Introduction
Lung cancer is an aggressive and heterogeneous
malignancy, and one of the leading causes of cancer-associated
mortalities in men throughout the world (1). Although lung cancers are treated by
surgical, radiotherapeutic and chemotherapeutic approaches, the
long-term survival rate is still not satisfactory (1,2).
Therefore, novel approaches/compounds are still required to
increase the success of treatment.
The use of medicinal plants for the treatment of
human diseases begins with the history of humanity, and represents
the oldest and most widespread form of medication due to their
healing properties (3,4). Several types of plants are important as
a source of effective anticancer agents, which indicates their
therapeutic values (5).
Pelargonium species are widely used as traditional remedies
for several diseases in Southern Africa (6). A number of herbs belonging to the genus
Pelargonium possess numerous properties that have medicinal
importance. For example, ‘Umckaloabo’, ethanol extracts of the root
of two species of Pelargonium (P. sidoides and P.
reniforme), have been used in gastrointestinal, hepatic and
respiratory diseases as a herbal remedy (7). Pelargonium species are rich in
essential oils that are the source of their therapeutic value
(8,9).
Among these, Geranium monoterpene oils exhibit anti-bacterial,
antifungal antioxidant, insecticidal, anthelmintic and anticancer
activity (7–13). There are also several
Pelargonium species used in folk medicine that have been
screened for their biological activities (Table I) (8,12,13,14–26).
 | Table I.Pelargonium species used in
folk medicine. |
Table I.
Pelargonium species used in
folk medicine.
| Genus:
Pelargonium plant species | Traditional uses
(ref) | Screened parts | Previously screened
activity (ref) |
|---|
| P. reniforme
and P. sidoides | Coughs, diarrhoea,
hepatic disorders and tuberculosis (14,15)
(roots) | Roots | Antimycobacterial
(12,16) |
| P.
graveolens | Antiasthmatic,
antiallergic, antidiarrhoeic, diuretic, tonic, hemostatic,
anti-hepatotoxic, stomachic and diabetic (17) (leaves and flowers) | Aerial parts | Antioxidant
(13) and anticancer (13,18) |
| P.
endlicherianum | Anthelmintic
(19) (roots and flowers) | Aerial parts and
roots | Antioxidant
(20) and antimicrobial (21) |
| P.
radula | Mosquito repellent
(22) (leaves and flowers) | Leaves | Antimicrobial
(23) |
| P.
betulinum | Coughs and other
chest problems, wound healing and gastrointestinal-related problems
(24) (leaves) | Aerial parts | Antibacterial and
antioxidant (8) |
| P.
cucullatum | Colic, diarrhoea
and wounds (24) (leaves) | Aerial parts | Antibacterial and
antioxidant (8) |
| P.
glutinosum | Astringent
(25) (all parts) | Stems and
leaves | Antibacterial,
antioxidant and cytotoxic (8) |
| P.
citronellum | Culinary (26) (leaves) | Stems and
leaves | Antibacterial and
cytotoxic (8) |
Pelargonium quercetorum Agnew (P.
quercetorum) belongs to Geraniaceae family, and is known as
Tolk in Gecitli, in the Hakkari province of Turkey (27). Kaval et al (27) reported that native people have used
this plant to treat intestinal worms, and that study was the first
report about the traditional use of P. quercetorum. However,
to the best of our knowledge, there is no information regarding the
cytotoxic activity of P. quercetorum against lung cancer
cell lines in the literature.
In the present study, the anti-growth/cytotoxic
effects of the methanol extract of P. quercetorum against
non-small cell lung cancer cell lines (A549, PC3 and H1299) were
investigated. The results demonstrated that P. quercetorum
had cytotoxic activity in a dose-dependent manner, and resulted in
an incomplete apoptosis, implying the requirement of further in
vivo experiments for the elucidation of its cell death
mechanism.
Materials and methods
Plant material
The whole plant of P. quercetorum was
collected from the Zap Valley at Sumbul Mountain in the Hakkari
province of Turkey, located in the C10 square according to the
Turkey's grid square system (28) in
May 2006 by Mr. Mehmet Firat (Department of Biology, Yuzuncu Yil
University, Van, Turkey). The specimen was identified using the
standard text ‘Flora of Turkey and the East Aegean lslands’
(29). A voucher specimen (number
MF.10111) was deposited in the Hacettepe University Herbarium
(Ankara, Turkey).
Extraction of P. quercetorum
sample
The sample was air-dried at room temperature,
cleaned of extraneous materials and then grounded into powder. A
total of 15 g of the material (trunk and flower parts) was
extracted by adding 150 ml of solvent methanol (Merck Millipore,
Darmstadt, Germany) in a Soxhlet apparatus for 24 h, and the crude
extract was concentrated in a rotary evaporator at 40°C. The
residues were lyophilized and stored at −80°C until used.
Determination of P. quercetorum
chemical compounds
Direct thermal desorption (DTD)
To evaluate the volatile compounds present in P.
quercetorum, DTD followed by analysis with comprehensive
two-dimensional gas chromatography-time-of-flight/mass spectrometry
(GCxGC-TOF/MS) was performed. The plant was directly loaded into
the system. A GCxGC-TOF/MS system was used together with a dual
stage commercial thermal desorption injector, which incorporated a
thermal desorption unit (TDU) connected to a
programmable-temperature vaporization injector [Cooled Injection
System (CIS)-4 Plus; Gerstel, Mülheim an der Ruhr, Germany], using
a heated transfer line. The injector was equipped with a
MultiPurpose Sample (Gerstel). Empty glass thermodesorption tubes
were conditioned at 400°C for 2 h prior to each use. Approximately
20–30 mg sample was placed into the thermodesorption tubes using
tweezers to ensure no contamination of the sample. The initial
desorption of the sample was conducted by heating the TDU from 40°C
(initial time, 0.2 min) to 150°C at a rate of 120°C/min with a
final hold time of 10 min under a helium flow of 1.5 ml/min in
splitless mode. Volatile analytes released from this heating were
cryo-focused at −40°C in the CIS, which was cooled with liquid
nitrogen prior to injection. The CIS was then heated at a rate of
10°C/sec to a final temperature of 150°C. Analytes were transferred
splitless to the GC column during the CIS temperature ramp.
Chromatographic analysis
The GCxGC-TOF/MS system consisted of a 6890 GC
(Agilent Technologies, Inc., Santa Clara, CA, USA) and a Pegasus
III TOF-MS system (LECO Corporation, Saint Joseph, MI, USA). The
modulator between the first and second GC columns was based on a
LECO liquid nitrogen two-stage cold jet system. Helium was used as
a carrier gas at a constant flow of 1.0 ml/min. The first column
was a non-polar BPX5 (30 mx0.32 mm i.d. ×0.25 µm film thickness),
while the second column was a BPX50 (1.5 mx0.10 mm i.d. ×0.10 µm
film thickness), both from SGE Analytical Science (Victoria,
Australia). The combination of separations produced the overall
two-dimensional chromatogram. Peak identification was performed
using TOF/MS with electron ionization.
