Introduction
Soft tissue sarcoma (STS) is a heterogeneous group
of neoplasms arising from degenerated cells of mesenchymal origin
(1). Currently, STS is differentiated
into >20 distinct subtypes, which are classified according to
their tissue of origin (2). The most
common type of STS observed in adults is undifferentiated
pleomorphic sarcoma not otherwise specified (NOS), which was
previously known as malignant fibrous histiocytoma, and presents
five histological subtypes (3–5).
Currently, surgical resection remains the only method of curative
treatment for undifferentiated pleomorphic sarcoma NOS, which often
occurs at a high malignancy grade, possesses a high risk of
metastasis, and exhibits resistance to radiotherapy and
chemotherapy (6–8). Previous studies have demonstrated that
additional radiation therapy improves local tumor control; however,
chemotherapy remains palliative, since there are no effective
chemotherapy drugs (9,10). Due to the limited number of patients
with specific subtypes of undifferentiated pleomorphic sarcoma NOS,
clinical studies often present limitations, and the majority of the
data available may not apply to certain subtypes. The application
of an anthracycline-based chemotherapy, including doxorubicin alone
or in combination with ifosfamide, is often the first-line
treatment of undifferentiated pleomorphic sarcoma NOS, and there
are no widely recognized second-line therapies available (11–13).
Recently, improved second-line drugs have been developed, including
histone deacetylase (HDAC) inhibitors, trabectedin and tyrosine
kinase inhibitors such as pazopanib, which are more effective than
those currently available (14–19). With
these drugs, a progression-free survival time of 3–5 months may be
achieved (14–19). However, the overall survival time is
not increased. Therefore, to prolong the survival time of patients
with undifferentiated pleomorphic sarcoma NOS, additional
improvements are required.
Cell lines and animal models are powerful tools for
the development of innovative therapeutics. In the past recent
years, numerous cell lines derived from undifferentiated
pleomorphic sarcoma have been generated and characterized (20–24). A
number of these cell lines were injected subcutaneously into
immunodeficient mice, and 4 weeks subsequent to injection
measurable tumor tissue was formed (20,21,24).
However, investigations comparing the effect of therapeutical
approaches in vivo and in vitro have not been
performed thus far. In other studies using xenotransplantation
models, original human tumor tissue was transplanted into
immunodeficient mice (25–27). These animal models appear to be more
similar to the tumors observed in humans, but hypoxia following
tissue transplantation remains a problem (25–27).
In the present study, two stable cell cultures were
generated from two patients with undifferentiated pleomorphic
sarcoma. These cells were subcutaneously injected into
immunodeficient mice, whereby remained tumorigenic. Following
chemotherapeutic treatment of the tumor, clear differences were
observed between the in vitro and in vivo models,
which confirms that it is imperative to test innovative
chemotherapeutics in appropriate animal models prior to clinical
use.
Materials and methods
Animals
In total, 4 immunodeficient non-obese diabetic (NOD)
severe combined immunodeficiency (SCID) gamma (NSG) mice
(NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) (2 male
and 2 female; 8 weeks-old) were purchased from the Jackson
Laboratory (Sacramento, ME, USA), and bred in the animal facility
at the Johannes Gutenberg University of Mainz (Mainz, Germany). The
mice were bred, maintained and manipulated under specific
pathogen-free conditions. All the food, water and litter were
sterilized prior to use. The temperature and humidity were
controlled at 20–24°C and 45–65%, respectively. Daily light cycles
consisted of 12 h light and dark cycles. The cages were fully
cleaned once or twice per week. Mice that were 6–8 weeks-old were
used for subcutaneous injections. All animal procedures were
conducted in accordance with the Institutional Guidelines of the
Johannes Gutenberg University of Mainz, and approved by the
responsible national authority (National Investigation Office
Rheinland-Pfalz; Koblenz, Germany; approval no. 23 177-07/G
13-1-027).
Isolation of sarcoma samples and cell
culture
Stable oligoclonal cell cultures, termed MZ-UPS-1
and MZ-UPS-2, were generated from freshly isolated tumor tissue
from two patients diagnosed with undifferentiated pleomorphic
sarcoma NOS. These two patients were treated in 2011 and 2012,
respectively, at the University Medical Center in Mainz and had
undergone surgeries where the local tumor tissue was removed.
Immediately following resection, the tumor tissue was minced,
placed onto 6-well plates (Sigma-Aldrich Chemie GmbH, Taufkirchen,
Germany) and cultivated at 37°C in a humidified atmosphere with 5%
CO2 with Gibco® Dulbecco's modified Eagle's
medium [Nutrient Mixture F-12 containing GlutaMAX™ Supplement
(DMEM/F12; Thermo Fisher Scientific, Inc., Waltham, MA, USA)], 1%
sodium pyruvate (100 mM; Thermo Fisher Scientific, Inc.), 10% fetal
calf serum (FCS; GE Healthcare Life Sciences, Chalfont, UK) and 1%
penicillin-streptomycin (Invitrogen™; Thermo Fisher Scientific,
Inc.). The cells were expanded to form a sub-confluent layer of
adherent cells 2–4 weeks subsequent to initial seeding.
Subsequently, the adherent tumor cells were digested using Accutase
(GE Healthcare Life Sciences) and transferred into 175
cm2 cell culture flasks (Sigma-Aldrich Chemie GmbH).
Since the MZ-UPS-1 cells were a fast-growing culture, they were
passaged and serially subcultured at a dilution of 1:3–1:5 every
week. By contrast, the MZ-UPS-2 cells were passaged every two weeks
and serially subcultured at a dilution of 1:2. The two
undifferentiated pleomorphic cell cultures MZ-UPS-1 and MZ-UPS-2
were maintained in vitro for ~30 passages for >1
year.
The two patients provided written informed consent
for biobanking. The present study was approved by the Ethics
Committee of the University Medical Center of Johannes Gutenberg
University of Mainz [Mainz, Germany; approval no. 837.250.13
(8935)].
