Introduction
Lung cancer is one of the most common types of
malignant tumor with a high mortality (1). Although surgical resection is currently
the optimal treatment for lung cancer, treatment of this disease
remains challenging as patients have not demonstrated long-term
clinical benefits (2). According to
the recent International Database, following complete resection of
lung cancer, the 5-year survival rate of patients with lung cancer
is 73% for pathological stage IA, 58% for stage IB, 46% for stage
IIA, 36% for stage IIB and 24% for stage IIIA (3). The failure of combined chemotherapy and
surgical resection is primarily attributed to cancer metastasis and
invasion (4,5). Therefore, it is necessary to seek more
effective anticancer agents targeting tumor invasion and
metastasis.
Plants and other natural products are important
chemical sources for chemotherapy (6,7).
Sinomenine is an active compound of the plant Sinomenium
acutum, which has been widely used as traditional medicine in
the treatment of various rheumatic diseases in China (8). It has been demonstrated that sinomenine
has a number of pharmacological activities, including
anti-inflammatory, antirheumatic, antiangiogenic and
immunosuppressive effects (9,10). Furthermore, there is strong evidence
to suggest that sinomenine has antineoplastic potential against a
variety of cancer cells, including synovial sarcoma, lung cancer
and hepatocellular carcinoma (11–13).
Chronic inflammation is undeniably a contributing
factor in tumour proliferation, survival, angiogenesis and
metastasis (14–16). To date, the majority of studies
investigating inflammation and cancer have focused on the
regulatory network dominated by nuclear factor (NF)-κB and signal
transducer and activator of transcription 3 (STAT3) (17–19). STAT3
is a key molecule in the promotion of tumorigenesis, functioning
via chronic inflammation, and also in the process of cancer-related
inflammation, which is able to regulate the biological behavior of
cancer and immune cells through mediation of extracellular
signaling of inflammatory factors (18). Given the roles of STAT3 in chronic
inflammation, and the regulation of the initiation and resolution
of epithelial-mesenchymal transition (EMT) in malignant cells
(20,21), decreasing or blocking its activity may
suppress malignant conversion. The present study hypothesized that
sinomenine may be able to inhibit lung cancer invasion via
modulation of the STAT3 signaling pathway; thus, the effect of
sinomenine on STAT3 signaling and EMT biomarkers in A549 cells was
investigated.
Materials and methods
Reagents
Sinomenine was obtained from the China's National
Institute for the Control of Pharmaceutical and Biological Products
(Beijing, China), and was dissolved in dimethyl sulfoxide
(Sigma-Aldrich, St. Louis, MO, USA) as a stock solution.
Cucurbitacin I, a potent pharmacological inhibitor of Janus kinase
(JAK)/STAT3 was provided by Gene Operation (Ann Arbor, MI,
USA).
Cell culture
The A549 human lung adenocarcinoma cell line was
obtained from the General Medical Cell Center of Peking Union
Medical College (Beijing, China). The cells were grown in RPMI-1640
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% inactivated fetal bovine serum
(Sigma-Aldrich) and 1% penicillin/streptomycin (Invitrogen; Thermo
Fisher Scientific, Inc.), and incubated at 37°C with 5%
CO2.
Cell viability assay
Cell viability was analyzed in 96-well plates
(Corning Incorporated, Corning, NY, USA) using Cell Counting kit-8
(CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan). A
total of 2×103 A549 cells/well were treated with the
indicated concentrations of sinomenine or cucurbitacin I, which
served as a positive control, for 24–72 h. Sinomenine was diluted
by RPMI-1640 culture medium, and the final concentration was 0.125,
0.25, 0.5, 1.0 or 2.0 mM in turn. The action concentration of
cucurbitacin I was 0.25 uM. Following treatment, 20 µl CCK-8 was
added, and incubation continued for 1 h. The optical density was
determined at the wavelength of 450 nm on a multi-mode microplate
reader (Synergy™ HT; BioTek Instruments, Inc., Winooski, VT,
USA).
