Introduction
Obesity is an independent risk factor for
cardiovascular diseases (1). Previous
studies have revealed that adipose tissue acts as an endocrine
organ, generating a range of secreted factors including leptin,
adiponectin, tumor necrosis factor-α (TNF-α), resistin, plasminogen
activator inhibitor-1 and visfatin (2–5). These
adipocytokines have been found to be expressed in atherosclerotic
plaques, modulating the inflammatory processes of atherosclerosis,
which implies that adipocytokines are vital for the development of
atherosclerosis (6–9).
Atherosclerosis is an inflammatory process that
starts with endothelial dysfunction, which is important in the
initial pathogenesis of atherosclerotic lesion formation (10,11).
Adipokines, including resistin and leptin, are proinflammatory
mediators that directly contribute to endothelial dysfunction and
atherogenesis (12–15).
Visfatin corresponds to a protein that was
previously identified as pre-B cell colony enhancing factor, which
is a 52-kDa cytokine expressed in lymphocytes. Visfatin is a newly
found visceral-fat-specific adipokine that is predominantly
produced in visceral adipose tissue (16,17).
Visfatin can upregulate the levels of interleukin (IL)-1β, IL-6,
IL-8 and TNF-α in cytokines (18–20).
Therefore, visfatin may provide an additional association between
adipose tissues and inflammation. Visfatin has previously been
found to markedly increase the expression of intercellular adhesion
molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)
through activating nuclear factor-κB (NF-κB), which directly
contributes to endothelial dysfunction (12,13,21).
Several studies have reported that the expression of visfatin is
increased at plaque rupture sites in patients with coronary artery
disease and that visfatin accelerates monocyte adhesion to
endothelial cells by upregulating adhesion molecules in vascular
endothelial cells (22). This finding
suggests a positive association between visfatin foam cell
expression, aortic inflammation and plaque destabilization.
Emerging evidence has established that visfatin is important for
the development of atherosclerosis (20,23,24).
However, the molecular mechanism underlying the association of
visfatin in the development of atherosclerosis remains unclear.
In addition, previous studies suggest that visfatin
can dysregulate the vascular endothelial function in a
dose-dependent manner. Visfatin can significantly increase the
expression of IL-6, ICAM-1, VCAM-1 and other cytokines in
endothelial dysfunction (21).
Whether the levels of visfatin in adipose tissue surrounding of the
coronary artery are associated with coronary endothelial
dysfunction has not yet been studied (25). Visfatin located in the adipose tissue
surrounding the coronary artery is presumed to initiate coronary
artery endothelial inflammation via the activation of the NF-κB
signal pathway, which then leads to coronary endothelial
dysfunction and promotes the development and progression of
atherosclerosis.
The present study demonstrates, for the first time,
that atorvastatin can decrease the visfatin-induced expression of
IL-6 and IL-8 in HCAECs. In addition, atorvastatin is found to
inhibit the visfatin-induced expression of ICAM-1 and VCAM-1 in
HCAECs. The present study also indicates that atorvastatin inhibits
the visfatin-activated NF-κB signaling pathway by preventing the
phosphorylation of extracellular signal-regulated kinase (ERK) in
HCAECs. Atorvastatin is shown to significantly inhibit
visfatin-induced NF-κB activity by decreasing the production of
reactive oxygen species (ROS). Atorvastatin, FK866 and BAY11-7082
are shown to decrease the visfatin-induced expression of
inflammatory mediators via the upregulation of NF-κB activation in
HCAECs. These results suggest that atorvastatin may inhibit
visfatin-induced upregulation of inflammatory mediators through
blocking the NF-κB signal pathway. The findings of the present
study provide a potential role of atorvastatin and visfatin in the
pathogenesis of HCAEC dysfunction, and this knowledge may
contribute to the development of novel therapies for
atherosclerosis.
Materials and methods
Cell culture
HCAECs were purchased from ScienCell Research
Laboratories (San Diego, CA, USA). Cells were grown in endothelial
cell growth medium (ScienCell Research Laboratories). In these
experiments, cells were seeded in 0.5 ml complete medium in 24-well
plates. The control cells were the vehicle cells, which were
created using HCAECs dissolved in dimethyl sulfoxide.
Drugs
Atorvastatin was purchased from the Shanghai Yijing
Industrial Co., Ltd. (Shanghai, China), FK866 was purchased from
Apexbio Technology LLC (Houston, TX, USA) and BAY11-7082 was
purchased from Selleck Chemicals LLC (Houston, TX, USA).
Antibodies
The following antibodies were purchased from Abcam
(Cambridge, MA, USA): Anti-VCAM1 rabbit polyclonal antibody against
human (predicted: mouse, rat and rabbit; dilution, 1:500–1,000;
catalog no., ab106777; localization, membrane); anti-ICAM1 rabbit
polyclonal antibody against mouse/human (predicted: rat, chimpanzee
and Chinese hamster; dilution, 1:500–1,000; catalog no., ab124759;
localization, membrane); anti-IL6 rabbit polyclonal antibody
against human/pig (dilution, 1:400–800; catalog no., ab6672;
localization, secreted); anti-IL8 rabbit polyclonal against
rat/human/cynomolgus monkey/rhesus monkey (dilution, 1:10; catalog
no., ab7747; localization, secreted); anti-NF-κB p65 rabbit
polyclonal antibody against mouse/rat/chicken/human/Indian muntjac
(dilution, 1:400–800; catalog no., ab16502; localization, nucleus);
and anti-ERK5 rabbit polyclonal antibody against mouse/rat/human
(dilution, 1:500–1,000; catalog no., ab196609; localization,
cytoplasm). The anti-phosphorylated-ERK (p-ERK) mouse monoclonal
antibody against mouse/rat/human/canine/bovine (dilution,
1:200–500; catalog no., sc-7383; localization, cytoplasm/nucleus)
was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). The anti-β-actin rabbit polyclonal antibody against
mouse/human (dilution, 1:100; catalog no., ab16039; localization,
cytoplasm) was purchased from Abcam and used as the control. The
secondary polyclonal goat anti-rabbit IgG-horseradish
peroxidase-conjugated antibody (dilution, 1:1,000–10,000; catalog
no., SE12) was purchased from Solarbio (Beijing, China).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol Reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and
reverse transcription was performed using a PrimeScript RT reagent
kit (Takara Bio, Inc., Otsu, Japan), according the manufacturer's
instructions. For RT-qPCR analysis, aliquots of complementary DNA
were amplified using SYBR Premix Ex Taq (Takara Bio, Inc.). PCR
reactions were performed in triplicate with the following
conditions: 95°C for 30 sec, then 40 cycles of 95°C for 5 sec, 60°C
for 15 sec and 72°C for 10 sec, on an MXP3000 cycler (Stratagene;
Agilent Technologies, Inc., Santa Clara, CA, USA) and repeated at
least three times. The primers were as follows: VCAM1 forward,
5′-GGGAAGATGGTCGTGATCCTT-3′ and reverse,
5′-TCTGGGGTGGTCTCGATTTTA-3′; ICAM1 forward,
5′-ATGCCCAGACATCTGTGTCC-3′ and reverse, 5′-GGGGTCTCTATGCCCAACAA-3′.
