Robinson SC, Schulz M, Trümper
L, Balkwill FR and Binder C: Enhanced invasiveness of breast cancer
cell lines upon co-cultivation with macrophages is due to TNF-alpha
dependent up-regulation of matrix metalloproteases. Carcinogenesis.
25:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A,
Schwall R, Schnitt SJ, Guida A, Hastings HM, Andreas J, et al:
Expression of interleukin-1beta in human breast carcinoma. Cancer.
80:421–434. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brüne B, Dehne N, Grossmann N, Jung M,
Namgaladze D, Schmid D, von Knethen A and Weigert A: Redox control
of inflammation in macrophages. Antioxid Redox Signal. 19:595–637.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Quatromoni JG and Eruslanov E:
Tumor-associated macrophages: Function, phenotype, and link to
prognosis in human lung cancer. Am J Transl Res. 4:376–389.
2012.PubMed/NCBI
|
|
11
|
Zhang Y, Sime W, Juhas M and Sjölander A:
Crosstalk between colon cancer cells and macrophages via
inflammatory mediators and CD47 promotes tumour cell migration. Eur
J Cancer. 49:3320–3334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calorini L, Bianchini F, Mannini A, Mugnai
G and Ruggieri S: Enhancement of nitric oxide release in mouse
inflammatory macrophages co-cultivated with tumor cells of a
different origin. Clin Exp Metastasis. 22:413–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herbeuval JP, Lelievre E, Lambert C, Dy M
and Genin C: Recruitment of STAT3 for production of IL-10 by colon
carcinoma cells induced by macrophage-derived IL-6. J Immunol.
172:4630–4636. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martinez FO, Gordon S, Locati M and
Mantovani A: Transcriptional profiling of the human
monocyte-to-macrophage differentiation and polarization: New
molecules and patterns of gene expression. J Immunol.
177:7303–7311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banciu M, Schiffelers RM, Fens MH,
Metselaar JM and Storm G: Anti-angiogenic effects of liposomal
prednisolone phosphate on B16 melanoma in mice. J Control Release.
113:1–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lixuan Z, Jingcheng D, Wenqin Y, Jianhua
H, Baojun L and Xiaotao F: Baicalin attenuates inflammation by
inhibiting NF-kappaB activation in cigarette smoke induced
inflammatory models. Pulm Pharmacol Ther. 23:411–419. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Del Rio D, Stewart AJ and Pellegrini N: A
review of recent studies on malondialdehyde as toxic molecule and
biological marker of oxidative stress. Nutr Metab Cardiovasc Dis.
15:316–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alupei MC, Licarete E, Patras L and Banciu
M: Liposomal simvastatin inhibits tumor growth via targeting
tumor-associated macrophages-mediated oxidative stress. Cancer
Lett. 356:946–952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahat MA and Hemmerlein B:
Macrophage-tumor cell interactions regulate the function of nitric
oxide. Front Physiol. 4:1442013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakamoto K, Maeda S, Hikiba Y, Nakagawa H,
Hayakawa Y, Shibata W, Yanai A, Ogura A and Omata M: Constitutive
NF-kappaB activation in colorectal carcinoma plays a key role in
angiogenesis, promoting tumor growth. Clin Cancer Res.
15:2248–2258. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uetsuka H, Haisa M, Kimura M, Gunduz M,
Kaneda Y, Ohkawa T, Takaoka M, Murata T, Nobuhisa T, Yamatsuji T,
et al: Inhibition of inducible NF-kappaB activity reduces
chemoresistance to 5-fluorouracil in human stomach cancer cell
line. Exp Cell Res. 289:27–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yucel A, Gulen S, Dincer S, Yucel A and
Yetkin G: Comparison of two different applications of the Griess
method for nitric oxide measurement. J Exp Integr Med. 2:12012.
View Article : Google Scholar
|
|
24
|
Nowis D, McConnell EJ, Dierlam L,
Palamarchuk A, Lass A and Wójcik C: TNF potentiates anticancer
activity of bortezomib (Velcade) through reduced expression of
proteasome subunits and dysregulation of unfolded protein response.
Int J Cancer. 121:431–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nai YJ, Jiang ZW, Wang ZM, Li N and Li JS:
Prevention of cancer cachexia by pyrrolidine dithiocarbamate (PDTC)
in colon 26 tumor-bearing mice. JPEN J Parenter Enteral Nutr.
31:18–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fernández-Majada V, Aguilera C, Villanueva
A, Vilardell F, Robert-Moreno A, Aytés A, Real FX, Capella G, Mayo
MW, Espinosa L and Bigas A: Nuclear IKK activity leads to
dysregulated notch-dependent gene expression in colorectal cancer.