Annexin V staining
Apoptosis was determined using an fluorescein
isothiocyanate (FITC) Annexin V apoptosis DTEC KIT (BD Biosciences,
Franklin Lakes, NJ, USA) according to the manufacturer's
instructions. First, A549 cells were incubated with sinomenine for
24 and 48 h. The cells were collected and washed twice with cold
PBS and then resuspended in 1X Binding Buffer at a concentration of
1×106 cells/ml. Subsequently, 100 µl of the suspension
(1×105 cells) was transferred to a 5 ml culture tube,
and 5 µl FITC-conjugated Annexin V with 10 µl propidium iodide (PI)
were added. The cells were gently vortexed and incubated for 15 min
at 25°C in the dark. Finally, 400 µl of 1X Binding Buffer was added
to each tube and analyzed using the FACSCalibur™ flow cytometer and
CellQuest Pro software version 6.0 (BD Biosciences) within 1 h.
Western blotting
Western blotting was performed as previously
described (22). Following treatment
with sinomenine for 48 h, A549 cells were lysed in
radioimmunoprecipitation assay lysis buffer (Applygen Technologies,
Inc., Beijing, China). The cell debris was removed by
centrifugation (15 min, 12,000 × g; Eppendorf, Hamburg, Germany),
and the supernatant was stored at −80°C. Protein concentrations
were estimated using the Pierce BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.).
Cell lysates (20 µg per lane) were subjected to 12%
sodium dodecyl sulfate polyacrylamide gel electrophoresis (Marker:
Amresco, LLC, Solon, OH, USA; Electrophoretic and transfer
equipment: Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
subsequently transferred to nitrocellulose membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked in 5%
skimmed milk, or bovine serum albumin (BSA; Vivantis Technologies,
Oceanside, CA, USA) for phosphorylated proteins, in Tris-buffered
saline with Tween 20 (50 mM Tris, 150 mM NaCl and 0.05% Tween 20),
and serially incubated with primary antibodies at 4°C overnight and
secondary antibodies for 1 h at room temperature. The antibodies
used were as follows: Rabbit monoclonal anti-JAK2 (dilution,
1:1,000; catalog no., 3230), anti-STAT3 (dilution, 1:1,000; catalog
no., 12640), anti-phosphorylated (p)-STAT3 (dilution, 1:1,000;
catalog no., 9145), anti-β-actin (dilution, 1:1,000; catalog no.,
4970), anti-Snail (dilution, 1:1,000; catalog no., 3879),
anti-vimentin (dilution, 1:1,000; catalog no., 5741),
anti-E-cadherin (dilution, 1:1,000; catalog no., 3195) (Cell
Signaling Technology, Inc., Danvers, MA, USA) and anti-N-cadherin
(1:1,000; catalog no., 180224; Thermo Fisher Scientific, Inc.)
antibodies, and horseradish peroxidase-linked anti-rabbit (catalog
no., 7054) or anti-mouse (catalog no., 7076) immunoglobulin (Ig)G
antibodies (dilution, 1:2,000; Cell Signaling Technology, Inc.).
The bands were visualized using a chemiluminescence reagent
(Applygen Technologies, Inc.) and recorded with a ChemiDoc™ XRS
imaging system (Bio-Rad Laboratories, Inc.). The band densities
were quantified using β-actin as a loading control, and analyzed
using Image J (https://imagej.nih.gov/ij/).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The extraction of 5 mg total RNA from the A549 cells
was performed using TRIzol® reagent (Thermo Fisher
Scientific, Inc.). The concentration and purity of the extracted
RNA were determined with a NanoDrop™ 2000 spectrophotometer (Thermo
Fisher Scientific, Inc.), while electrophoresis was conducted to
verify its integrity. A 2% agarose gel was used, the markers were
supplied by Amresco, LLC, and the electrophoretic tank and
visualization equipment were from Beyotime Institute of
Biotechnology (Jiangsu, China).