Relative messenger RNA (mRNA) levels were calculated using the ΔΔCq
method, using β-actin as a control, and expressed as
2(−ΔΔCq) (26).
Measurement of VCAM-1 and ICAM-1 in
HCAECs by enzyme-linked immunosorbent assay (ELISA)
The concentration of VCAM-1 and ICAM-1 in
conditioned media was measured using Human ELISA Kits (Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions.
Western blot analysis
Total protein of tissues and cells was obtained
using radioimmunoprecipitation assay lysis buffer containing a
mixture of proteinase inhibitors (Sigma-Aldrich, St. Louis, MO,
USA). The protein concentration was determined using BCA protein
assay reagent (Beyotime Institute of Biotechnology, Haimen, China).
Equivalent amounts of proteins were separated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and then transferred
onto nitrocellulose membranes (Invitrogen; Thermo Fisher
Scientific, Inc.). After being blocked in Tris-buffered saline
containing 5% fat-free milk, the membranes were incubated with the
aforementioned VCAM-1, ICAM-1, IL-6, IL-8, NF-κB p65, ERK or p-ERK
primary antibodies at 4°C overnight, washed using
phosphate-buffered saline (PBS)-Tween buffer [0.01 mol/l PBS (pH
7.2–7.4); 0.2 mol/l NaH2PO4 19 ml; 0.2 mol/l
Na2HPO4 81 ml; NaCl 17 g; 2,000 ml
ddH2O; 20% Tween 20] and then incubated with horseradish
peroxidase-conjugated secondary antibody at room temperature for 2
h. A β-actin antibody was used as a loading control. Signals were
detected on X-ray film using the Pierce ECL detection system
(Thermo Fisher Scientific, Inc.).
Measurement of ROS production
ROS production was measured by using
2′,7′-diclorofluorescein diacetate (H2DCFDA; Invitrogen;
Thermo Fisher Scientific, Inc,). In total, 4 HCAECs were incubated
in the absence or presence of 50 ng/ml visfatin, with or without 50
nM FK866, 50 µM BAY11-0782 or 10 µM atorvastatin, for 24 h. The
HCAECs were then incubated with H2DCFDA (10 µM) for 30
min at 37°C. Fluorescence images were obtained using a fluorescence
microscope (BX-51; Olympus Corporation, Tokyo, Japan). The
fluorescence intensity was measured using Image J software version
2.1.4.7 (imagej.nih.gov/ij/; National
Institutes of Health, Bethesda, MD, USA); then the mean
fluorescence was calculated and normalized to the control
value.
Statistical analysis
Statistical analysis was performed with SPSS 13.0
for Windows (SPSS Inc., Chicago, IL, USA). The Pearson
χ2 test or Fisher's exact test was used to compare
qualitative variables, and the Student's t-test for
quantitative variables. All statistical tests were two-sided, and a
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
Visfatin improves the production of
cytokines in HCAECs, but BAY11-7082 and atorvastatin can antagonize
this process
IL-6 and IL-8 have been previously reported to be
involved in the pathogenesis of obesity, insulin resistance and
atherosclerosis (27,28). Therefore, the present study aimed to
examine whether visfatin, FK866, BAY11-7082 and atorvastatin could
affect cytokine and adhesion molecule gene expression in HCAECs.
Compared with the control, HCAECs incubated in the presence of
visfatin caused a significant increase in IL-6 and IL-8 mRNA
expression. However, the IL-6 and IL-8 mRNA levels of the visfatin
group were reduced in the presence of FK866, BAY11-7082 and
atorvastatin (Fig. 1A and B). An
ELISA was used to measure the levels of IL-6 and IL-8 released from
HCAECs. Compared with the control group, visfatin group showed
significant increases in the release of IL-6 and IL-8. However,
levels of IL-6 and IL-8 in the FK866, BAY11-7082 and atorvastatin
groups were decreased compared with the visfatin group and
increased compared with the control group (Fig. 1C and D). Overall, these results
suggest that FK866, BAY11-7082 and atorvastatin decrease the
visfatin-induced upregulation of IL-6 and IL-8 in HCAECs.