Chemotherapeutic treatment in vitro
and cell viability assay
MZ-UPS-1 and MZ-UPS-2 cells from the fourth passage
were harvested, washed in phosphate-buffered saline (PBS; Thermo
Fisher Scientific, Inc.) and resuspended in DMEM/F12-GlutaMAX™
Supplement with 10% FCS. One day prior to chemotherapeutic
treatment, the cells were seeded onto 96-well plates
(1×104 cells/100 µl medium/well; Greiner Bio-One GmbH,
Frickenhausen, Germany) to ensure adherence. On day 1, the
supernatants were discarded, and a cell viability assay was
conducted with alamarBlue™ Cell Viability Assay Reagent (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Briefly, alamarBlue™ solution was diluted 1:10 in
DMEM/F12-GlutaMAX™ Supplement, and 100 µl/well was added to the
cells, which were incubated for 1–2 h. Subsequently, the
supernatants were removed, and the absorbance was measured at 570
nm (reference wavelength, 600 nm) in a multiplate spectophotometer
(Sunrise™; Tecan Group Ltd., Männedorf, Switzerland). In parallel
assays, the plated tumor cells were cultured in 200 µl
DMEM/F12-GlutaMAX™ Supplement in the presence of the appropriate
concentrations of the chemotherapeutic agents for 1, 2, 4, 7, 10
and 14 days. alamarBlue™ assays were performed at each of the above
time points to determine cell viability. At least three experiments
were performed.
Xenotransplantation
Cultured MZ-UPS-1 and MZ-UPS-2 cells from the fourth
passage were harvested by detachment with Accutase and washed twice
in PBS. Live cells were counted using trypan blue staining
(Sigma-Aldrich Chemie GmbH) and a Neubauer counting chamber
(Sigma-Aldrich Chemie GmbH). In total, 1×106 cells were
injected subcutaneously into the right flank of NSG mice. Tumor
growth was verified 4 weeks later. The effectiveness of the
xenograft transplant was 100% for the two undifferentiated
pleomorphic sarcoma cell cultures MZ-UPS-1 and MZ-UPS-2.
Histology
Samples from the original tumors of the two patients
and isolated xenografts from the mice were fixed in 4%
phosphate-buffered formaldehyde solution
(Roti®-Histofix; Carl Roth GmbH + Co. KG, Karlsruhe,
Germany) and embedded in paraffin (Sigma-Aldrich Chemie GmbH). The
tissue sections (5 µm) were subsequently deparaffinized using xylol
(Sigma-Aldrich Chemie GmbH) and a descending sequence of ethanol
(100, 90 and 70% and distilled water), stained with hematoxylin and
eosin (Sigma-Aldrich Chemie GmbH) and viewed under a microscope
(Axioskop 40; Zeiss GmbH, Jena, Germany).
Immunohistochemical staining were performed
according to manufacturers's protocol on a Ventana BenchMark XT
platform (Ventana Medical Systems, Inc., Tucson, AZ, USA).
Following deparaffinization, antigen retrieval was performed using
peroxidase and alkaline phosphatase blocking reagent (Dako,
Glostrup, Denmark) for 10 min at 95–99°C. The tissue sections were
incubated with mouse anti-human monoclonal vimentin (for 32 min;
catalog no., M0725), mouse anti-human monoclonal actin (for 32 min;
catalog no., M0851) and rabbit monoclonal anti-human Ki-67
antibodies (for 36 min; catalog no., M7240) at room temperature.
All primary antibodies were purchased from Dako. Following washing
two times with reaction buffer (Ventana Medical Systems, Inc.), the
slides were then incubated with anti-mouse secondary antibody
(ultraView Universal Alkaline Phosphatase Red Detection Kit;
Ventana Medical Systems, Inc.) for 30 min at room temperature and
visualized according to manufacturer's instructions.
Animal treatment and tumor
measurement
The size of the tumors in the mice were measured
using a digital caliper 6–8 weeks subsequent to subcutaneous
injection of 1×106 MZ-UPS-1 or MZ-UPS-2 cells. Tumor
size was measured from caudal to cranial and dorsal to ventral, and
the values were multiplied to calculate the tumor area.
Chemotherapeutic agents were prepared prior to application.
Ready-to-use doxorubicin was purchased at a concentration of 2
mg/ml (Hexal AG, Holzkirchen, Germany), and suberoylanilide
hydroxamic acid (SAHA; Sigma-Aldrich Chemie GmbH) was dissolved in
dimethyl sulfoxide (DMSO; Sigma-Aldrich Chemie GmbH) at a
concentration of 25 mg/ml. Pazopanib was purchased as 400 mg
capsules Votrient® (GlaxoSmithKline, Brentford, UK). One
Votrient® capsule was ground using a pestel and mortar,
and dissolved in DMSO at a concentration of 30 mg/ml upon mixing
overnight. The mice were weighed and treated as described in
Table I. Tumor size was measured
every 2 days.
 | Table I.Chemotherapeutic treatment regimen of
xenograft mouse models of undifferentiated pleomorphic sarcoma. |
Table I.
Chemotherapeutic treatment regimen of
xenograft mouse models of undifferentiated pleomorphic sarcoma.