To prepare the reaction master mix, 2 ml 10X RT
buffer, 4 ml 25 mM MgCl2, 2 ml 0.1 M dithiothreitol and
1 ml RNAaseOUT were combined. The reaction mixture was added to the
RNA/primer mixture, mixed briefly, and then incubated at room
temperature for 2 min. SuperScript II RT (1 ml; 50 units) was added
each tube, mixed and incubated at 25°C for 10 min. Tubes were
incubated at 42°C for 50 min, heat inactivated at 70°C for 15 min,
and then chilled on ice. RNase H (1 ml) was added and incubated at
37°C for 20 min. The first strand of complementary DNA (cDNA) was
synthesized by RT using the High-Capacity RNA-to-cDNA kit (Thermo
Fisher Scientific, Inc.) stored at −20°C until use. qPCR was
performed using TaqMan® Gene Expression Master Mix
(Thermo Fisher Scientific, Inc.), template cDNA and primers. The
primers were as follows: STAT3-RT5, 5′-CATCATGGGCTTTATCAGTAAGGA-3′
and STAT3-RT3, 5′-GTCAATGGTATTGCTGCAGGTCGT-3′; E-cadherin-RT5,
5′-CCCACCACGTACAAGGGTC-3′ and E-cadherin-RT3,
5′-CTGGGGTATTGGGGGCATC-3′; N-cadherin-RT5,
5′-GCGGAGAGGAAGACCAGGA-3′ and N-cadherin-RT3,
5′-TAGTTGGGCTCCGAGTGCAT-3′; GAPDH forward,
5′-TGCACCACCAACTGCTTAGC-3 and reverse,
5′-GGCATGGACTGTGGTCATGAG-3′.
To prepare the mixture, 12.5 ml SYBR Green Mix (2X),
0.2 ml cDNA, 1 ml primer pair mix (5 pmol/ml each primer) and 11.3
ml H2O were combined. The total volume of each combined
reaction mixture was 20 µl. Gene-specific, fluorogenic RT-qPCR for
STAT3, E-cadherin, N-cadherin and glyceraldehyde 3-phosphate
dehydrogenase was performed using a 3PrimeX Progene thermal cycler
(Techne; Bibby Scientific Limited, Stone, UK). The thermocycling
protocol was as follows: 50°C for 2 min, 1 cycle; 95°C for 10 min,
1 cycle; 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec, 40
cycles; then 72°C for 10 min, 1 cycle. The results were normalized
according to the method by Liu et al (23). The ABI Prism SDS 7300 (Thermo Fisher
Scientific, Inc.) was used for quantification and the results were
viewed and analyzed with Applied Biosystems 7500 Fast system
(Thermo Fisher Scientific, Inc.). The assay was repeated 3
times.
Immunofluorescence
A549 cells were treated with 1.0 mM sinomenine for
24 h and then fixed in 4% paraformaldehyde (Santa Cruz
Biotechnology, Inc., Dallas, Texas, USA) for 20 min at room
temperature. The samples were washed 2 times in phosphate-buffered
saline (PBS) to remove residual paraformaldehyde. Cells were
permeabilized with 0.1% Triton X-100 made in PBS solution for 15
min, and then washed 3 times with PBS. Subsequent to blocking with
2% BSA for 1 h, cells were washed with PBS and BSA prior to being
incubated with anti-STAT3 (1:500), anti-E-cadherin (1:500),
anti-N-cadherin (1:500) or anti-vimentin (1:500) primary antibodies
overnight at 4°C. Samples were then washed 5 times with PBS and
BSA, followed by incubation with anti-rabbit or anti-mouse IgG
secondary antibodies for 1 h at room temperature. Cells were then
stained with 4′,6-diamidino-2-phenylindole (Santa Cruz
Biotechnology, Inc.) and mounted with anti-fading agent.
Immunostaining was analyzed using the widefield high-content
screening system ImageXpress® Micro XLS (Molecular
Devices, LLC, Sunnyvale, CA, USA).
Statistical analysis
All experiments were performed three times unless
otherwise stated, and data are presented as the mean ± standard
deviation. Statistical significance was evaluated using one-way
analysis of variance, followed by Fisher's least significant
difference test. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS software version 18.0 (SPSS, Inc., Chicago,
IL, USA).
Results
Sinomenine reduces A549 cell
viability
To determine the effect of sinomenine on A549 cell
viability, cells were treated with various concentrations of
sinomenine (0–2 mM) for 24–72 h. The results demonstrated that
sinomenine-treated cells exhibited a decreased cell count compared
with untreated cells, and the inhibitory effect was dose-dependent
(Fig. 1). As a positive control,
cucurbitacin I (0.25 µM) was used, which significantly reduced the
number of A549 cells (P<0.05) (Fig.