Determination of cytotoxic
activity
Cell culture and chemicals
Non-small cell lung cancer cell lines A549, H1299
(Dr Donner, Walther Oncology Center, Indiana University School of
Medicine, Indianapolis, IN, USA) and PC3 (Dr Yokota, National
Cancer Center Research Institute, Division of Genome Biology,
Tokyo, Japan) were cultured in RPMI-1640 (Lonza Bioscience,
Verviers, Belgium) medium supplemented with L-glutamine
(Gibco®; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 10% fetal bovine serum (Lonza Bioscience), penicillin G (100
U/ml) and streptomycin (100 µg/ml) (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) at 37°C in a humidified atmosphere
containing 5% CO2. According to the American Type
Culture Collection (Manassas, VA, USA), PC3 is often known as a
prostate cancer cell line (CRL1435), but in the present study, it
represents a non-small cell lung cancer cell line derived from the
Japanese Collection Research Resources Bank (Osaka, Japan; JCRB,
JCRB0077).
The lyophilized P. quercetorum extract (PQE)
was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St.
Louis, MO, USA) at a concentration of 100 mg/ml as a stock
solution, aliquoted and stored at −80°C. PQE was used at different
concentrations ranging from 3.13 to 100 µg/ml, and the dilutions
were made in culture medium.
Adenosine triphosphate (ATP) assay
The ATP assay, a highly sensitive
luciferin:luciferase-based assay, was performed to determine the
level of cellular ATP as an indirect marker of the number of alive
cells present in the sample (30).
A549, PC3 and H1299 cells were seeded at a density of 1×104
cells/well in a 96-well plate in 200 µl medium. Cells were
incubated either alone (as control) or in the presence of PQE for
48 h. The untreated/control cells received vehicle only (0.1% DMSO)
without any drugs (which represented the maximum viability). Each
experiment was conducted at least twice in triplicates. At the end
of the treatment period (48 h), cell viability was determined by
ATP assay with an ATP bioluminescent somatic cell assay kit
(Sigma-Aldrich), according to the manufacturer's protocol with a
slight modification, as explained previously (31). Morphological changes of cells were
also observed under a phase-contrast microscope (CKX41; Olympus
Corporation Tokyo, Japan).
Determination of cell death mode
Annexin-V-fluorescein isothiocyanate (FITC)
fluorescence imaging for apoptosis
The translocation of phosphatidylserine (PS)
molecules from the inner to the outer side of the cell membrane is
one of the earlier events of apoptosis (32). Annexin-V-FITC is able to bind to PS,
thus allowing the apoptotic cells to become visible. Propidium
iodide (PI) is normally used as a second dye to distinguish between
early and late apoptosis as well as necrosis (33). In addition, a nucleus-staining
fluorescent dye, Hoechst 33342 (200 µg/ml, 1:40; AppliChem GmbH,
Darmstadt, Germany) was used in the present study to detect
apoptosis on the basis of nuclear morphology. Hoechst 33342 dye
stains all types of cells (alive and dead) (34). While early apoptotic cells are
considered only Annexin-V-FITC-positive, late apoptotic cells (or
secondary necrotic cells) are considered both Annexin-V-FITC- and
PI-positive, with the presence of pyknotic nuclei and/or condensed
chromatin (33,34). Briefly, A549, PC3 and H1299 cells were
seeded in a 96-well plate at a density of 1×104 cells/well, and
treated for 12 and 24 h with PQE at a dose of 100 µg/ml. Upon
treatment, cells were stained with Annexin-V-FITC and PI using the
Annexin-V-FLUOS Staining kit (Roche Diagnostics GmbH, Mannheim,
Germany), according to the manufacturer's protocol. The cells were
then visualized under a fluorescence microscope.
M30 and M65 assays
Intact cytokeratin 18 (CK18, also known as M65) and
caspase-cleaved CK18 (also known as M30) were measured using
M30-Apoptosense® and M65 EpiDeath®
enzyme-linked immunosorbent assay (ELISA) kits (Vivalavida AB,
Nacka, Sweden). The M30-Apoptosense® ELISA kit measures
the levels of M30 produced during apoptosis, while the M65
EpiDeath® ELISA kit measures the levels of both
caspase-cleaved and intact CK18, which is released from cells
undergoing necrosis (35). A total of
1×104 A549, PC3 or H1299 cells were seeded per well in a
96-well plate in 200 µl culture medium in triplicates. After 24 h,
cells were treated with PQE (100 µg/ml) for 48 h. At the end of the
treatment period, cells were lysed with 10% NP-40 (Sigma-Aldrich)
for 10 min on a shaker to perform the M30 assay, while the
supernatants were collected for the M65 assay, according to the
manufacturer's protocol. The absorbance was determined with an
ELISA reader at 450 nm (FLASH Scan S12®; Analytik Jena
AG, Jena, Germany).
Western blot analysis for further dissection of
the apoptosis mechanism
A549, PC3 and H1299 cells were seeded in
25-cm2 flasks, and treated with PQE (100 µg/ml) for 24 h
when the cells reached 70% confluency. Additionally, cisplatin (20
µM) for A549 and PC3 cells, and etoposide (5 µM) for H1299 cells,
were used as positive controls for cleaved-poly (adenosine
diphosphate-ribose) polymerase (PARP) (36–38). Cells
were lysed in radioimmunoprecipitation assay lysis buffer (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), containing protease
inhibitors. Equal amounts of protein (20 µg protein/lane) were
subjected to 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred to a nitrocellulose
membrane (Thermo Fisher Scientific, Inc.). Western blotting was
performed using rabbit anti-β-actin and anti-PARP monoclonal
antibodies at 1:1,000 dilution (catalog nos., 4970 and 9532,
respectively; Cell Signaling Technology, Inc., Danvers, MA, USA) in
5% (w/v) bovine serum albumin (Amresco, LLC, Solon, OH, USA).
Horseradish peroxidase (HRP)-linked anti-rabbit immunoglobulin G
antibody (1:2,000 dilution; catalog no., 7074; Cell Signaling
Technology, Inc.) was used to detect anti-β-actin and anti-PARP
antibodies. Secondary antibody detection was performed according to
the instructions of Phototope®-HRP Western Blot
Detection System (Cell Signaling Technology, Inc.). Stripping was
performed according to the manufacturer's reprobing protocol (Cell
Signaling Technology, Inc.). Bound antibodies were visualized on a
FUSION-FX7 imaging device (Vilber Lourmat, Marne-la-Vallée,
France).
Statistical analysis
All statistical analysis were performed using SPSS
20.0 statistical software (IBM SPSS, Armonk, NY, USA). The
significance was calculated using one-way analysis of variance.