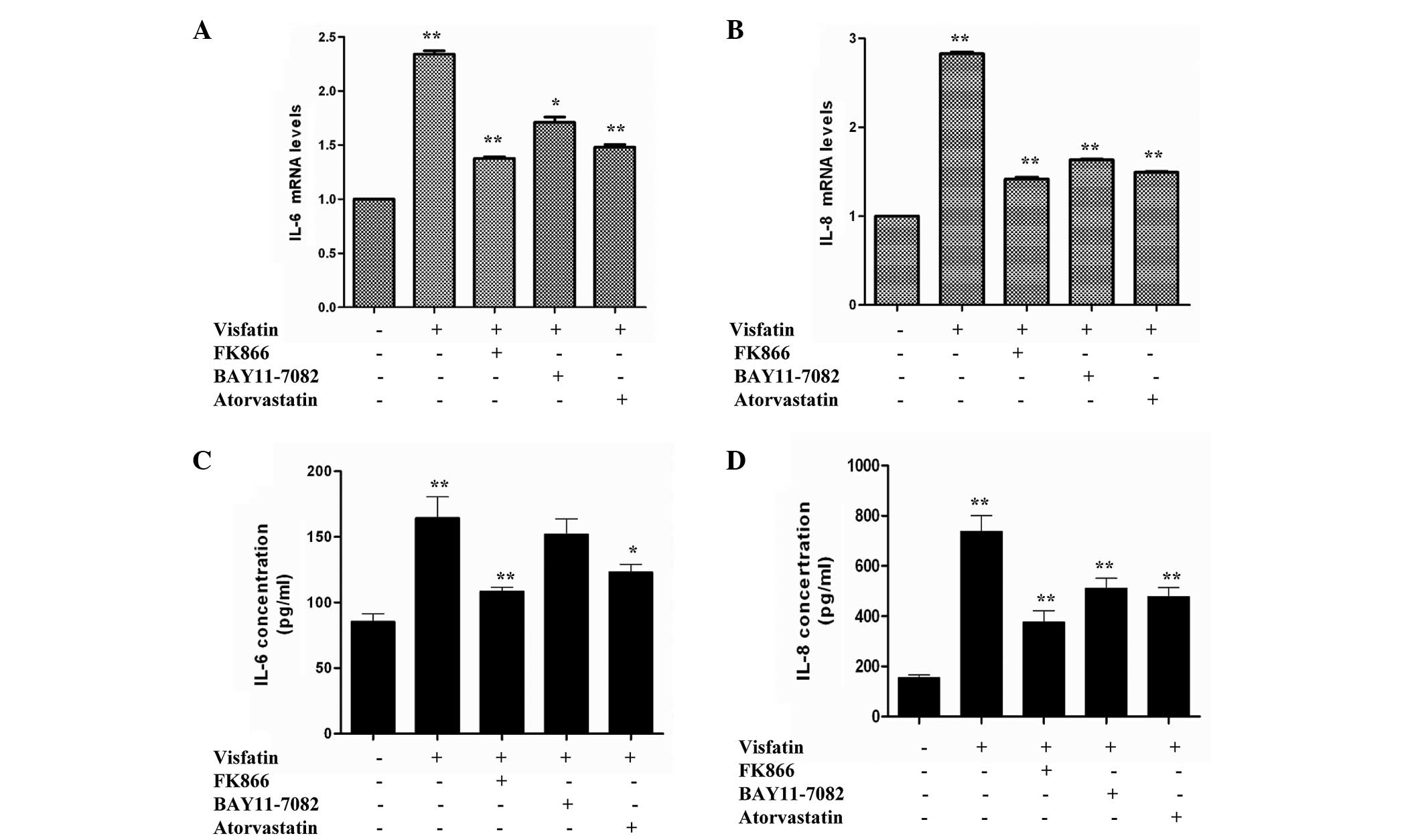 | Figure 1.Reverse transcription-quantitative
polymerase chain reaction assays revealed the effect of
atorvastatin on visfatin-induced inflammation. Human coronary
artery endothelial cells were incubated in the absence or presence
of 50 ng/ml visfatin, with or without 50 nM FK866, 50 µM BAY11-0782
or 10 µM atorvastatin, for 24 h. (A) Atorvastatin downregulated
visfatin-induced IL-6 gene expression (P-values from left to right
vs. control: 0.0081, 0.0045, 0.0233 and 0.0057). (B) Atorvastatin
decreased visfatin-induced IL-8 gene expression (P-values from left
to right vs. control: 0.0096, 0.0037, 0.0043 and 0.0031). (C)
Atorvastatin downregulated visfatin-induced IL-6 release (P-values
from left to right vs. control: 0.0078, 0.0022, 0.0783 and 0.0382).
(D) Atorvastatin inhibited visfatin-induced IL-8 release (P-values
from left to right vs. control: 0.0094, 0.0032, 0.0039 and 0.0035).
Significant differences were determined using Student's
t-test; *P<0.05, **P<0.01 vs. visfatin
control. IL-6, interleukin-6; IL-8, interleukin-8. |
Atorvastatin inhibits the
visfatin-induced expression of adhesion molecules in HCAECs
The expression of adhesion molecules, including
ICAM-1 and VCAM-1, on the endothelial cell surface is an initial
step in atherogenesis (29). ICAM-1
and VCAM-1 can mediate leukocyte adhesion to the endothelium during
inflammation. Therefore, the present study investigated whether
visfatin could induce the expression of ICAM-1 and VCAM-1 in
HCAECs. The data revealed that visfatin strongly increased ICAM-1
and VCAM-1 mRNA levels compared with the control group. However,
the ICAM-1 and VCAM-1 mRNA levels in the visfatin group were
decreased in the presence of FK866, BAY11-7082 and atorvastatin
(Fig. 2A and B). Western blot
analysis assays also indicated that the treatment of HCAECs with
visfatin increased ICAM-1 and VCAM-1 protein levels compared with
control the group. However, the ICAM-1 and VCAM-1 protein levels of
the visfatin group were decreased in the presence of FK866,
BAY11-7082 and atorvastatin (Fig. 2C and
D). Overall, these results suggest that atorvastatin inhibited
the visfatin-induced expression of ICAM-1 and VCAM-1 in HCAECs.