1).
Sinomenine induces A549 cell
apoptosis
To determine whether the decreased viability of A549
cells following sinomenine treatment occurs as a result of
apoptosis, Annexin V-FITC/PI staining was performed. As presented
in Fig. 2A, sinomenine induced
apoptosis in a dose- and time-dependent manner in A549 cells. The
minimum dose of sinomenine required to induce apoptosis was 0.25
mM, while 1 mM sinomenine produced a similar effect to that caused
by 0.25 µM cucurbitacin I (Fig. 2B and
C). These results suggest that sinomenine-induced apoptosis may
result in decreased cell viability.
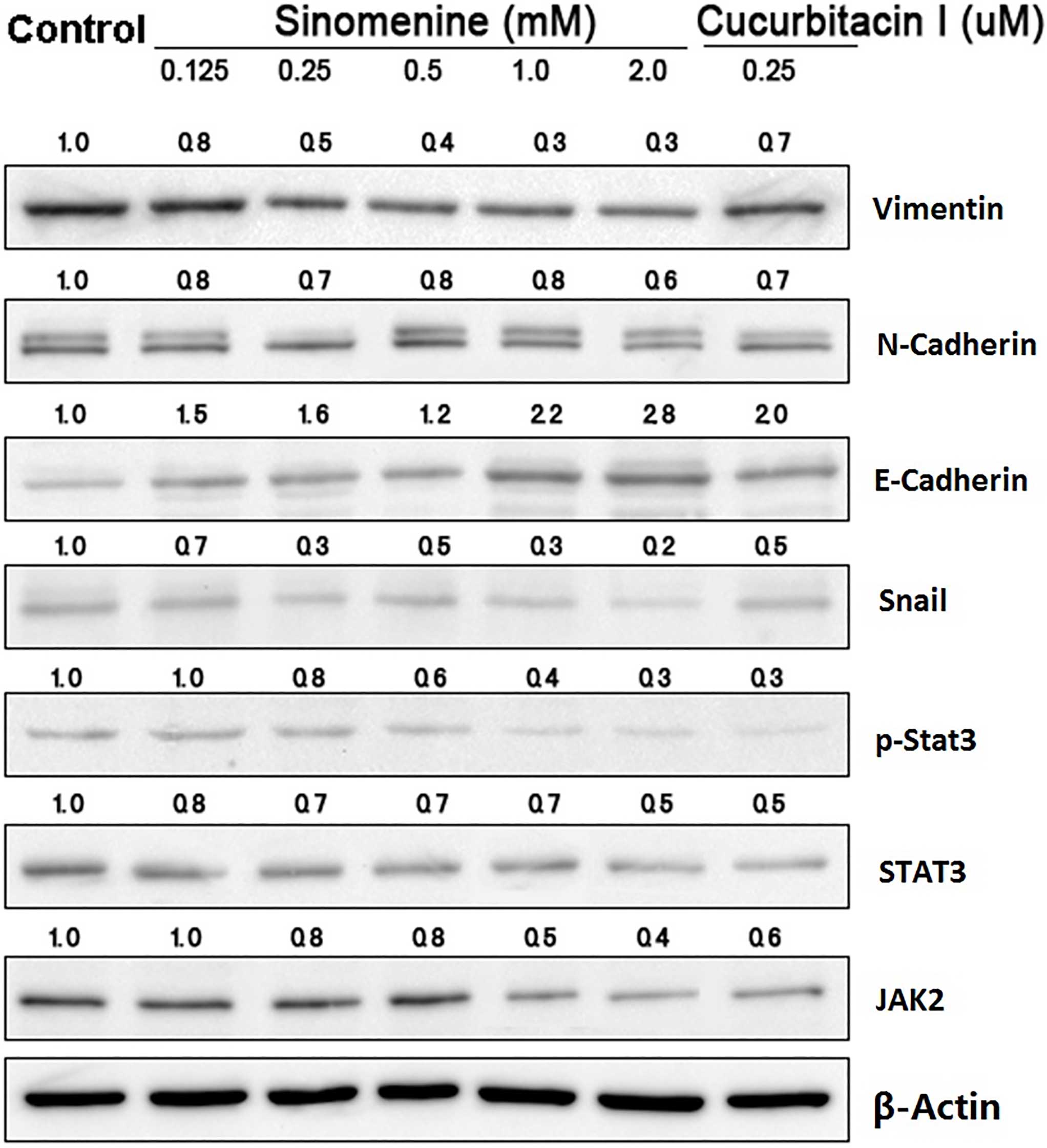
Sinomenine regulates the protein
expression of JAK2, STAT3 and EMT markers
To investigate the mechanisms underlying the
biological effects of sinomenine on A549 cells, the upstream and
downstream targets of STAT3 signaling were examined in A549 cells
treated with sinomenine for 48 h by western blotting. The results
demonstrated that sinomenine and cucurbitacin I downregulated the
expression of JAK2, STAT3, p-STAT3, Snail, N-cadherin and vimentin
compared with the untreated controls, while E-cadherin expression
was upregulated (Fig. 3).