| Chemotherapy | Body concentration,
mg/kg | Type of
application | Application
mode |
|---|
| Doxorubicin |
6 | Intravenous | Weekly |
| Suberoylanilide
hydroxamic acid | 50 |
Intraperitoneal | Daily |
| Pazopanib | 100 | Oral | Daily |
Statistical analysis
Student's t test was used to compare the mean
values between two experimental groups where appropriate using
GraphPad Prism version 6.0f (GraphPad Software, Inc., La Jolla, CA,
USA). For non-Gaussian distributions, the Mann Whitney U test was
used for the calculation of statistical significance. Gaussian
distribution was analyzed with the Kolmogorov Smirnov test. Data
are expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Two undifferentiated pleomorphic
sarcoma cell lines were established
Histology of the biopsies and resected tumors of the
two patients provided a diagnosis of undifferentiated pleomorphic
sarcoma NOS grade 3, according to the FNCLCC classification
(28). The tumors were located in the
left axillary region or the gluteus maximus. The two primary tumor
tissues histologically exhibited a storiform and pleomorphic growth
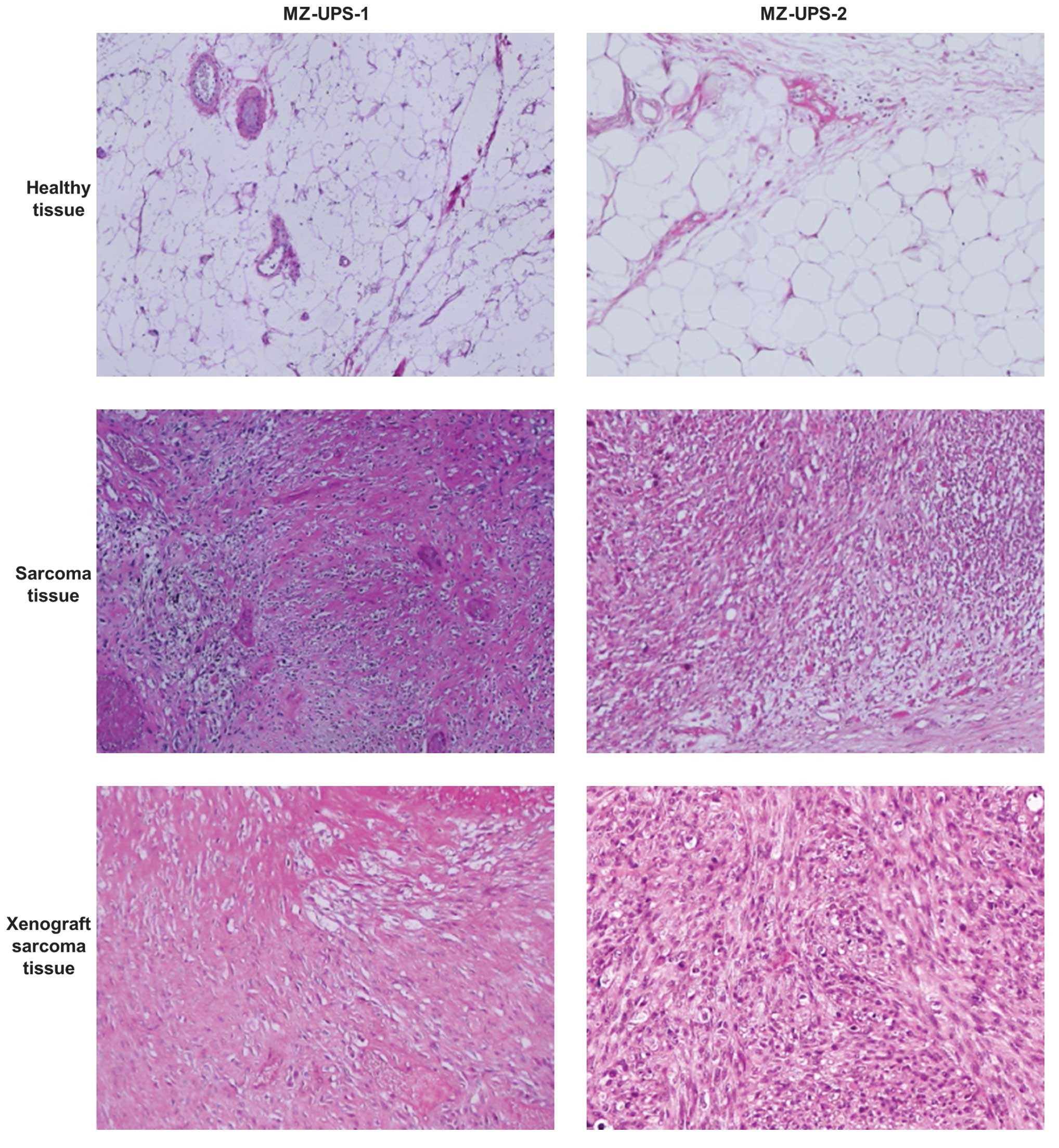
pattern with specific myxoid regions (Fig. 1).
Immunohistochemical staining of the MZ-UPS-1 cells
and the original tumor from which they were derived, revealed that
the cells expressed vimentin and actin and possessed a Ki-67 index
of >20%. The MZ-UPS-2 cells and the cells from their original
tumor expressed vimentin and possessed a Ki-67 index of >20%
(data not shown). The features of the two undifferentiated
pleomorphic sarcoma cell lines are revealed in Table II.
 | Table II.Two novel undifferentiated
pleomorphic sarcoma cell lines. |
Table II.
Two novel undifferentiated
pleomorphic sarcoma cell lines.