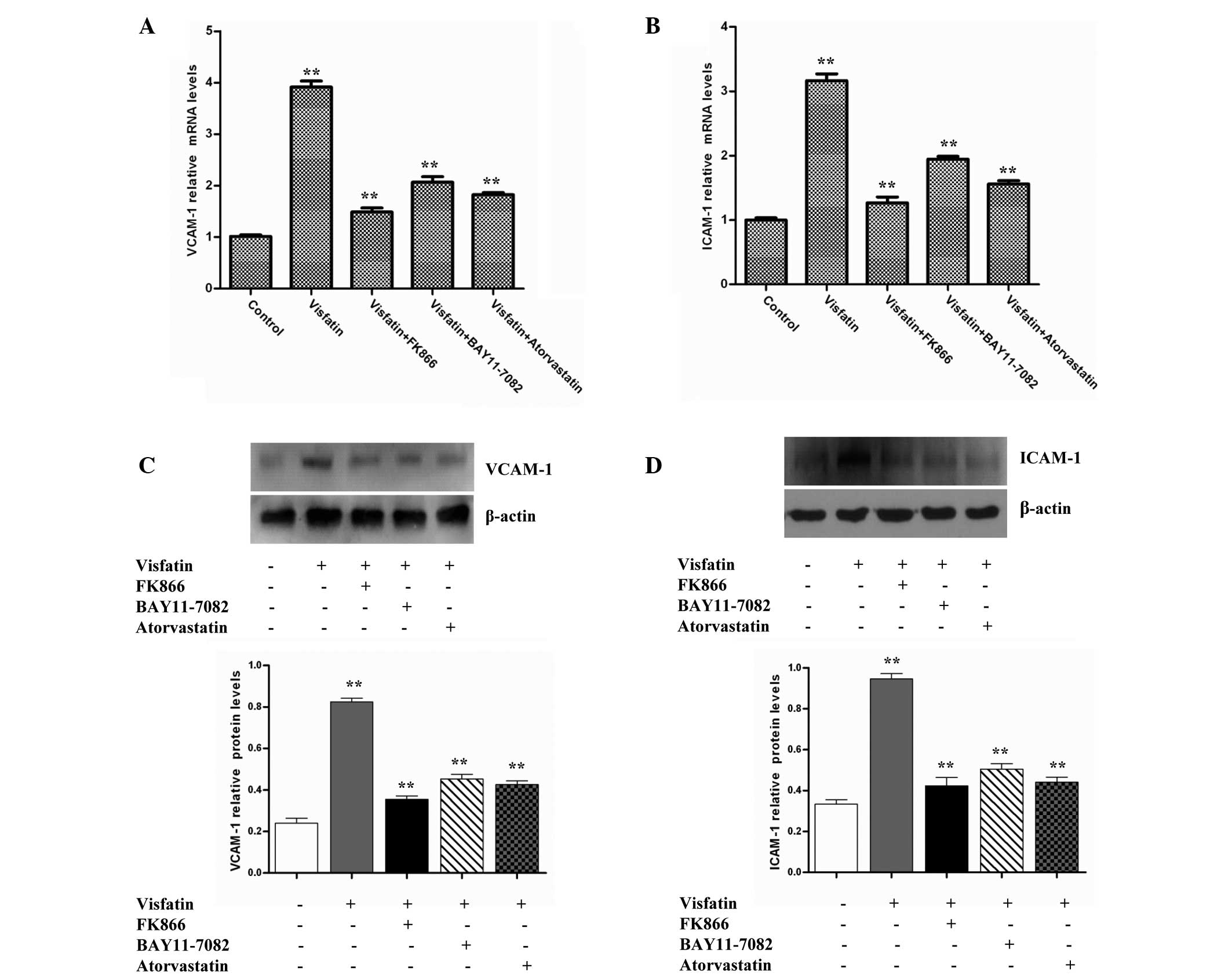 | Figure 2.Atorvastatin and signaling inhibitors
decreased visfatin-induced VCAM-1 and ICAM-1 expression in HCAECs.
HCAECs were incubated in the absence or presence of 50 ng/ml
visfatin, with or without 50 nM FK866, 50 µM BAY11-0782 or 10 µM
atorvastatin, for 24 h. (A) RT-qPCR assays revealed that
atorvastatin downregulated visfatin-induced VCAM-1 gene expression
(P-values from left to right vs. control: 0.0068, 0.0015, 0.0021
and 0.0017). (B) RT-qPCR assays revealed that atorvastatin
decreased visfatin-induced ICAM-1 gene expression (P-values from
left to right vs. control: 0.0073, 0.0011, 0.0026 and 0.0019). (C)
Western blotting assays revealed that atorvastatin downregulated
the visfatin-upregulated VCAM-1 protein level (P-values from left
to right vs. control: 0.0089, 0.0016, 0.0023 and 0.0018). (D)
Western blotting assays revealed that atorvastatin inhibited the
visfatin-upregulated ICAM-1 protein level (P-values from left to
right vs. control: 0.0097, 0.0014, 0.0020 and 0.0016). Total cell
lysates were separated by 12% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and immunoblots were analyzed with anti-VCAM-1,
anti-ICAM-1 and anti-β-actin antibodies. β-actin was measured as an
internal control. Significant differences were determined using
Student's t-test, **P<0.01. VCAM-1, vascular
adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1;
HCAECs, human coronary artery endothelial cells; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Visfatin induces the expression of
cytokines and adhesion molecules through the NF-κB signaling
pathway, but atorvastatin can antagonize the process
Numerous cytokines, such as IL-1, IL-6, IL-8 and
TNF-α, and cell adhesion molecules, such as CD4, CD8, CD22, CD28,
CTLA-4, ICOS, ICAM-1, CD80 and CD86, can induce gene expression
through the NF-κB signaling pathway (30). NF-κB is a potent proinflammatory
nuclear transcription factor, and the activation of NF-κB is a
major part of the initiation and amplification of inflammatory
responses. NF-κB activity can be modulated via phosphorylation by
mitogen-activated protein kinases (MAPKs), including ERK1/2
(31–33). In addition, visfatin has been shown to
promote angiogenesis through the activation of the ERK1/2 in
endothelial cells. Therefore, the present study investigated
whether visfatin could activate NF-κB activity via the ERK
signaling pathway in HCAECs.