To further confirm the effect of sinomenine on the
STAT3 signal transduction pathway, the messenger (m)RNA expression
of STAT3, E-cadherin and N-cadherin was examined by RT-qPCR. The
results demonstrated that the mRNA levels of STAT3 and N-cadherin
were significantly reduced in the A549 cells following treatment
with sinomenine and cucurbitacin I, whereas the level of E-cadherin
mRNA was markedly increased (P<0.05) (Fig. 4). These results indicate that
sinomenine may interfere with EMT in A549 cells by reducing the
expression levels of the functional targets of the STAT3 signal
transduction pathway.
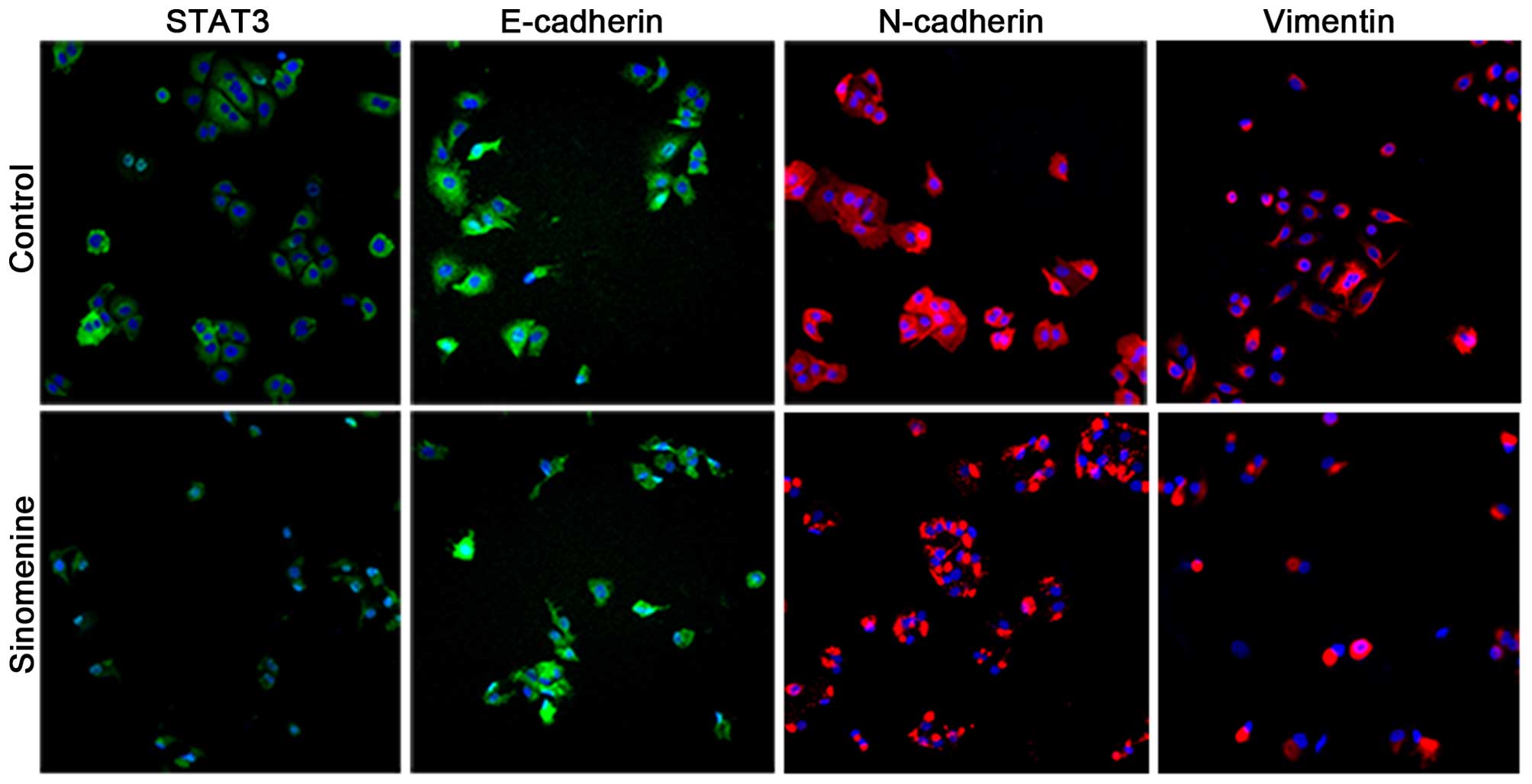
Sinomenine prevents the invasion of
A549 cells
To examine whether sinomenine affects the invasion
of A549 cells, biomarkers for EMT were examined by
immunofluorescence. It was observed that E-cadherin staining under
the plasma membrane was significantly increased in the A549 cells
treated with sinomenine compared with the untreated control cells,
whilst STAT3, N-cadherin and vimentin exhibited a decreased level
of staining (Fig. 5). These results
are consistent with the aforementioned western blotting and RT-qPCR
data, and strongly suggest that sinomenine is able to attenuate
A549 cell invasion through regulation of the STAT3 signaling
pathway.
Discussion
The present study observed that sinomenine induced
strong cytotoxicity, as indicated by decreased cell viability and
apoptosis induction in A549 human lung adenocarcinoma cells. These
results are consistent with previous observations in liver, breast
and colon tumor cells (13,24,25).
STAT3 is a key transcription factor that is widely
expressed in various tissues and cell types, and is primarily
activated by the JAK-STAT and mitogen-activated protein kinase
signal transduction pathways (26).
Increasing evidence suggests that STAT3 is involved in
proliferation, differentiation, invasion, metastasis, angiogenesis
and resistance to apoptosis (27).
The reported abnormal upregulation of STAT3 in hematological
malignancies and solid tumors, including leukemia, hepatoma, and