| Features | MZ-UPS-1 | MZ-UPS-2 |
|---|
| Organism | Human | Human |
| Ethnicity | Caucasian | Caucasian |
| Age, years | 47 | 48 |
| Gender | Male | Female |
| Tissue | Mesenchymal | Mesenchymal |
| Morphology |
Fibroblastic/myofibroblastic |
Fibroblastic/myofibroblastic |
| Cell type | Pleomorphic sarcoma
NOS, G3 | Pleomorphic sarcoma
NOS, G3 |
| Growth
properties | Monolayer | Monolayer |
| Culture medium | DMEM/F12-GlutaMAX™
Supplement with sodium pyruvate and 10% FCS | DMEM/F12- GlutaMAX™
Supplement with sodium pyruvate and 10% FCS |
| Split ratio | 1:3–1:5 every
week | 1:2 every 2
weeks |
| Medium renewal | 2–3 times
weekly | 2–3 times
weekly |
| Tumorigenic | Yes, in NSG
mice | Yes, in NSG
mice |
Tumor formation following subcutaneous
xenotransplantation
Early passages of the two undifferentiated
pleomorphic sarcoma cells were used for subcutaneous
xenotransplantation into NSG mice. Tumors from the MZ-UPS-1 cells
grew ≤1.5 cm3 in diameter 4–6 weeks subsequent to a
subcutaneous injection of 1×106 cells. The histology and
type of growth of the tumor was similar to the original human
resected tumor (Fig. 1). In the
boundary region, the tumor tissue exhibited a solid and
predominantly storiform growth pattern with increased myxoid
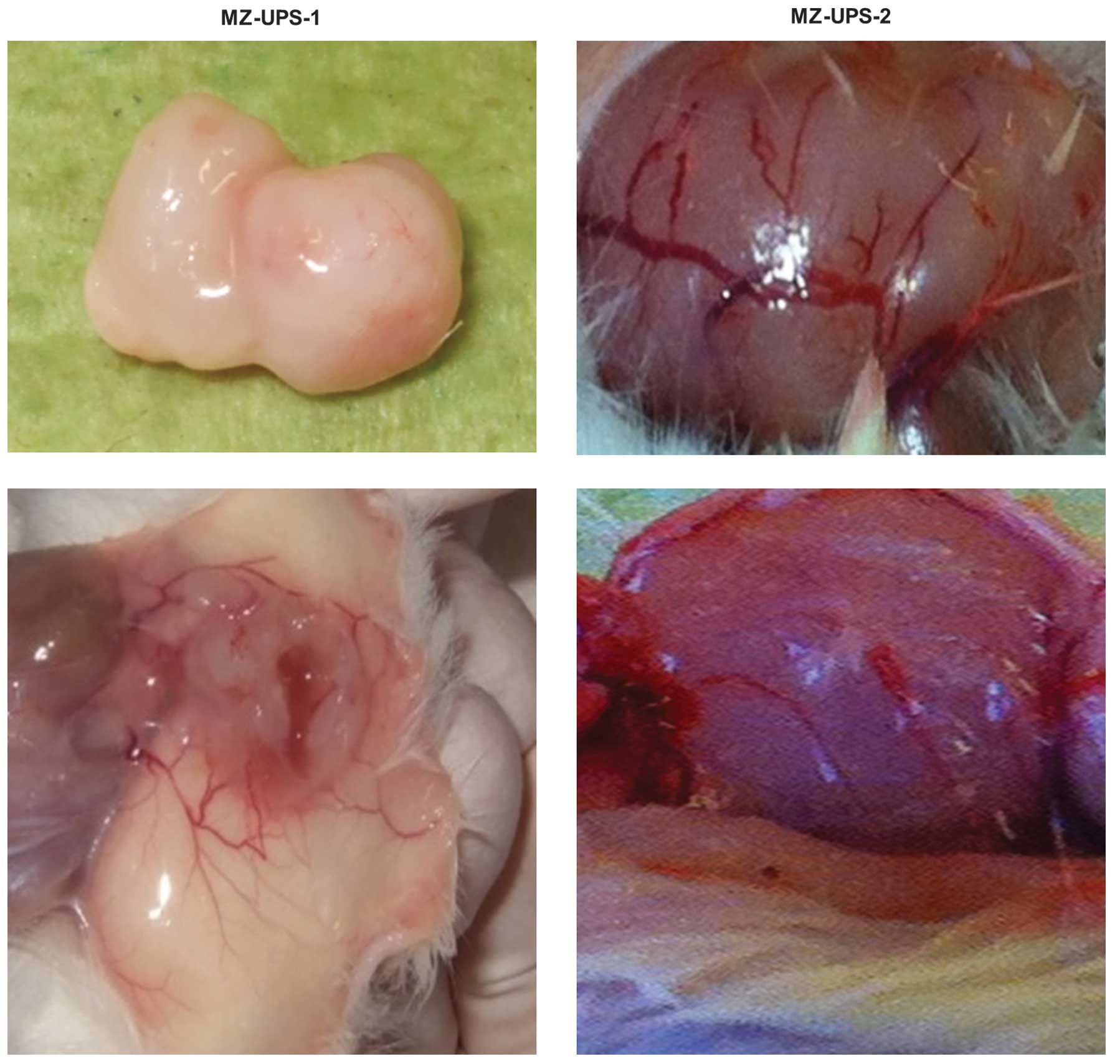
regions and a fluid-filled chamber. On the surface of the tumor and
the surrounding tissue, neovascularization was clearly observed
(Fig. 2). Similarly to the in
vitro findings, the in vivo tumor formation of MZ-UPS-2
cells was slower, compared with MZ-UPS-1 cells. Histologically, the
MZ-UPS-2 xenograft exhibited a predominantly storiform growth
pattern; however, no myxoid regions or fluid-filled chambers were
observed (Fig. 1), contrarily to the
original MZ-UPS-2 tumor tissue. Similarly to the xenograft tumor
from the MZ-UPS-1 cells, the MZ-UPS-2 tumor exhibited clear
neovascularization on the surface.
Chemotherapeutic treatment of
undifferentiated pleomorphic sarcoma cell cultures in vitro
Doxorubicin is commonly used as the first-line
treatment for patients with undifferentiated pleomorphic sarcoma.
There is no widely accepted second-line treatment. However, novel
therapeutic approaches have been recently identified as second-line
treatments for patients with recurrent or incurable sarcoma,
including HDAC inhibitors and tyrosine kinase inhibitors, such as
pazopanib, which has been approved in USA and Europe since 2012 for
the treatment of distinct STS subtypes (14–19).
The two patients in the present study were
administered doxorubicin; however, this treatment did not prevent
tumor progression. Pazopanib was also used in the two patients as a
second-line treatment, which resulted in the tumor becoming stable.
During pazopanib treatment, symptom relief with increased tumor
necrosis was observed in one patient (MZ-UPS-2), and in the other
patient a mild tumor regression was observed (data not shown).