Significant differences in the M30 and M65 assays were determined
using the Student's t test. P<0.05 was considered to
indicate a statistically significant difference. The results were
expressed as the mean ± standard deviation.
Results
Chemical composition of P.
quercetorum
The volatile compounds of P. quercetorum were
analyzed by GCxGC-TOF/MS (39), and
the qualitative and quantitative compositions were presented in
Table II. A total of 23 compounds
were identified in P. quercetorum, and the major components
were tetracosane (40.92%), heneicosane (16.23%), 2-methyleicosane
(12.37%), eicosane (5.15%), 2-pyrrolidinone (2.70%), octadecanol
acetate (2.63%), 1-tetracosanol (2.61%), ylangene (1.36%) and
1-hexadecanol (1.06%). The rate of unknown compounds was 7.86%. All
other components were present in <1%.
 | Table II.Chemical composition of
Pelargonium quercetorum extract. |
Table II.
Chemical composition of
Pelargonium quercetorum extract.
|
Compounda |
1tRb |
2tRb | % Areac |
|---|
| Acetic acid |
430 | 1.30 |
0.65 |
| 2,5-Dimethyl
pyrazine |
510 | 2.05 |
0.61 |
|
γ-Butyrolactone |
545 | 2.68 |
0.47 |
|
Benzeneacetaldehyde |
740 | 2.35 |
0.11 |
|
4-Hydroxy-2,5-dimethyl-3(2H)-furanone |
760 | 2.27 |
0.15 |
|
2-Pyrrolidinone |
805 | 3.17 |
2.70 |
|
5-Hydroxymethyldihydrofuran-2(3H)-one | 1025 | 3.19 |
0.36 |
| Hexahydrofarnesyl
acetone | 1980 | 1.54 |
0.73 |
| Allyl octadecyl
oxalate | 2000 | 1.37 |
0.57 |
|
n-Tridecan-1-ol | 2045 | 1.57 |
0.94 |
| Ylangene | 2075 | 1.70 |
1.36 |
| Iso-palmitic methyl
ester | 2090 | 1.52 |
0.14 |
| Nerolidol | 2490 | 1.96 |
0.11 |
| Farnesane | 2810 | 1.37 |
0.68 |
| Eicosane | 2900 | 1.42 |
5.15 |
| 1-Tetracosanol | 2920 | 1.56 |
2.61 |
|
3,7-Dimethylnonane | 2965 | 1.48 |
0.70 |
| 1-Hexadecanol | 2990 | 1.45 |
1.06 |
| 1-Octadecanol
acetate | 3005 | 1.53 |
2.63 |
| Heneicosane | 3075 | 1.56 | 16.23 |
|
2-Methyleicosane | 3150 | 1.45 | 12.37 |
| Tetracosane | 3235 | 1.59 | 40.92 |
| Oleic acid | 3315 | 3.81 |
0.85 |
| Unknown | – | – |
7.86 |
Anti-growth/cytotoxic effect of PQE by
ATP assay
The cytotoxic effect of PQE on non-small cell lung
cancer cell lines (A549, PC3 and H1299) were screened by ATP
viability assay. Cells were treated with increasing doses of PQE
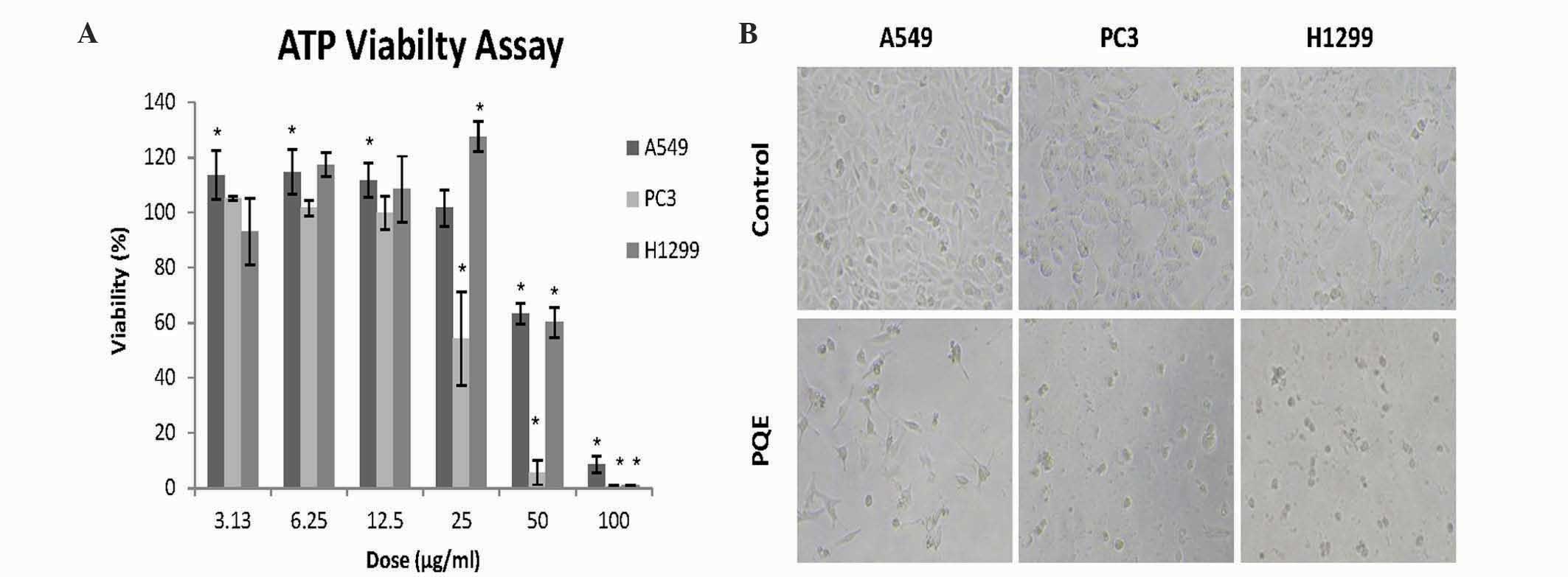
(3.13–100 µg/ml) for 48 h. As shown in Fig. 1A, PQE significantly reduced the cell
viability levels in a dose-dependent manner (P<0.05). PQE
exhibited stronger anti-growth effect on PC3 cells at relatively
lower doses, compared with A549 and H1299 cells. The cell death was
clearly evident in all cell lines by phase-contrast microscopy
(Fig. 1B). The inhibitory
concentration (IC)50 and IC90 values were
calculated on the basis of the results of the ATP assay (Table III).
 | Table III.IC50 and IC90
values of Pelargonium quercetorum extract in non-small cell
lung cancer cells lines. |
Table III.