lung, prostate and breast cancer (28–32),
indicates that STAT3 is important in the pathogenesis of such
tumors. With regard to sinomenine, it has been reported that the
drug possesses immunosuppressive properties, such as inhibiting the
nuclear translocation of NF-κB (33).
Notably, STAT3 and NF-κB interact and crosstalk between their
associated pathways (34,35). The present study attempted to clarify
whether the growth inhibition of A549 cells caused by sinomenine is
mediated through the regulation of the STAT3 signaling pathway. The
results demonstrated that STAT3 expression and activation were
significantly suppressed in A549 cells following sinomenine
treatment, which suggested that the inhibition of STAT3 signaling
resulted in growth inhibition of A549 cells.
During the process of EMT, epithelial cells lose
polarity and gain cell motility, subsequently resulting in cell
invasion (36–39). A number of studies have reported that
EMT is involved in tumor invasion, metastasis and chemoresistance
(36,37). During tumorigenesis, epithelial-type
markers, including β-catenin, E-cadherin and cytokeratin, are
typically downregulated, while mesenchymal markers such as
N-cadherin and vimentin are upregulated (40). Therefore, understanding how to
effectively restrain the process of EMT is vital for successful
treatment of cancer. In the current study, it was observed that
sinomenine reversed the protein expression of EMT biomarkers,
indicating that sinomenine inhibited cell invasion. In addition,
sinomenine may inhibit EMT through the regulation of STAT3 and its
downstream target, Snail. It has been demonstrated that STAT3 is
required for EMT via upregulation of the downstream gene Snail
(41). Snail is able to directly
inhibit the expression of E-cadherin, and subsequently interacts
with the COOH-terminal region and the 5′-CACCTG-3′ motif in the
E-cadherin promoter sequence to activate EMT (42,43). Yadav
et al (41) reported that EMT
may be induced by Snail, which is activated by the JAK/STAT3
signaling pathway, in head and neck tumor cells, thus resulting in
tumor metastasis.