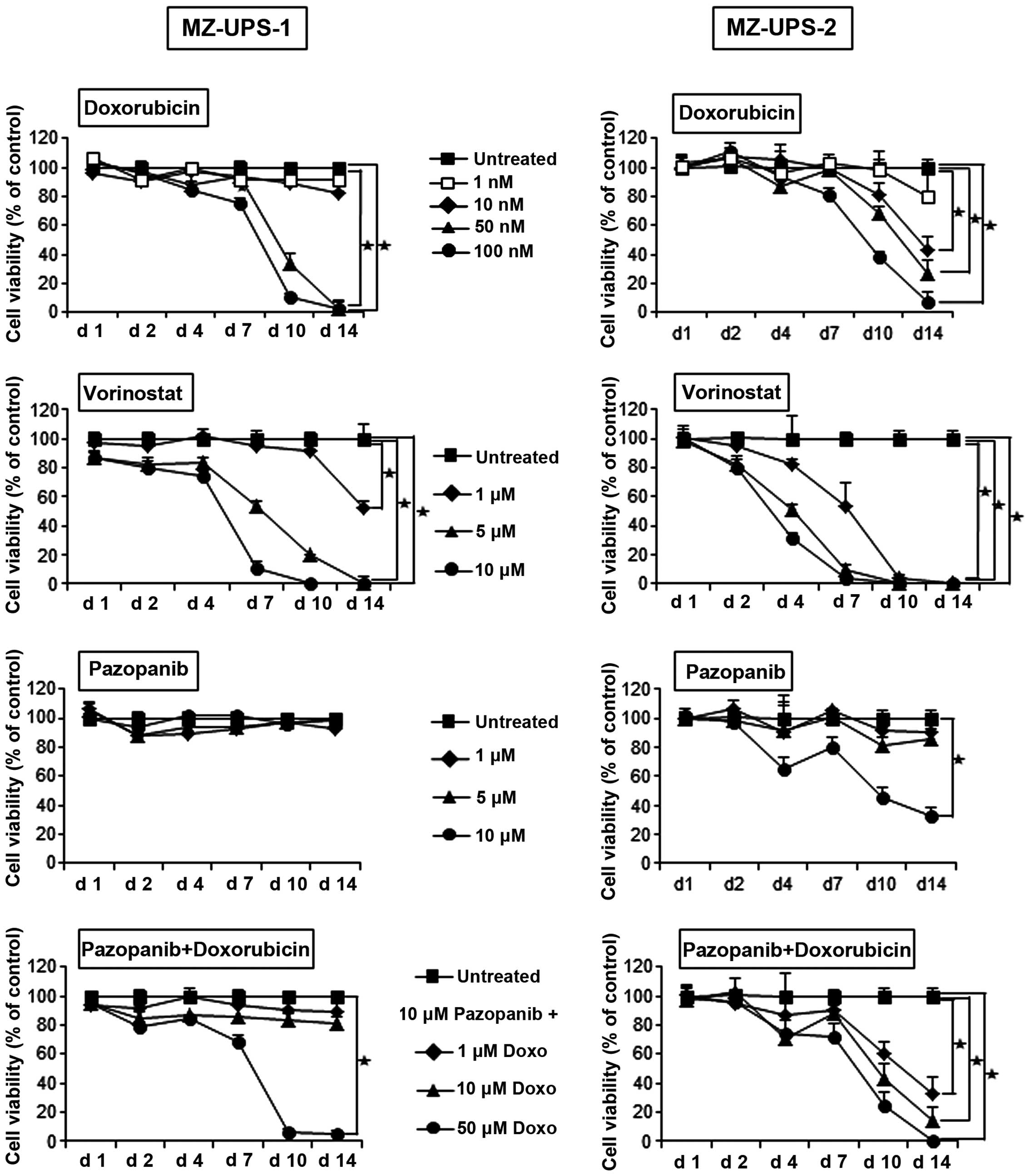
Prior to the in vivo experiments, the fourth
passages of the two cell cultures, MZ-UPS-1 and MZ-UPS-2, were
incubated with various concentrations of doxorubicin, pazopanib and
the HDAC inhibitor SAHA, also known as vorinostat (Fig. 3). The anthracycline doxorubicin is a
remarkably potent cytostatic drug, and caused the death of the
MZ-UPS-1 cells within 14 days, even at a concentration of 50 nM.
The viability of the MZ-UPS-2 cells began to decrease on day 7
following incubation with the highest concentration of doxorubicin
tested (100 nM). On day 14, the viability of the MZ-UPS-2 cells was
considerably decreased, even at a concentration of 10 nM
doxorubicin. The viability of the two cell cultures was decreased
on day 4 following incubation in the presence of 5 or 10 µM
vorinostat. Pazopanib had no inhibitory effect on MZ-UPS-1 cells,
and slightly inhibited the growth of MZ-UPS-2 cells. The
combination of doxorubicin and pazopanib had no synergistic effect
on MZ-UPS-1 cells, and only caused a small synergistic reduction in
the viability of MZ-UPS-2 cells.
Chemotherapeutic treatment in
vivo
Immunodeficient NSG mice with xenografts of MZ-UPS-1
or MZ-UPS-2 cells were generated in the present study to compare
the efficacy of different chemotherapeutic treatments in
vivo (which is expected to be comparable to human patient
response) with the cytostatic effects demonstrated by these
chemotherapeutic drugs in vitro.
Cells from the same passage were isolated and
injected into NSG mice. The concentrations of the chemotherapeutic
agents used and the frequency of application corresponded to
treatment regimens that are normally employed to treat human
patients with undifferentiated pleomorphic sarcoma (Fig. 4A), according to the guidelines from
the German Society for Hematology and Medical Oncology (www.onkopedia-guidelines.info/en/onkopedia/guidelines).
Tumor size from the mouse models was initially measured and
normalized to 100% 6–8 weeks subsequent to the injection of the
human undifferentiated pleomorphic sarcoma cells. The tumor size of
MZ-UPS-1 mice that were not treated with chemotherapy had more than
doubled within 8 days. By contrast, the tumor growth of mice
treated with doxorubicin or SAHA was markedly decelerated, and
treatment with pazopanib resulted in a stabilization of tumor size.
Combined therapy with doxorubicin and pazopanib significantly
reduced the tumor to <50% of its initial size (Fig. 4B).