Western blot analyses indicated that the treatment
of HCAECs with visfatin increased p-ERK and p65 protein levels, but
did not increase ERK protein levels, compared with the control
group. However, the protein levels of p-ERK and p65 in the visfatin
group were reduced in the presence of FK866, BAY11-7082 and
atorvastatin (Fig. 3A). The present
study then aimed to determine whether visfatin could enhance the
nuclear accumulation of p65 protein. A western blot analysis of p65
demonstrated that there was no significant difference in the
cytoplasm protein levels of p65 compared with control cells;
however, the nuclear protein levels of p65 significantly increased
with the treatment of HCAECs with visfatin. However, this effect
was inhibited in the presence of FK866, BAY11-7082 and atorvastatin
(Fig. 3B). Overall, the results
suggest that visfatin upregulates the expression of p-ERK and p65
and promotes the translocation of p65 to the nucleus in HCAECs, but
that atorvastatin could antagonize this effect.
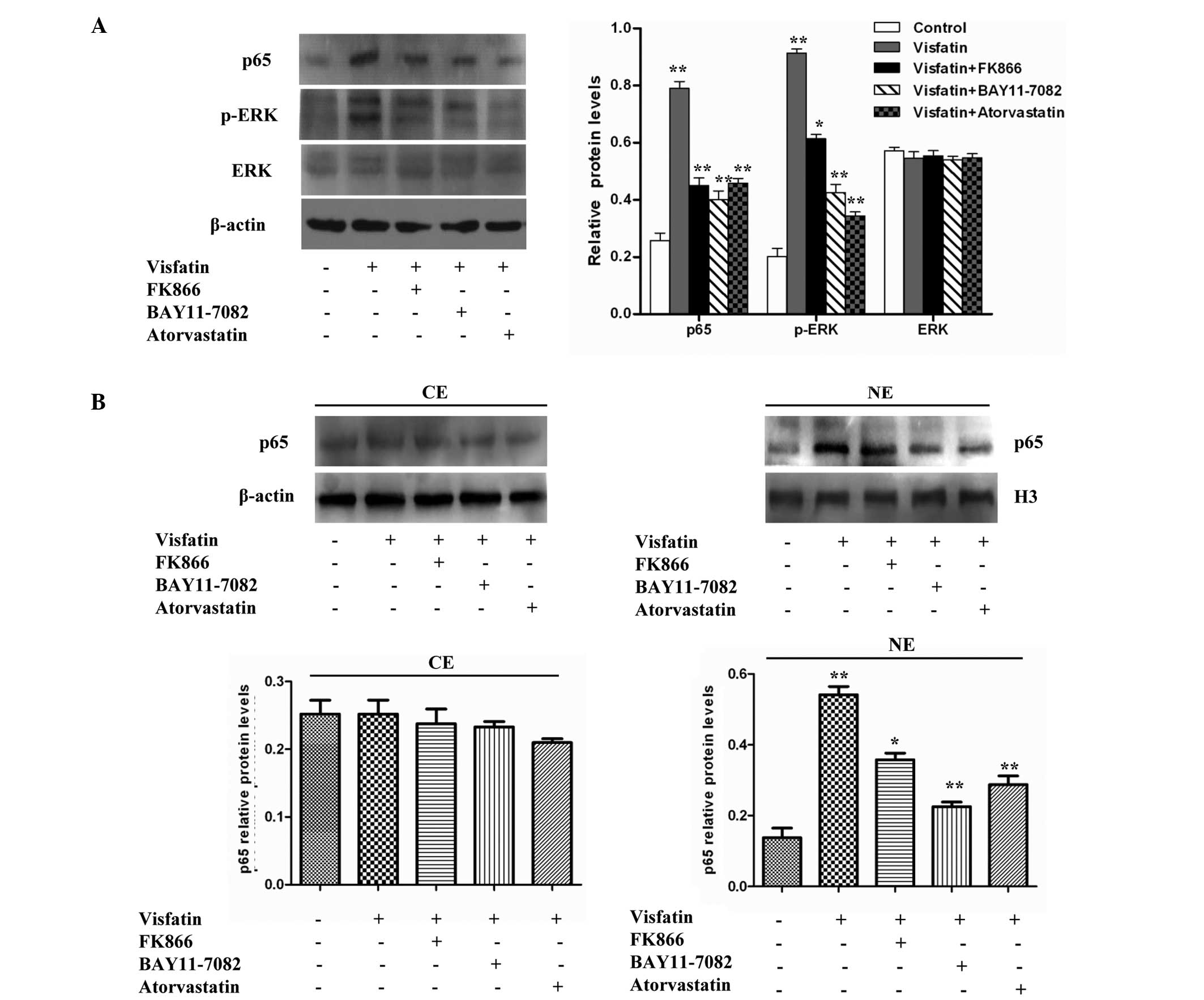 | Figure 3.Effect of atorvastatin on the
visfatin-induced NF-κB signaling pathway in HCAECs. (A) Western
blotting assays revealed that visfatin increased p-ERK and p65
protein levels, but did not increase ERK protein levels compared
with the control group. However, FK866, BAY11-7082 and atorvastatin
inhibited visfatin-induced NF-κB activity (P-values from left to
right vs. controls: 0.0082, 0.0055, 0.0047 and 0.0058; 0.0090,
0.0061, 0.0053 and 0.0037; 0.0632, 0.0689, 0.0620 and 0.0627). (B)
Cell fractionation assays revealed that atorvastatin decreased the
visfatin-increased nuclear p65 protein levels in HCAECs (P-values
from left to right vs. control, left image: 0.0728, 0.0675, 0.0622
and 0.0530; right image: 0.0082, 0.0056, 0.0024 and 0.0037).