Proc Natl Acad Sci USA. 104:276–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Azuma M, Yamashita T, Aota K, Tamatani T
and Sato M: 5-Fluorouracil suppression of NF-KappaB is mediated by
the inhibition of IKappab kinase activity in human salivary gland
cancer cells. Biochem Biophys Res Commun. 282:292–296. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karin M: NF-kappaB as a critical link
between inflammation and cancer. Cold Spring Harb Perspect Biol.
1:a0001412009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin Y, Bai L, Chen W and Xu S: The
NF-kappaB activation pathways, emerging molecular targets for
cancer prevention and therapy. Expert Opin Ther Targets. 14:45–55.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choo MK, Sakurai H, Koizumi K and Saiki I:
Stimulation of cultured colon 26 cells with TNF-alpha promotes lung
metastasis through the extracellular signal-regulated kinase
pathway. Cancer Lett. 230:47–56. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zins K, Abraham D, Sioud M and Aharinejad
S: Colon cancer cell-derived tumor necrosis factor-alpha mediates
the tumor growth-promoting response in macrophages by up-regulating
the colony-stimulating factor-1 pathway. Cancer Res. 67:1038–1045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gutschalk CM, Yanamandra AK, Linde N,
Meides A, Depner S and Mueller MM: GM-CSF enhances tumor invasion
by elevated MMP-2, −9, and −26 expression. Cancer Med. 2:117–129.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hagemann T, Lawrence T, McNeish I, Charles
KA, Kulbe H, Thompson RG, Robinson SC and Balkwill FR:
‘Re-educating’ tumor-associated macrophages by targeting NF-kappaB.
J Exp Med. 205:1261–1268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ryan AE, Colleran A, O'Gorman A, O'Flynn
L, Pindjacova J, Lohan P, O'Malley G, Nosov M, Mureau C and Egan
LJ: Targeting colon cancer cell NF-κB promotes an anti-tumour
M1-like macrophage phenotype and inhibits peritoneal metastasis.
Oncogene. 34:1563–1574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Casanovas O, Hicklin DJ, Bergers G and
Hanahan D: Drug resistance by evasion of antiangiogenic targeting
of VEGF signaling in late-stage pancreatic islet tumors. Cancer
Cell. 8:299–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Igney FH and Krammer PH: Tumor
counterattack: Fact or fiction? Cancer Immunol Immunother.
54:1127–1136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kundu N, Zhang S and Fulton AM: Sublethal
oxidative stress inhibits tumor cell adhesion and enhances
experimental metastasis of murine mammary carcinoma. Clin Exp
Metastasis. 13:16–22. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siegert A, Denkert C, Leclere A and
Hauptmann S: Suppression of the reactive oxygen intermediates
production of human macrophages by colorectal adenocarcinoma cell
lines. Immunology. 98:551–556. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wartenberg M, Schallenberg M, Hescheler J
and Sauer H: Reactive oxygen species-mediated regulation of eNOS
and iNOS expression in multicellular prostate tumor spheroids. Int
J Cancer. 104:274–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hwang PM, Bunz F, Yu J, Rago C, Chan TA,
Murphy MP, Kelso GF, Smith RA, Kinzler KW and Vogelstein B:
Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced
apoptosis in colorectal cancer cells. Nat Med. 7:1111–1117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lamberti M, Porto S, Marra M, Zappavigna
S, Grimaldi A, Feola D, Pesce D, Naviglio S, Spina A, Sannolo N and
Caraglia M: 5-fluorouracil induces apoptosis in rat cardiocytes
through intracellular oxidative stress. J Exp Clin Cancer Res.
31:602012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fu Y, Yang G, Zhu F, Peng C, Li W, Li H,
Kim HG, Bode AM and Dong Z and Dong Z: Antioxidants decrease the
apoptotic effect of 5-Fu in colon cancer by regulating
Src-dependent caspase-7 phosphorylation. Cell Death Dis.
5:e9832014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sun L, Luo C and Liu J: Hydroxytyrosol
induces apoptosis in human colon cancer cells through ROS
generation. Food Funct. 5:1909–1914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fiaschi T and Chiarugi P: Oxidative
stress, tumor microenvironment, and metabolic reprogramming: A
diabolic liaison. Int J Cell Biol. 2012:7628252012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Riganti C, Miraglia E, Viarisio D,
Costamagna C, Pescarmona G, Ghigo D and Bosia A: Nitric oxide
reverts the resistance to doxorubicin in human colon cancer cells
by inhibiting the drug efflux. Cancer Res. 65:516–525.
2005.PubMed/NCBI
|
|
46
|
Leung EL, Fraser M, Fiscus RR and Tsang
BK: Cisplatin alters nitric oxide synthase levels in human ovarian
cancer cells: Involvement in p53 regulation and cisplatin
resistance. Br J Cancer. 98:1803–1809. 2008. View Article : Google Scholar : PubMed/NCBI
|