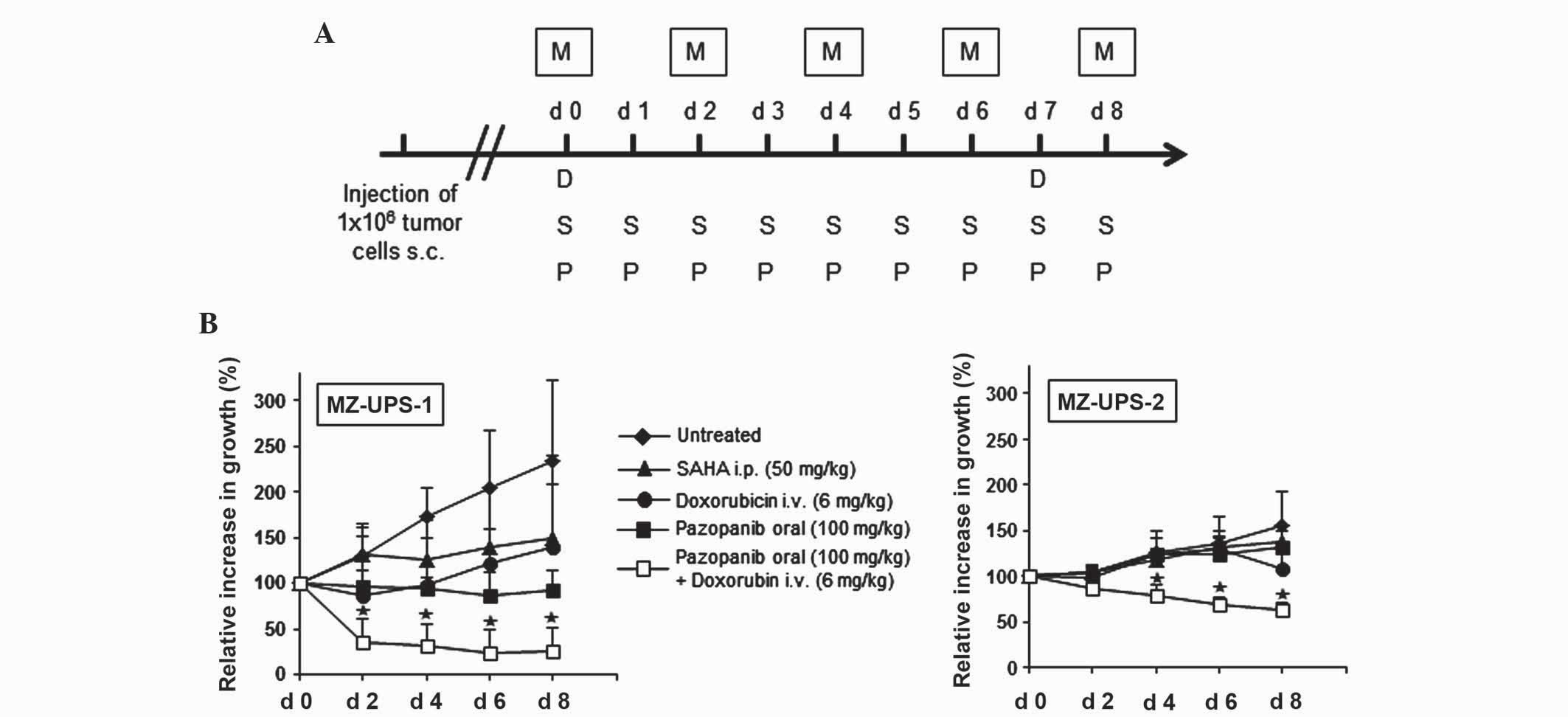 | Figure 4.Combined therapy with doxorubicin and
pazopanib resulted in a reduction of tumor size in the two
xenograft sarcoma mouse models. In vitro cultured MZ-UPS-1
and MZ-UPS-2 cells were washed twice in phosphate-buffered saline,
and 1×106 cells were injected subcutaneously into NSG
mice. Chemotherapeutic treatment was started 6–8 weeks following
xenotransplantation. (A) The chemotherapeutic regimen administered
to the xenograft sarcoma mouse models was as follows: Doxorubicin
was injected intravenously, while suberoylanilide hydroxamic acid
and pazopanib were administered intraperitoneally and orally,
respectively. (B) The initial tumor size of each mouse was
normalized to 100%. Tumor size was measured every 2 days, and
compared to the initial size. Relative tumor growth was averaged in
each group. Data are presented as the mean ± standard deviation.
n=5-10/group. *P≤0.05 vs. untreated. M, measurement; s.c.,
subcutaneously; D, doxorubicin; S/SAHA, suberoylanilide hydroxamic
acid; P, pazopanib; i.p., intraperitoneally; i.v.,
intravenously. |
The xenograft development of the MZ-UPS-2 cells was
slower compared to that of MZ-UPS-1 cells (Fig. 4B), which was comparable to the growth
of the MZ-UPS-2 cells in vitro. The tumor size of MZ-UPS-2
mice that were not treated with chemotherapy increased ~0.5 times
in size 8 days subsequent to the initial measurement of the tumor.
Following treatment with doxorubicin, SAHA and pazopanib, the
pleomorphic tumor xenograft only marginally decreased in size. The
combined therapy of doxorubicin and pazopanib led to a significant
reduction in the tumor size, and therefore was the most promising
therapeutic treatment in vivo. The in vivo results
obtained in the present study are markedly different to the in
vitro results, where doxorubicin and the HDAC inhibitor SAHA
were the most potent chemotherapeutic agents, while pazopanib only
marginally influenced tumor cell viability.
Discussion
Undifferentiated pleomorphic sarcoma is an extremely
heterogeneous aggressive subgroup of soft tissue sarcoma (2). Due to the heterogeneity of
undifferentiated pleomorphic sarcoma, numerous studies are focused
on developing individualized therapeutic strategies for patients,
instead of administering a standard chemotherapy to all patients
(29). Since there is a limited
number of patients affected by each of the undifferentiated
pleomorphic sarcoma subgroups, no large clinical trial has been
conducted to date to evaluate the efficiency of chemotherapeutic
treatment. Therefore, it is crucial to develop preclinical tools
that allow the evaluation of individualized therapeutic approaches.