Significant differences were determined using Student's
t-test, *P<0.05, **P<0.01. NF-κB,
nuclear factor-κB; HCAECs, human coronary artery endothelial cells;
p-ERK, phosphorylated-extracellular signal-regulated kinase; ERK,
extracellular signal-regulated kinase; CE, cytoplasmic extract; NE,
nuclear extract. |
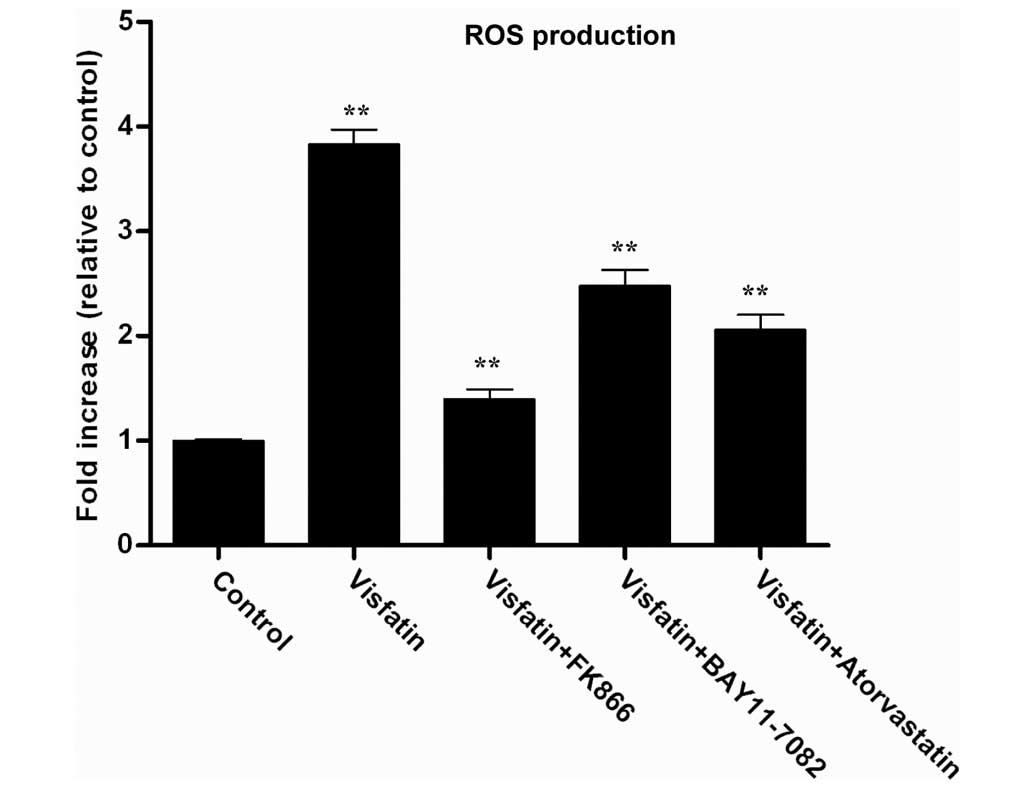
Visfatin promotes NF-κB activation
associated with ROS production, but atorvastatin can antagonize the
process
Visfatin has been previously found to upregulate
NF-κB activity, indicating that visfatin may induce the expression
of pro-inflammatory and adhesion molecules (34). ROS are known to be produced by
cytokines, growth factors and vasoactive agents, and contribute to
the intracellular signaling cascades associated with inflammatory
response (35). ROS can induce the
activation of the NF-κB signaling pathway by modifying the
activation of one or more of the kinase enzymes involved in NF-κB
activation cascades (36). Therefore,
the present study investigated whether visfatin could induce ROS
generation in HCAECs. The H2DCFDA assay was used to
assess the effect of visfatin on ROS generation. Visfatin
significantly increased ROS generation in HCAECs; however the
visfatin-induced ROS generation was decreased in the presence of
FK866, BAY11-7082 and atorvastatin (Fig.
4). Overall, these results suggest that atorvastatin decreased
visfatin-induced ROS generation in HCAECs.
Discussion
Obesity is a significant risk factor for
cardiovascular diseases, including atherosclerosis and hypertension
(37,38). Atherosclerosis is an inflammatory
process that begins with systemic endothelial dysfunction, a
pivotal event in the early pathogenesis of atherosclerotic lesion
formation (10,11). Adipokines regulate the endothelial
expression of cell adhesion molecules and chemoattractant
chemokines, one of the early important steps in atherosclerosis
(14,15). Previous studies have reported that the
expression of visfatin is increased at plaque rupture sites in
patients with coronary artery disease, which suggests a possible
role for visfatin in the development of atherosclerosis (21,25). The
present study demonstrated that visfatin increased the production
of IL-6 and IL-8 in HCAECs. The treatment of HCAECs with visfatin
resulted in the upregulation of ICAM-1 and VCAM-1 expression. Using
western blotting assays, the treatment of HCAECs with visfatin was
found to result in increased p-ERK and p65 protein levels and
decreased ERK protein levels compared with the control group. In
addition, visfatin enhanced p65 protein nuclear accumulation in
HCAECs. Finally, visfatin was shown to induce ICAM-1 and VCAM-1
expression through the NF-κB signal pathway and promote NF-κB
activation, in association with ROS production. However,
atorvastatin was indicated to antagonize the function of visfatin
in HCAECs. Overall, these results suggest that atorvastatin may be
vital for the prevention and therapy of atherosclerosis.