IC50 and IC90
values of Pelargonium quercetorum extract in non-small cell
lung cancer cells lines.
| Dose (µg/ml) | A549 | PC3 | H1299 |
|---|
|
IC50 | 62.13±1.90 | 27.18±3.21 | 58.53±2.96 |
|
IC90 | 98.70±1.64 | 47.73±1.36 | 92.43±0.60 |
Fluorescence staining for confirmation
of cell death mode
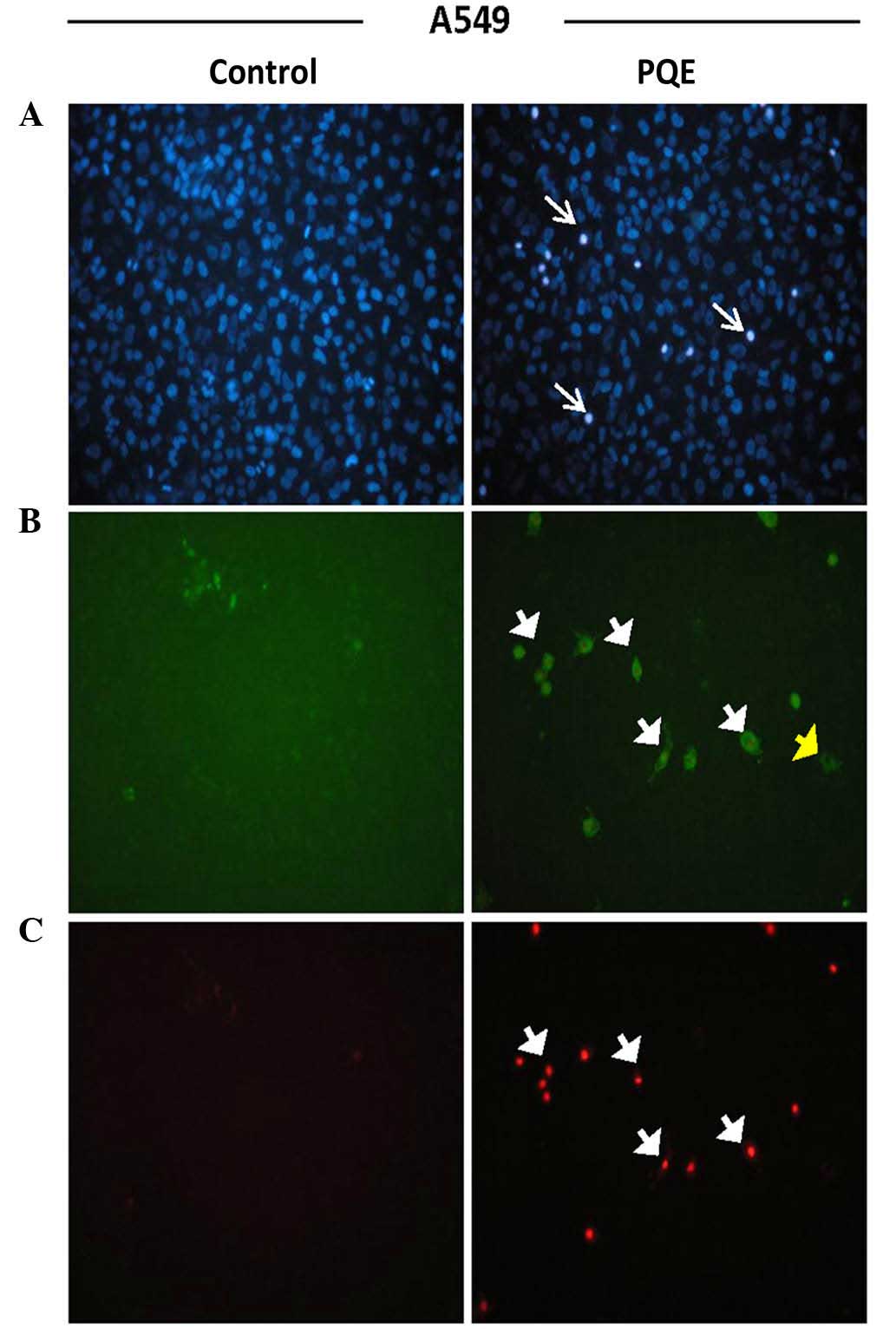
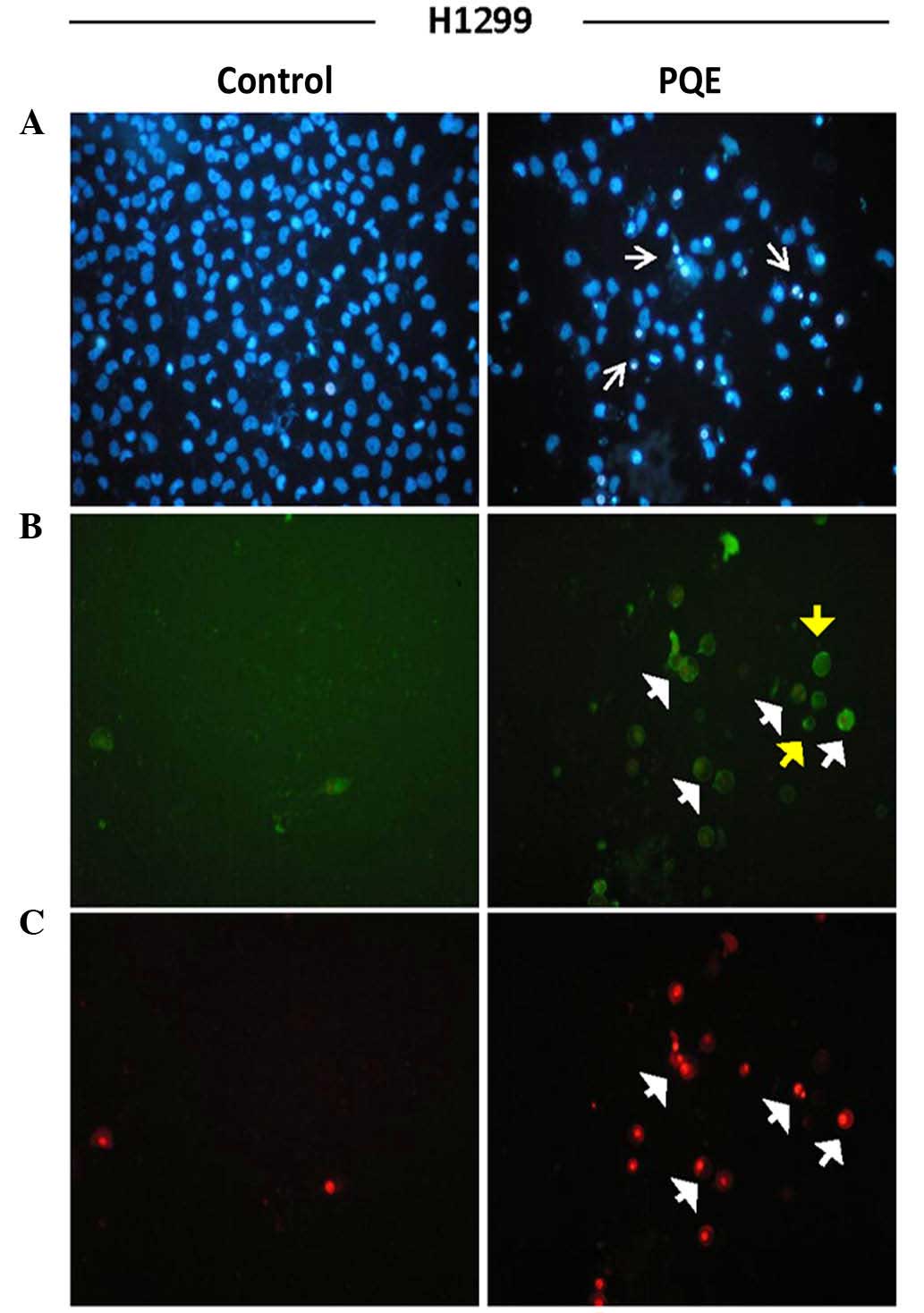
Annexin-V-FITC staining was performed in order to
determine the presence of apoptosis and to distinguish early- from
late-stage apoptosis. Hoechst dye 33342 was additionally used.