In conclusion, the present study demonstrated that
sinomenine affects apoptosis, and inhibits tumor cell death and
invasion by suppressing the activation of the STAT3 signaling
pathway in A549 cells. To the best of our knowledge, the present
study is the first to report that sinomenine is able to reverse EMT
changes in A549 cells. These results may aid the understanding of
the underlying mechanisms of sinomenine in treating non-small cell
lung cancer.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (Beijing, China; grant nos.
81273718 and 81403346) and the China Postdoctoral Science
Foundation (Beijing, China; grant no. 2014M550132).
References
|
1
|
Yano T, Okamoto T, Fukuyama S and Maehara
Y: Therapeutic strategy for postoperative recurrence in patients
with non-small cell lung cancer. World J Clin Oncol. 5:1048–1054.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pirker R: Adjuvant chemotherapy in
patients with completely resected non-small cell lung cancer.
Transl Lung Cancer Res. 3:305–310. 2014.PubMed/NCBI
|
|
3
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of malignant tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Byron E and Pinder-Schenck M: Systemic and
targeted therapies for early-stage lung cancer. Cancer Control.
21:21–31. 2014.PubMed/NCBI
|
|
5
|
Akbari-Birgani S, Paranjothy T, Zuse A,
Janikowski T, Cieślar-Pobuda A, Likus W, Urasińska E, Schweizer F,
Ghavami S, Klonisch T and Łos MJ: Cancer stem cells,
cancer-initiating cells and methods for their detection. Drug
Discov Today. 21:836–842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brower V: Back to nature: Extinction of
medicinal plants threatens drug discovery. J Natl Cancer Inst.
100:838–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li JW and Vederas JC: Drug discovery and
natural products: End of an era or an endless frontier? Science.
325:161–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu Q, Sun Y, Zhu J, Fang T, Zhang W and
Li JX: Antinociceptive effects of sinomenine in a rat model of
neuropathic pain. Sci Rep. 4:72702014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qian L, Xu Z, Zhang W, Wilson B, Hong JS
and Flood PM: Sinomenine, a natural dextrorotatory morphinan
analog, is anti-inflammatory and neuroprotective through inhibition
of microglial NADPH oxidase. J Neuroinflammation. 4:232007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kok TW, Yue PY, Mak NK, Fan TP, Liu L and
Wong RN: The anti-angiogenic effect of sinomenine. Angiogenesis.
8:3–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li XJ, Yue PY, Ha WY, Wong DY, Tin MM,
Wang PX, Wong RN and Liu L: Effect of sinomenine on gene expression
of the IL-1 beta-activated human synovial sarcoma. Life Sci.
79:665–673. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang T, Zhou L, Zhang W, Qu D, Xu X, Yang
Y and Li S: Effects of sinomenine on proliferation and apoptosis in
human lung cancer cell line NCI-H460 in vitro. Mol Med Rep.
3:51–56. 2010.PubMed/NCBI
|
|
13
|
Lu XL, Zeng J, Chen YL, He PM, Wen MX, Ren
MD, Hu YN, Lu GF and He SX: Sinomenine hydrochloride inhibits human
hepatocellular carcinoma cell growth in vitro and in
vivo: Involvement of cell cycle arrest and apoptosis induction.
Int J Oncol. 42:229–238. 2013.PubMed/NCBI
|
|
14
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuraishy A, Karin M and Grivennikov SI:
Tumor promotion via injury- and death-induced inflammation.