IL-6 and IL-8 have been reported to be involved in
the pathogenesis of obesity, insulin resistance and atherosclerosis
(27,28). In the present study, visfatin was
found to induce the production of IL-6 and IL-8 in HCAECs. This
finding is in agreement with previous studies, which showed that
visfatin induced the production of the IL-1β, IL-6, IL-8 and TNF-α
cytokines (18–20). However, for the first time, the
present study found that atorvastatin could antagonize the
visfatin-promoted production of IL-6 and IL-8. The expression of
adhesion molecules such as VCAM-1 and ICAM-1 on the endothelial
cell surface is the initial step in atherogenesis (29). A previous study reported that the
upregulation of ICAM-1 and VCAM-1 has been found in human
atherosclerotic plaques, and was associated with upregulated
leukocyte recruitment and accumulation (21). The present study demonstrated that
visfatin increased ICAM-1 and VCAM-1 mRNA and protein levels in
HCAECs, but that atorvastatin could antagonize this visfatin
effect. These results suggest that atorvastatin can use
anti-inflammatory actions to attenuate the dysfunction of HCAECs
and atherosclerosis.
NF-κB can induce the expression of numerous
cytokines and cell adhesion molecules (32). NF-κB activity can be modulated through
phosphorylation by MAPKs, including ERK1/2, JNK and p38 (30). The present study demonstrated that
visfatin upregulated p-ERK and p65 protein levels, suggesting that
visfatin could activate p65 via activation of the MAPK/ERK
signaling pathway. Visfatin also promoted the translocation of p65
to the nucleus in HCAECs, but atorvastatin could antagonize this
effect. In the present study, visfatin upregulated NF-κB activity,
which indicates that visfatin induced the expression of
inflammatory and adhesion molecules, possibly through the NF-κB
signaling pathway. ROS were produced by cytokines, growth factors
and vasoactive agents, which contribute to the intracellular
signaling cascades associated with inflammatory response (39). ROS induced NF-κB activation by
modifying the activation of one or more of the kinase enzymes in
the NF-κB activation cascades (40,41). In
the present study, visfatin significantly increased ROS generation
in HCAECs, but the visfatin-induced ROS generation was reduced in
the presence of FK866, BAY11-7082 and atorvastatin. These results
suggest that the inhibition of the visfatin-induced expression of
cytokines and cell adhesion molecules by atorvastatin may be
mediated by the downregulation of NF-κB activation due to decreased
ROS production.
In conclusion, the present study indicates that
visfatin causes in the dysfunction of HCAECs by upregulating the
expression of IL-6, IL-8, ICAM-1 and VCAM-1, probably through
activation of the NF-κB pathway. The activation of the NF-κB
pathway may be mainly due to visfatin-induced p-ERK expression and
ROS generation. However, atorvastatin can antagonize the
visfatin-promoted development of atherosclerosis, suggesting that
atorvastatin may be vital for the prevention and therapy of
atherosclerosis.
References
|
1
|
Rashid MN, Fuentes F, Touchon RC and
Wehner PS: Obesity and the risk for cardiovascular disease. Prev
Cardiol. 6:42–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ajuwon KM and Spurlock ME: Adiponectin
inhibits LPS-induced NF-kappaB activation and IL-6 production and
increases PPARgamma2 expression in adipocytes. Am J Physiol Regul
Integr Comp Physiol. 288:R1220–R1225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anfossi G, Russo I, Doronzo G, Pomero A
and Trovati M: Adipocytokines in atherothrombosis: Focus on
platelets and vascular smooth muscle cells. Mediators Inflamm.
2010:1743412010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawanami D, Maemura K, Takeda N, Harada T,
Nojiri T, Imai Y, Manabe I, Utsunomiya K and Nagai R: Direct
reciprocal effects of resistin and adiponectin on vascular
endothelial cells: A new insight into adipocytokine-endothelial
cell interactions. Biochem Biophys Res Commun. 314:415–419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lau DC, Dhillon B, Yan H, Szmitko PE and
Verma S: Adipokines: Molecular links between obesity and
atheroslcerosis. Am J Physiol Heart Circ Physiol. 288:H2031–H2041.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berg AH and Scherer PE: Adipose tissue,
inflammation and cardiovascular disease. Circ Res. 96:939–949.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guerre-Millo M: Adipose tissue and
adipokines: For better or worse. Diabetes Metab. 30:13–19. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sethi JK and Vidal-Puig A: Visfatin: The
missing link between intra-abdominal obesity and diabetes? Trends
Mol Med. 11:344–347. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu ZH and Zhao SP: Adipocyte: A potential
target for the treatment of atherosclerosis. Med Hypotheses.
67:82–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blankenberg S, Barbaux S and Tiret L:
Adhesion molecules and atherosclerosis. Atherosclerosis.
170:191–203. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kougias P, Chai H, Lin PH, Yao Q, Lumsden
AB and Chen C: Effects of adipocyte-derived cytokines on
endothelial functions: Implication of vascular disease. J Surg Res.
126:121–129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kralisch S, Sommer G, Stangl V, Köhler U,
Kratzsch J, Stepan H, Faber R, Schubert A, Lössner U, Vietzke A, et
al: Secretory products from human adipocytes impair endothelial
function via nuclear factor kappaB. Atherosclerosis. 196:523–531.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cooke JP and Oka RK: Does leptin cause
vascular disease? Circulation. 106:1904–1905. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kougias P, Chai H, Lin PH, Lumsden AB, Yao
Q and Chen C: Adipocyte-derived cytokine resistin causes
endothelial dysfunction of porcine coronary arteries. J Vasc Surg.
41:691–698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samal B, Sun Y, Stearns G, Xie C, Suggs S
and McNiece I: Cloning and characterization of the cDNA encoding a
novel human pre-B-cell colony-enhancing factor. Mol Cell Biol.