Cells were treated with PQE (100 µg/ml) for 12 and 24 h. The images
of 24-h treatment are shown in Figs.
2–4. The early apoptotic cells
were positively stained for only Annexin-V-FITC (green), while the
late apoptotic cells (also called secondary necrotic cells) were
positively stained for PI (red). The presence of pyknotic nuclei
(thin white arrows) on either early (yellow short arrows) or late
(white short arrows) apoptotic cells was a well-known proof of
apoptotic cell death (Figs.
2–4).
Levels of total/intact and
caspase-cleaved CK18 for cell death modes
Two different ELISAs were used, one for the
estimation of total CK18 (M65, for primary or secondary necrosis)
and the other one for the estimation of caspase-cleaved CK18 (M30,
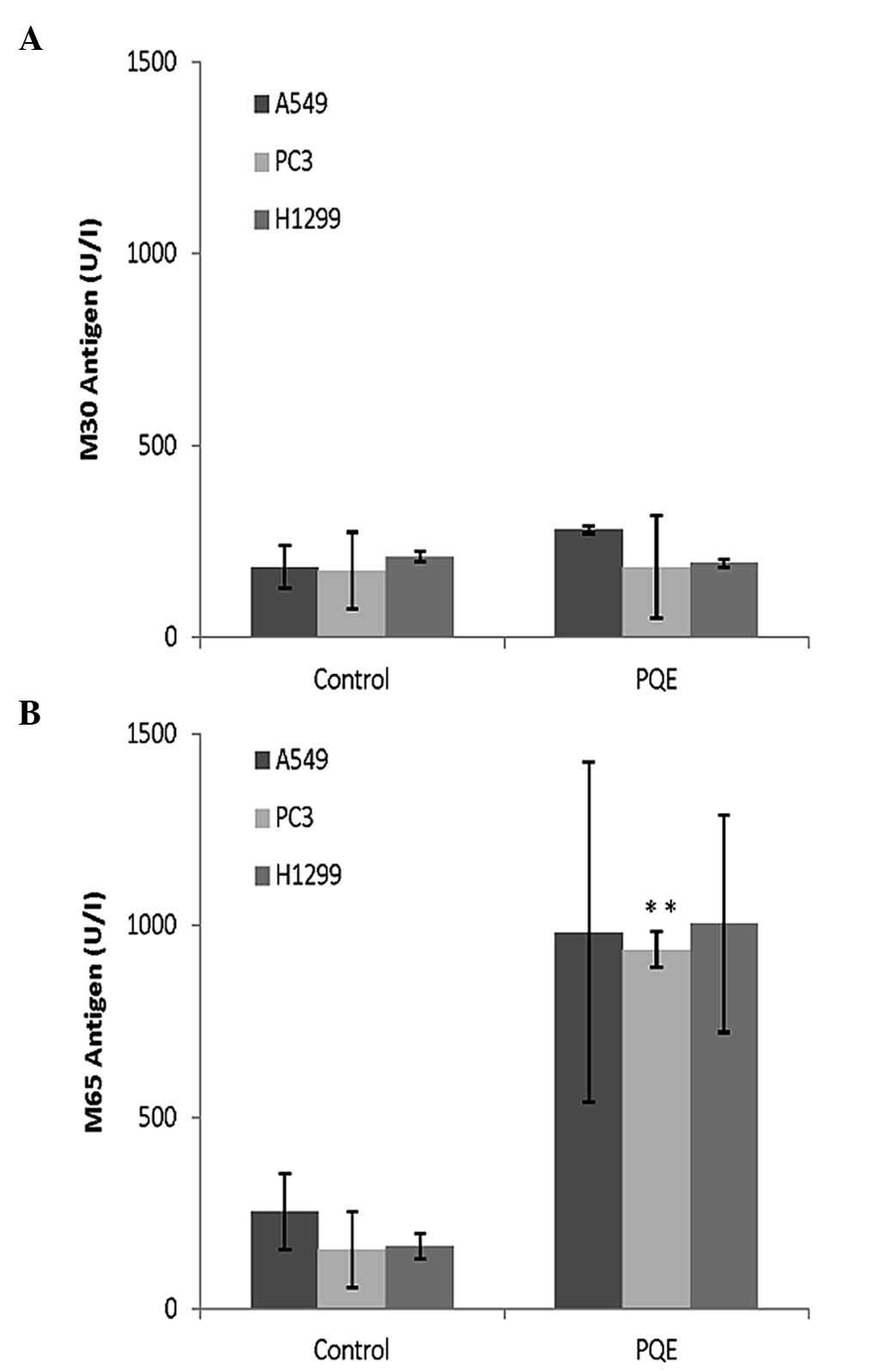
for apoptosis). Fig. 5A indicates
that the levels of M30 did not change in A549, PC3 or H1299 cells
after 48-h PQE treatment, implying that the apoptosis resulted from
PQE may not reach the fragmentation of the cytoskeleton. However,
it is notable that M30 was substantially increased in A549 cells
after treatment with paclitaxel, which is a positive control for
caspase-cleaved CK18 (P=0.053) (data not shown).
With regard to M65 levels, they were observed to
increase 6-fold in PC3 and H1299 cells, and ~4-fold in A549 cells,
after treatment with PQE (100 µg/ml) for 48 h, compared with
untreated control cells (Fig. 5B).
These increments may have resulted from secondary necrosis that
normally occurs following apoptosis.