Previous studies have demonstrated that there are distinct
histopathological differences between the different subtypes of
undifferentiated pleomorphic sarcoma, and have revealed a panel of
molecular markers that may significantly aid the development of an
optimal management regimen for patients with undifferentiated
pleomorphic sarcoma (30,31). Consequently, reliable and reproducible
preclinical animal models are required, which are similar to the
oligoclonal biological diversity observed in human patients, for
testing various targeted therapeutic approaches for individual
patients.
The present study established two xenograft animal
models generated from stable undifferentiated pleomorphic sarcoma
cell cultures to investigate the efficacy of chemotherapeutic
regimens for the treatment of undifferentiated pleomorphic sarcoma
in vivo vs. in vitro. The results of the present
study demonstrated that there is a clear discrepancy between the
in vitro cell culture and the in vivo xenograft
model, which is comparable to a human treatment scenario. The mouse
model reflects the local microenvironment of a human tumor, which
appears to be crucial to allow a predictive analysis of treatment
regimens in addition to monitoring direct cytotoxic effects of
drugs (32).
Understanding the various biological sensitivities
of the various histological subtypes of undifferentiated
pleomorphic sarcoma may lead to the development of individual
therapeutic targeted approaches (33).
In contrast to other approaches using solid tumor
tissue or silicon chambers to place tumor fragments around the
superficial epigastric vessels (26,27), the
present study generated stable oligoclonal cell cultures from
freshly isolated tumor tissue of two patients with undifferentiated
pleomorphic sarcoma, which were similar to the oligoclonal variety
exhibited by the original tumors. Additionally, these cultures were
subcutaneously injected into immunodeficient mice to establish
xenograft animal models. The present study observed that
neovascularization was identical between the original tumor and the
xenograft tumor. There were no regions of hypoxemia in the
xenograft tumor, which is important, as it allows the analysis of
anti-angiogenic therapeutic approaches and rules out the
possibility of anomalous results that hypoxic conditions may
generate during homing and engraftment of the tumor (34).
Furthermore, the present study treated the tumors
in vivo and in vitro with the most common or
innovative chemotherapeutic agents currently available. Only in the
xenograft mouse model the results observed were comparable to the
treatment results of the two patients from whom the original tumors
were resected. Therefore, tumor derived cell cultures do not
reflect the actual treatment condition that is observed in
patients. Notably, the combination of doxorubicin and pazopanib
significantly reduced the tumor size with an acceptable toxicity
level, in terms of weight loss (<20%), movement disorder and
apathy (data not shown).
In addition, the novel xenograft models allow
chemotherapeutic analysis at various time points, which may lead to
the identification of molecular mechanisms associated with
pleomorphic sarcoma development and progression, and other local
tumor-tissue interactions.
In conclusion, there is a discrepancy in tumor
growth and cell viability between in vitro and in
vivo models concerning chemotherapeutic treatments. The novel
and reproducible xenograft animal models generated in the present
study have demonstrated that in vivo models are required to
test potential chemotherapeutic agents for the treatment of
undifferentiated pleomorphic sarcoma, since they provide similar
results to those observed in human patients, compared with in
vitro models.
Acknowledgements
The present study was supported by the Internal
University Initial Research Founding of the Johannes Gutenberg
University of Mainz (Mainz, Germany).
References
|
1
|
Iwasaki H, Isayama T, Johzaki H and
Kikuchi M: Malignant fibrous histiocytoma. Evidence of perivascular
mesenchymal cell origin immunocytochemical studies with monoclonal
anti-MFH antibodies. Am J Pathol. 128:528–537. 1987.PubMed/NCBI
|
|
2
|
Katenkamp K and Katenkamp D: Soft tissue
tumors: New perspectives on classification and diagnosis. Dtsch
Arztebl Int. 106:632–636. 2009.PubMed/NCBI
|
|
3
|
Poremba C: Soft tissue sarcomas: The role
of histology and molecular pathology for differential diagnosis.
Verh Dtsch Ges Pathol. 90:59–72. 2006.(In German). PubMed/NCBI
|
|
4
|
Al-Agha OM and Igbokwe AA: Malignant
fibrous histiocytoma: Between the past and the present. Arch Pathol
Lab Med. 132:1030–1035. 2008.PubMed/NCBI
|
|
5
|
Matushansky I, Charytonowicz E, Mills J,
Siddiqi S, Hricik T and Cordon-Cardo C: MFH classification:
Differentiating undifferentiated pleomorphic sarcoma in the 21st
Century. Expert Rev Anticancer Ther. 9:1135–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spira AI and Ettinger DS: The use of
chemotherapy in soft-tissue sarcomas. Oncologist. 7:348–359. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grimer R, Judson I, Peake D and Seddon B:
Guidelines for the management of soft tissue sarcomas. Sarcoma.
2010:5061822010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Italiano A, Mathoulin-Pelissier S, Cesne
AL, Terrier P, Bonvalot S, Collin F, Michels JJ, Blay JY, Coindre
JM and Bui B: Trends in survival for patients with metastatic
soft-tissue sarcoma. Cancer. 117:1049–1054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Daigeler A, Klein-Hitpass L, Stricker I,
Müller O, Kuhnen C, Chromik AM, Steinstraesser L, Goertz O, Steinau
HU and Lehnhardt M: Malignant fibrous histiocytoma - pleomorphic
sarcoma, NOS gene expression, histology, and clinical course. A
pilot study. Langenbecks Arch Surg. 395:261–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fletcher CDM, Unni KK and Mertens F: World
Health Organisation Classification of Tumours. Pathology and
Genetics of Tumours of Soft Tissue and Bone. IARC Press. (Lyon).