14:1431–1437. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sommer G, Garten A, Petzold S,
Beck-Sickinger AG, Blüher M, Stumvoll M and Fasshauer M:
Visfatin/PBEF/Nampt: Structure, regulation and potential function
of a novel adipokine. Clin Sci (Lond). 115:13–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moschen AR, Kaser A, Enrich B, Mosheimer
B, Theurl M, Niederegger H and Tilg H: Visfatin, an adipocytokine
with proinflammatory and immunomodulating properties. J Immunol.
178:1748–1758. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee WJ, Wu CS, Lin H, Lee IT, Wu CM, Tseng
JJ, Chou MM and Sheu WH: Visfatin-induced expression of
inflammatory mediators in human endothelial cells through the
NF-kappaB pathway. Int J Obes (Lond). 33:465–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guzik TJ, Mangalat D and Korbut R:
Adipocytokines-novel link between inflammation and vascular
function? J Physiol Pharmacol. 57:505–528. 2006.PubMed/NCBI
|
|
21
|
Kim SR, Bae YH, Bae SK, Choi KS, Yoon KH,
Koo TH, Jang HO, Yun I, Kim KW, Kwon YG, et al: Visfatin enhances
ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB
activation in endothelial cells. Biochim Biophys Acta.
1783:886–895. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buldak RJ, Gowarzewski M, Buldak L,
Skonieczna M, Kukla M, Polaniak R and Zwirska-Korczala K: Viability
and oxidative response of human colorectal HCT-116 cancer cells
treated with visfatin/eNampt in vitro. J Physiol Pharmacol.
66:557–566. 2016.
|
|
23
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SR, Bae SK, Choi KS, Park SY, Jun HO,
Lee JY, Jang HO, Yun I, Yoon KH, Kim YJ, et al: Visfatin promotes
angiogenesis by activation of extracellular signal-regulated kinase
1/2. Biochem Biophys Res Commun. 357:150–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dahl TB, Yndestad A, Skjelland M, Øie E,
Dahl A, Michelsen A, Damås JK, Tunheim SH, Ueland T, Smith C, et
al: Increased expression of visfatin in macrophages of human
unstable carotid and coronary atherosclerosis: Possible role in
inflammation and plaque destabilization. Circulation. 115:972–980.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kotsopoulos J, Zhang S, Akbari M, Salmena
L, Llacuachaqui M, Zeligs M, Sun P and Narod SA: BRCA1 mRNA levels
following a 4–6-week intervention with oral 3,3′-diindolylmethane.
Br J Cancer. 111:1269–1274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simonini A, Moscucci M, Muller DW, Bates
ER, Pagani FD, Burdick MD and Strieter RM: IL-8 is an angiogenic
factor in human coronary atherectomy tissue. Circulation.
101:1519–1526. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yudkin JS, Kumari M, Humphries SE and
Mohamed-Ali V: Inflammation, obesity, stress and coronary heart
disease: Is interleukin-6 the link? Atherosclerosis. 148:209–214.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi M, Ikeda U, Masuyama J, Kitagawa
S, Kasahara T, Shimpo M, Kano S and Shimada K: Monocyte-endothelial
cell interaction induces expression of adhesion molecules on human
umbilical cord endothelial cells. Cardiovasc Res. 32:422–429. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han SG, Newsome B and Hennig B: Titanium
dioxide nanoparticles increase inflammatory responses in vascular
endothelial cells. Toxicology. 306:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adya R, Tan BK, Chen J and Randeva HS:
Nuclear factor-kappaB induction by visfatin in human vascular
endothelial cells: Its role in MMP-2/9 production and activation.
Diabetes Care. 31:758–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adya R, Tan BK, Punn A, Chen J and Randeva
HS: Visfatin induces human endothelial VEGF and MMP-2/9 production
via MAPK and PI3K/Akt signalling pathways: Novel insights into
visfatin-induced angiogenesis. Cardiovasc Res. 78:356–365. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Collins T, Read MA, Neish AS, Whitley MZ,
Thanos D and Maniatis T: Transcriptional regulation of endothelial
cell adhesion molecules: NF-kappaB and cytokine-inducible
enhancers. FASEB J. 9:899–909. 1995.PubMed/NCBI
|
|
34
|
Said RS, El-Demerdash E, Nada AS and Kamal
MM: Resveratrol inhibits inflammatory signaling implicated in
ionizing radiation-induced premature ovarian failure through
antagonistic crosstalk between silencing information regulator 1
(SIRT1) and poly(ADP-ribose) polymerase 1 (PARP-1). Biochem
Pharmacol. 103:140–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Görlach A, Bertram K, Hudecova S and
Krizanova O: Calcium and ROS: A mutual interplay. Redox Biol.
6:260–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chaudhari N, Talwar P, Parimisetty A,
d'Hellencourt Lefebvre C and Ravanan P: A molecular web:
endoplasmic reticulum stress, inflammation, and oxidative stress.
Front Cell Neurosci. 8:2132014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shirai K: Obesity as the core of the
metabolic syndrome and the management of coronary heart disease.
Curr Med Res Opin. 20:295–304. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van Gaal LF, Mertens IL and De Block CE:
Mechanisms linking obesity with cardiovascular disease. Nature.
444:875–880. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim YR, Koh HJ, Kim JS, Yun JS, Jang K,
Lee JY, Jung JU and Yang CS: Peptide inhibition of p22phox and
Rubicon interaction as a therapeutic strategy for septic shock.
Biomaterials. 101:47–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ruiz-Ojeda FJ, Gomez-Llorente C, Aguilera
CM, Gil A and Rupérez AI: Impact of 3-amino-1,2,4-triazole
(3-AT)-derived increase in hydrogen peroxide levels on inflammation
and metabolism in human differentiated adipocytes. PLoS One.
11:e01525502016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Satoh R, Kakugawa K, Yasuda T, Yoshida H,
Sibilia M, Katsura Y, Levi B, Abramson J, Koseki Y, Koseki H, et
al: PLoS Genet. 12:e10057762016. View Article : Google Scholar : PubMed/NCBI
|