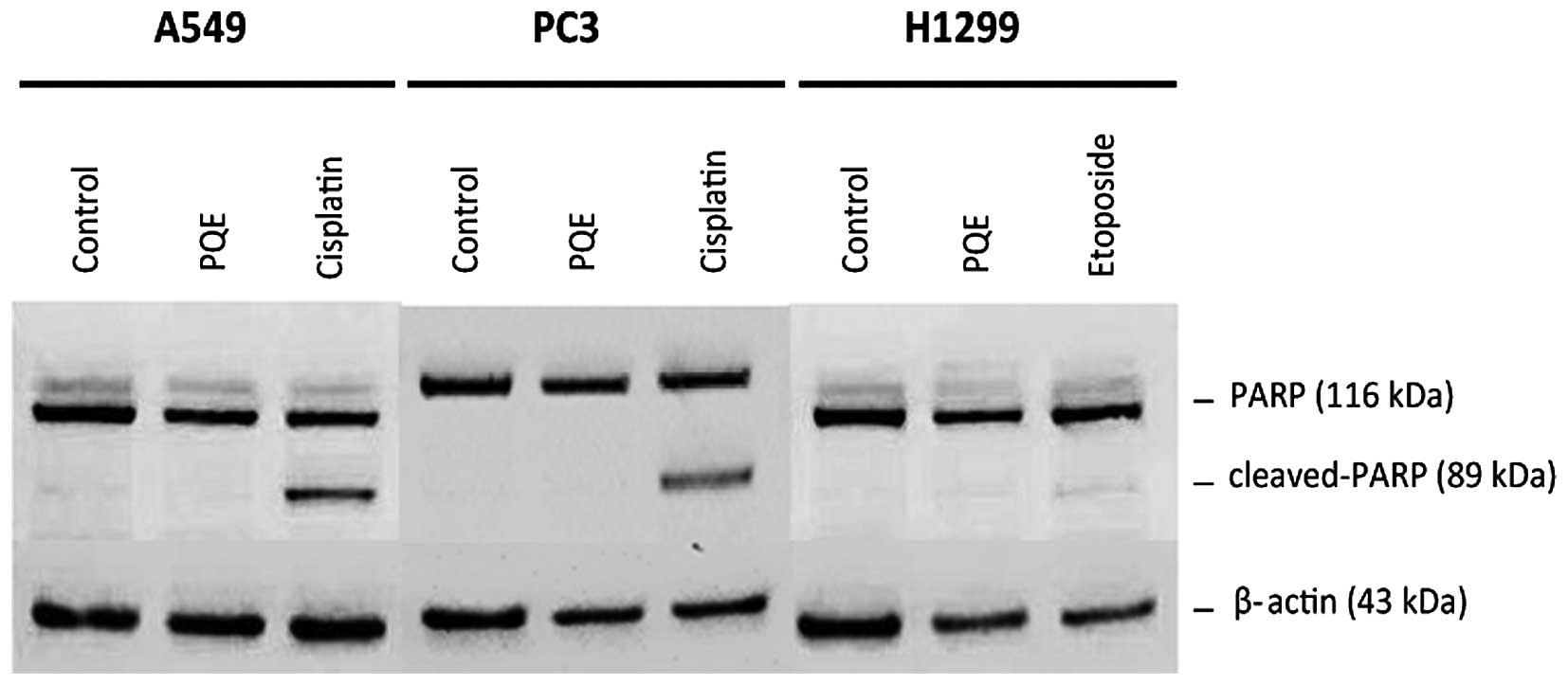
Detection of PARP cleavage by western
blotting
The cleavage of PARP was assayed by western blotting
to further dissect the mechanism of apoptosis. Cisplatin and
etoposide were used as positive controls for PARP-cleavage. It was
observed that PARP was not cleaved in A549, PC3 or H1299 cell lines
following the treatment with PQE (100 µg/ml) for 24 h (Fig. 6). The lack of cleavage of PARP was
considered as incomplete apoptosis, rather than typical
apoptosis.
Discussion
Lung cancer is the leading cause of
cancer-associated mortalities worldwide due to its high incidence
and mortality (40). Despite novel
chemotherapy regimens, there is not sufficient success in its
treatment. Therefore, new therapeutic approaches would be
instrumental for better management of lung cancer patients. Since
natural products are important in cancer therapy, the present study
was conducted to evaluate the possible anti-growth/cytotoxic
activity of PQE on lung cancer cell lines. Despite the fact that
there are several studies on the anticancer activity of the
Pelargonium genus (13,41), the
present study is the first one in the literature to demonstrate the
cytotoxic activity of P. quercetorum on non-small cell lung
cancer cell lines (A549, PC3 and H1299).
In the present study, P. quercetorum was
observed to exert a significant anti-growth effect against A549,
PC3 and H1299 lung cancer cell lines in a dose-dependent manner.
According to the ATP assay results, the IC50 values were
62.1, 27.2 and 58.5 µg/ml for A549, PC3 and H1299 cell lines,
respectively. In a previous study in which the crude acetone
extracts of different Pelargonium species were used against
transformed human kidney epithelium (Graham) cells, it was reported
that the IC50 values of P. sublignosum, P.
citronellum, P. graveolens, P. betulinum, P. capitatum
and P. tomentosum were 11.9, 59.9, 83.3, 88.5, 101.5 and
195.1 µg/ml, respectively (8). This
variability of IC50 values may be due to the use of
different solvents for the extraction process or the different
composition of the plant itself. In fact, biological activities of
different species may vary by essential oil components.
A large number of Pelargonium species are
aromatic and comprise geranium essential oil, which is one of the
top 20 essential oils in the world (9,42). It is
known that geranium essential oils exhibit anticancer activity
(43,44). Therefore, in addition to certain
unknown compounds, P. quercetorum-derived essential oils may
be responsible for this cytotoxic effect. Despite the fact that
these oils could not be analyzed in the present study, an extensive
analysis of the chemical composition identified by GCxGC-TOF/MS was
conducted (Table II). On the basis
of this analysis, it was noticed that tetracosane, heneicosane and
2-methyleicosane were the most abundant components in P.
quercetorum.
Furthermore, the present study investigated the mode
of cell death resulted from PQE. Firstly, the morphology of the
cells nuclei was evaluated for the presence of any apoptosis. The
nuclei were observed to be pyknotic, implying that the mode of cell
death was apoptosis, which was confirmed by Annexin-V positivity.
To dissect further the mechanism of cell death/apoptosis, both the
caspase-cleaved CK18 (M30) and intact CK18 (M65) levels were also
measured. These assays perfectly discriminate two main cell death
modes, apoptosis or necrosis (35).