Immunity. 35:467–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
He G and Karin M: NF-κB and STAT3 - key
players in liver inflammation and cancer. Cell Res. 21:159–168.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bosch-Barrera J and Menendez JA: Silibinin
and STAT3: A natural way of targeting transcription factors for
cancer therapy. Cancer Treat Rev. 41:540–546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Ma G, Lu D, Lin F, Xu HJ, Liu J and
Arlinghaus RB: Bcr: A negative regulator of the Bcr-Abl
oncoprotein. Oncogene. 18:4416–4424. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Z, Duan ZJ, Chang JY, Zhang ZF, Chu R,
Li YL, Dai KH, Mo GQ and Chang QY: Sinomenine sensitizes
multidrug-resistant colon cancer cells (Caco-2) to doxorubicin by
downregulation of MDR-1 expression. PLoS One. 9:e985602014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Wang K, Ren Y, Zhang L, Tang XJ,
Zhang HM, Zhao CQ, Liu PJ, Zhang JM and He JJ: MAPK signaling
mediates sinomenine hydrochloride-induced human breast cancer cell
death via both reactive oxygen species-dependent and -independent
pathways: An in vitro and in vivo study. Cell Death Dis.
5:e13562014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang JX, Yang ZR, Wu DD, Song J, Guo XF,
Wang J and Dong WG: Suppressive effect of sinomenine combined with
5-fluorouracil on colon carcinoma cell growth. Asian Pac J Cancer
Prev. 15:6737–6743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spitzner M, Ebner R, Wolff HA, Ghadimi BM,
Wienands J and Grade M: STAT3: A novel molecular mediator of
resistance to chemoradiotherapy. Cancers (Basel). 6:1986–2011.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
28
|
Ishida F, Matsuda K, Sekiguchi N,
Makishima H, Taira C, Momose K, Nishina S, Senoo N, Sakai H, Ito T
and Kwong YL: STAT3 gene mutations and their association with pure
red cell aplasia in large granular lymphocyte leukemia. Cancer Sci.
105:342–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geletu M, Guy S and Raptis L: Effects of
SRC and STAT3 upon gap junctional, intercellular communication in
lung cancer lines. Anticancer Res. 33:4401–4410. 2013.PubMed/NCBI
|
|
30
|
Ramakrishna G, Rastogi A, Trehanpati N,
Sen B, Khosla R and Sarin SK: From cirrhosis to hepatocellular
carcinoma: New molecular insights on inflammation and cellular
senescence. Liver Cancer. 2:367–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsu FN, Chen MC, Lin KC, Peng YT, Li PC,
Lin E, Chiang MC, Hsieh JT and Lin H: Cyclin-dependent kinase 5
modulates STAT3 and androgen receptor activation through
phosphorylation of Ser727 on STAT3 in prostate cancer
cells. Am J Physiol Endocrinol Metab. 305:E975–E986. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ibrahim SA, Hassan H, Vilardo L, Kumar SK,
Kumar AV, Kelsch R, Schneider C, Kiesel L, Eich HT, Zucchi I, et
al: Syndecan-1 (CD138) modulates triple-negative breast cancer stem
cell properties via regulation of LRP-6 and IL-6-mediated STAT3
signaling. PLoS One. 8:e857372013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao Y, Li J, Yu K, Liu Y and Chen X:
Sinomenine inhibits maturation of monocyte-derived dendritic cells
through blocking activation of NF-kappa B. Int Immunopharmacol.
7:637–645. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
De Simone V, Franzè E, Ronchetti G,
Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald
TT, Pallone F, et al: Th17-type cytokines, IL-6 and TNF-α
synergistically activate STAT3 and NF-κB to promote colorectal
cancer cell growth. Oncogene. 34:3493–3503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II,
Yang SH, Kim CH and Lee JC: Epithelial to mesenchymal transition
derived from repeated exposure to gefitinib determines the
sensitivity to EGFR inhibitors in A549, a non-small cell lung
cancer cell line. Lung Cancer. 63:219–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ceppi P, Mudduluru G, Kumarswamy R, Rapa
I, Scagliotti GV, Papotti M and Allgayer H: Loss of miR-200c
expression induces an aggressive, invasive, and chemoresistant
phenotype in non-small cell lung cancer. Mol Cancer Res.
8:1207–1216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yadav A, Kumar B, Datta J, Teknos TN and
Kumar P: IL-6 promotes head and neck tumor metastasis by inducing
epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling
pathway. Mol Cancer Res. 9:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peinado H, Portillo F and Cano A:
Transcriptional regulation of cadherins during development and
carcinogenesis. Int J Dev Biol. 48:365–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View Article : Google Scholar : PubMed/NCBI
|