2002.
|
|
11
|
Casali PG and Blay JY:
ESMO/CONTICANET/EUROBONET Consensus Panel of experts: Soft tissue
sarcomas: ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 21(Suppl 5): v198–v203. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Italiano A, Le Cesne A, Mendiboure J, Blay
JY, Piperno-Neumann S, Chevreau C, Delcambre C, Penel N, Terrier P,
Ranchere-Vince D, et al: Prognostic factors and impact of adjuvant
treatments on local and metastatic relapse of soft-tissue sarcoma
patients in the competing risks setting. Cancer. 120:3361–3369.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leahy M, Del Garcia Muro X, Reichardt P,
Judson I, Staddon A, Verweij J, Baffoe-Bonnie A, Jönsson L, Musayev
A, Justo N, et al: SABINE Investigators: Chemotherapy treatment
patterns and clinical outcomes in patients with metastatic soft
tissue sarcoma. The SArcoma treatment and Burden of Illness in
North America and Europe (SABINE) study. Ann Oncol. 23:2763–2770.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: EORTC Soft Tissue and Bone Sarcoma Group; PALETTE
study group: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ray-Coquard I and Thomas D: Targeted
therapies: Pazopanib for soft-tissue sarcoma: A PALETTE of data
emerges. Nat Rev Clin Oncol. 9:431–432. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nguyen DT and Shayahi S: Pazopanib:
Approval for soft-tissue sarcoma. J Adv Pract Oncol. 4:53–57.
2013.PubMed/NCBI
|
|
17
|
Ranieri G, Mammì M, Di Donato Paola E,
Russo E, Gallelli L, Citraro R, Gadaleta CD, Marech I, Ammendola M
and De Sarro G: Pazopanib a tyrosine kinase inhibitor with strong
anti-angiogenetic activity: A new treatment for metastatic soft
tissue sarcoma. Crit Rev Oncol Hematol. 89:322–329. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tavallai S, Hamed HA, Grant S, Poklepovic
A and Dent P: Pazopanib and HDAC inhibitors interact to kill
sarcoma cells. Cancer Biol Ther. 15:578–585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schöffski P, Cornillie J, Wozniak A, Li H
and Hompes D: Soft tissue sarcoma: An update on systemic treatment
options for patients with advanced disease. Oncol Res Treat.
37:355–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakatani T, Marui T, Yamamoto T, Kurosaka
M, Akisue T and Matsumoto K: Establishment and characterization of
cell line TNMY1 derived from human malignant fibrous histiocytoma.
Pathol Int. 51:595–602. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krause AK, Hinrichs SH, Orndal C, DeBoer
J, Neff JR and Bridge JA: Characterization of a human myxoid
malignant fibrous histiocytoma cell line, OH931. Cancer Genet
Cytogenet. 94:138–143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mairal A, Chibon F, Rousselet A, Couturier
J, Terrier P and Aurias A: Establishment of a human malignant
fibrous histiocytoma cell line, COMA. Characterization by
conventional cytogenetics, comparative genomic hybridization, and
multiplex fluorescence In situ hybridization. Cancer Genet
Cytogenet. 121:117–123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hakozaki M, Hojo H, Sato M, Tajino T,
Yamada H, Kikuchi S and Abe M: Establishment and characterization
of a new cell line, FPS-1, derived from human undifferentiated
pleomorphic sarcoma, overexpressing epidermal growth factor
receptor and cyclooxygenase-2. Anticancer Res. 26(5A): 3393–3401.
2006.PubMed/NCBI
|
|
24
|
Nishio J, Iwasaki H, Nabeshima K, Ishiguro
M, Isayama T and Naito M: Establishment of a new human pleomorphic
malignant fibrous histiocytoma cell line, FU-MFH-2: Molecular
cytogenetic characterization by multicolor fluorescence in
situ hybridization and comparative genomic hybridization. J Exp
Clin Cancer Res. 29:1532010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steinstraesser L, Jacobsen F, Schubert C,
Gevers K, Stricker I, Steinau HU and Al-Benna S: Establishment of a
primary human sarcoma model in athymic nude mice. Hum Cell.
23:50–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tilkorn DJ, Daigeler A, Hauser J, Ring A,
Stricker I, Schmitz I, Steinstraesser L, Steinau HU and Al-Benna S:
A novel xenograft model with intrinsic vascularisation for growing
undifferentiated pleomorphic sarcoma NOS in mice. J Cancer Res Clin
Oncol. 138:877–884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tilkorn DJ, Stricker I, Hauser J, Ring A,
Schmitz I, Steinstraesser L, Steinau HU, Daigeler A and Al-Benna S:
Experimental murine model of primary high grade undifferentiated
pleomorphic sarcoma not otherwise specified. In Vivo.
26:559–563. 2012.PubMed/NCBI
|
|
28
|
Fletcher CDM, Bridge JA, Hogendoorn P and
Mertens F: World Health Organization Classification of Tumours of
Soft Tissue and Bone (4th). 5:IARC. Lyon: 2013.
|
|
29
|
Radaelli S, Stacchiotti S, Casali PG and
Gronchi A: Emerging therapies for adult soft tissue sarcoma. Expert
Rev Anticancer Ther. 14:689–704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reichardt P: Soft tissue sarcomas, a look
into the future: Different treatments for different subtypes.
Future Oncol. 10(Suppl 8): s19–s27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Villacis RA, Silveira SM, Barros-Filho MC,
Marchi FA, Domingues MA, Scapulatempo-Neto C, Aguiar S Jr, Lopes A,
Cunha IW and Rogatto SR: Gene expression profiling in
leiomyosarcomas and undifferentiated pleomorphic sarcomas: SRC as a
new diagnostic marker. PLoS One. 9:e1022812014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Villacis RA, Silveira SM, Barros-Filho MC,
Marchi FA, Domingues MA, Scapulatempo-Neto C, Aguiar S Jr, Lopes A,
Cunha IW and Rogatto SR: Gene expression profiling in
leiomyosarcomas and undifferentiated pleomorphic sarcomas: SRC as a
new diagnostic marker. PLoS One. 9:e1022812014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Versleijen-Jonkers YM, Vlenterie M, van de
Luijtgaarden AC and van der Graaf WT: Anti-angiogenic therapy, a
new player in the field of sarcoma treatment. Crit Rev Oncol
Hematol. 91:172–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tentler JJ, Tan AC, Weekes CD, Jimeno A,
Leong S, Pitts TM, Arcaroli JJ, Messersmith WA and Eckhardt SG:
Patient-derived tumour xenografts as models for oncology drug
development. Nat Rev Clin Oncol. 9:338–350. 2012. View Article : Google Scholar : PubMed/NCBI
|