No increase in M30 levels was observed in these cell lines after
treatment with PQE, despite the fact that the Annexin-V-FITC
staining of the cell membrane and the morphological evaluation of
the cell nuclei clearly implied an apoptotic cell death. For the
lack of M30 increase, there could be two reasons, one being the
lack of CK18 in the cells, and the other one being no fragmentation
of CK18 actually occurring. The present authors recently reported
that all the cell lines evaluated in the present study expressed
CK18, being A549 cells the ones that exhibited the highest levels
(45). This result implies that the
apoptotic process may not include the fragmentation of CK18. That
is why the type of cell death observed in the present study was
described as incomplete apoptosis. Regarding the M65 levels, the
explanation of increases in M65 levels is that the cells should be
undergoing secondary necrosis following apoptosis (46).
In order to additionally investigate the mechanism
of apoptosis, the levels of PARP, which are normally cleaved by
active caspase-3 during apoptosis, were evaluated by the present
study (47,48). No significant alterations in the
cleaved PARP levels were observed compared with untreated control
cells by western blot analysis. Therefore, the present authors
would like to refer to the resultant cell death in the present
study as either incomplete apoptosis or apoptosis-like cell death.
The latter is highly possible, since numerous different types of
cell death have recently emerged (49). However, there are no data in the
literature that could be used for comparison with results of the
present study. Since both M30 production and PARP cleavage have
been demonstrated to result from caspase-activation (50), it is reasonable to conclude that in
the present study the cell death resulted from PQE may be
caspase-independent. However, this should be the topic of future
studies, as additional extensive experiments are required to
elucidate this point.
To the best of our knowledge, the present is the
first study to demonstrate the anti-growth/cytotoxic activity of
P. quercetorum in non-small cell lung cancer cells,
warranting further evaluation in vivo for proof of
concept.
Acknowledgements
The authors would like to thank the Research Fund of
Uludag University (Bursa, Turkey) for funding the present study
[project number UAP (F)-2012/17] and for providing the
kits/chemicals required. The authors would also like to thank Dr
Aysegul Cebi (Giresun University, Giresun, Turkey) for providing
the plant; Dr Serap Celikler (Uludag University, Bursa, Turkey) for
contributing to the extraction and lyophilization of the plant; and
Dr Hakan Akca (Pamukkale University, Denizli, Turkey) for providing
the cell lines used in the present study.
References
|
1
|
Sanders HR and Albitar M: Somatic
mutations of signaling genes in non-small cell lung cancer. Cancer
Genet Cytogenet. 203:7–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halberstein RA: Medicinal plants:
Historical and cross-cultural usage patterns. Ann Epidemiol.
15:686–699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ayo RG: Phytochemical constituents and
bioactivities of the extracts of Cassia nigricans Vahl. J
Med Plant Res. 14:1339–1348. 2010.
|
|
5
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Latté KP and Kolodziej H: Antioxidant
properties of phenolic compounds from Pelargonium reniforme. J
Agric Food Chem. 52:4899–4902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kolodziej H: Pelargonium reniforme
and Pelargonium sidoides: Their botany, chemistry and
medicinal use. Geranium and Pelargonium, Series: Medicinal
and Aromatic Plants - Industrial Profiles. Lis-Balchin M: 27:Taylor
and Francis. (London). 262–290. 2002.
|
|
8
|
Lalli JYY, Van Zyl RL, Van Vuuren SF and
Viljoen AM: In vitro biological activities of South African
Pelargonium (Geraniaceae) species. South African Journal of
Botany. 74:153–157. 2008. View Article : Google Scholar
|
|
9
|
Saraswathi J, Venkatesh K, Baburao N,
Hilal MH and Rani AR: Phytopharmacological importance of
Pelargonium species. J Med Plant Res. 5:2587–2598. 2011.
|
|
10
|
Yanishlieva NV, Marinova E, Gordon MH and
Raneva VG: Antioxidant activity and mechanism of action of thymol
and carvacrol in two lipid systems. Food Chem. 64:59–66. 1999.
View Article : Google Scholar
|
|
11
|
Kris-Etherton PM, Hecker VK, Bonanome A,
Coval SM, Binkoski AE, Hilpert KF, Griel AE and Etherton TD:
Bioactive compounds in foods: Their role in the prevention of
cardiovascular disease and cancer. Am J Med. 113(Suppl 9B):
71S–88S. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seidel V and Taylor PW: In vitro activity
of extracts and constituents of Pelargonium against rapidly growing
mycobacteria. Int J Antimicrob Agents. 23:613–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fayed S: Antioxidant and anticancer
activities of Citrus reticulate (Petitgrain mandarin)
and Pelargonium graveolens (Geranium) essential oils.
Research Journal of Agriculture and Biological Sciences. 5:740–747.
2009.
|
|
14
|
Kolodziej H: Fascinating metabolic pools
of Pelargonium sidoides and Pelargonium reniforme, traditional and
phytomedicinal sources of the herbal medicine Umckaloabo.
Phytomedicine. 14(Suppl 6): 9–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moyo M and Van Staden J: Medicinal
properties and conservation of Pelargonium sidoides DC. J
Ethnopharmacol. 152:243–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mativandlela SPN, Lall N and Meyer JJM:
Antibacterial, antifungal and antitubercular activity of (the roots
of) Pelargonium reniforme (CURT) and Pelargonium
sidoides (DC) (Geraniaceae) root extracts. South African
Journal of Botany. 72:232–237. 2006. View Article : Google Scholar
|
|
17
|
Boukhris M, Bouaziz M, Feki I, Jemai H, El
Feki A and Sayadi S: Hypoglycemic and antioxidant effects of leaf
essential oil of Pelargonium graveolens L'Hér. in alloxan induced
diabetic rats. Lipids in health and disease. 11:812012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang HJ, Su XL, Liu HY, Chen YH and Ni JH:
Studies on the chemical components and anti-tumour action of the
volatile oils from Pelargonium graveoleus. Yao Xue Xue Bao.
24:366–371. 1989.(In Chinese). PubMed/NCBI
|
|
19
|
Bozan B, Ozek T, Kurkcuoglu M, Kirimer N
and Can Baser KH: The analysis of essential oil and head space
volatiles of the flowers of Pelargonium endlicherianum used as an
anthelmintic in folk medicine. Planta Medica. 65:781–782. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tepe B, Sokmen M, Akpulat HA, Yumrutas O
and Sokmen A: Screening of antioxidative properties of the
methanolic extracts of Pelargonium endlicherianum Fenzl.,
Verbascum wiedemannianum Fisch. and Mey., Sideritis
libanotica Labill. subsp. lineraris (Bentham) Borm.,
Centaurea mucronifera DC. and Hieracium cappadocicum
Freyn from Turkish flora. Food Chemistry. 98:9–13. 2006. View Article : Google Scholar
|
|
21
|
Ozbilge H, Kaya EG, Taskin OM and Kosar M:
Antimicrobial activity of Pelargonium endlicherianum Fenzl.
(Geraniaceae) roots against some microorganisms. J Med Plant Res.
4:2647–2650. 2010.
|
|
22
|
Asnawi S, Mohd ZZ, Aziz Abdul A, Khamis AK
and Abdul Aziz B: Evaluation of the potential of Pelargonium Radula
extract in repelling Aedes Aegypti. Journal of Chemical &
Natural Resources Engineering. 2:11–19. 2008.
|
|
23
|
Lis-Balchin M, Buchbauer G, Ribisch K and
Wenger MT: Comparative antibacterial effects of novel Pelargonium
essential oils and solvent extracts. Lett Appl Microbiol.
27:135–141. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scott G, Springfield EP and Coldrey N: A
pharmacognostical study of 26 South African plant species used as
traditional medicines. Pharmaceutical Biology. 42:186–213. 2004.
View Article : Google Scholar
|
|
25
|
Grieve M: Tansy. Modern Herbal: The
Medicinal, Culinary, Cosmetic and Economic Properties, Cultivation
and Folklore of Herbs, Grasses, Fungi, Shrubs and Trees with All
Their Modern Scientific Uses. Leyel CF: Penguin. (Middleburg).
789–790. 1984.
|
|
26
|
Bown D: The Herb Society of America New
Encyclopedia of Herbs and Their Uses. Dorling Kindersley Publishing
Inc. New York, NY: 1995.
|
|
27
|
Kaval I, Behçet L and Cakilcioglu U:
Ethnobotanical study on medicinal plants in Geçitli and its
surrounding (Hakkari-Turkey). J Ethnopharmacol. 155:171–184. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davis PH: Flora of Turkey and the East
Aegean Islands. 1:Edinburgh University Press. Edinburgh: 11965.
|
|
29
|
Davis PH, Mill RR and Tan K: Flora of
Turkey and the east Aegean Islands. 10(Suppl 1)Edinburgh
Universitys Press. Edinburgh: 1061988.
|
|
30
|
Andreotti PE, Cree IA, Kurbacher CM,
Hartmann DM, Linder D, Harel G, Gleiberman I, Caruso PA, Ricks SH,
Untch M, et al: Chemosensitivity testing of human tumors using a
microplate adenosine triphosphate luminescence assay: Clinical
correlation for cisplatin resistance of ovarian carcinoma. Cancer
Res. 55:5276–5282. 1995.PubMed/NCBI
|
|
31
|
Ari F, Aztopal N, Icsel C, Yilmaz VT,
Guney E, Buyukgungor O and Ulukaya E: Synthesis, structural
characterization and cell death-inducing effect of novel
palladium(II) and platinum(II) saccharinate complexes with
2-(hydroxymethyl) pyridine and 2-(2-hydroxyethyl) pyridine on
cancer cells in vitro. Bioorg Med Chem. 21:6427–6434. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ulukaya E, Acilan C, Ari F, Ikitimur E and
Yilmaz Y: A glance at the methods for detection of apoptosis
qualitatively and quantitatively. Turkish Journal of Biochemistry.
36:261–269. 2011.
|
|
33
|
Hammill AK, Uhr JW and Scheuermann RH:
Annexin V staining due to loss of membrane asymmetry can be
reversible and precede commitment to apoptotic death. Exp Cell Res.
251:16–21. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang G, Gurtu V, Kain SR and Yan G: Early
detection of apoptosis using a fluorescent conjugate of annexin V.
Biotechniques. 23:525–531. 1997.PubMed/NCBI
|
|
35
|
Linder S, Olofsson MH, Herrmann R and
Ulukaya E: Utilization of cytokeratin-based biomarkers for
pharmacodynamic studies. Expert Rev Mol Diagn. 10:353–359. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chiu CC, Lin CH and Fang K: Etoposide
(VP-16) sensitizes p53-deficient human non-small cell lung cancer
cells to caspase-7-mediated apoptosis. Apoptosis. 10:643–650. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dasgupta P, Kinkade R, Joshi B, DeCook C,
Haura E and Chellappan S: Nicotine inhibits apoptosis induced by
chemotherapeutic drugs by up-regulating XIAP and survivin. Proc
Natl Acad Sci USA. 103:6332–6337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Ling MT, Wong YC and Wang X:
Evidence of a novel antiapoptotic factor: Role of inhibitor of
differentiation or DNA binding (Id-1) in anticancer drug-induced
apoptosis. Cancer Sci. 98:308–314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
NIST: Automated Mass Spectral
Deconvolution and Identification System 2005 version. National
Institute of Standards and Technology. US Department of Commerce.
(USA). 2005.
|
|
40
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global Cancer Statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Moura MD, Silva JDS, de Oliveira RAG,
Diniz MDFFM and Barbosa-Filho JM: Natural products reported as
potential inhibitors of uterine cervical neoplasia. Acta
Farmacéutica Bonaerense. 21:67–74. 2002.
|
|
42
|
Lis-Balchin M: Geranium oil. International
Journal of Aromatherapy. 7:18–20. 1996. View Article : Google Scholar
|
|
43
|
Haag JD, Lindstrom MJ and Gould MN:
Limonene-induced regression of mammary carcinomas. Cancer Res.
52:4021–4026. 1992.PubMed/NCBI
|
|
44
|
Zhuang SR, Chen SL, Tsai JH, Huang CC, Wu
TC, Liu WS, Tseng HC, Lee HS, Huang MC, Shane GT, et al: Effect of
citronellol and the Chinese medical herb complex on cellular
immunity of cancer patients receiving chemotherapy/radiotherapy.
Phytother Res. 23:785–790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cevatemre B, Ulukaya E, Sarimahmut M, Oral
AY and Frame FM: The M30 assay does not detect apoptosis in
epithelial-derived cancer cells expressing low levels of
cytokeratin 18. Tumor Biol. 36:6857–6865. 2015. View Article : Google Scholar
|
|
46
|
Ulukaya E, Acilan C and Yilmaz Y:
Apoptosis: Why and how does it occur in biology? Cell Biochem
Funct. 29:468–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nicholson DW, Ali A, Thornberry NA,
Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle
M, Lazebnik Y, et al: Identification and inhibition of the
ICE/CED-3 protease necessary for mammalian apoptosis. Nature.
376:37–43. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Alnemri ES: Mammalian cell death
proteases: A family of highly conserved aspartate specific cysteine
proteases. J Cell Biochem. 64:33–42. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Galluzzi L, Vitale I, Abrams JM, Alnemri
ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry
WS, Fulda S, et al: Molecular definitions of cell death
subroutines: Recommendations of the Nomenclature Committee on Cell
Death 2012. Cell Death Differ. 19:107–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gown AM and Willingham MC: Improved
detection of apoptotic cells in archival paraffin sections:
Immunohistochemistry using antibodies to cleaved caspase 3. J
Histochem Cytochem. 50:449–454. 2002. View Article : Google Scholar : PubMed/NCBI
|