Introduction
Various types of cancer induce bone metastasis,
which leads to serious bone loss and fractures. Bone metastasis
occurs in 70–80% of patients with advanced stage breast cancer
(1–4),
and induces severe pathological bone fractures, pain,
hypercalcemia, and spinal cord and nerve-compression syndromes
(3,5).
This bone disorder is frequently causes morbidity and mortality.
Tumor invasion of the bone tissues is associated with the
recruitment of osteoclasts and osteoblasts, resulting in growth
factor liberation from the bone matrix. Furthermore, these growth
factors can enhance tumor growth, resulting in a cycle of bone
metastasis (4,5).
Breast cancer cells promote osteoclast formation via
the secretion of osteoporotic cytokines, including parathyroid
hormone-related peptide, tumor necrosis factor-α (TNF-α),
prostaglandin E2, leukemia inhibitory factor, and
interleukin-1, −6, −8, −11, −15 and −17 (4,6).
Constitutively-activated nuclear factor-κB (NF-κB) in breast cancer
cells has been shown to play a crucial role in osteolysis, which
stimulates osteoclastogenesis. Moreover, breast cancer cells
stimulate the production of granulocyte macrophage
colony-stimulating factor, which enhances development from
monocytes to osteoclasts (7). In
addition, progesterone receptor-positive mammary epithelial cancer
cells express receptor activator of NF-κB ligand (RANKL), which
mediates the proliferation of epithelial cells and carcinogenesis
(8). In addition, breast cancer cells
suppress the function of osteoblasts. This is demonstrated by an
increase in apoptosis and a decrease in proteins required for new
bone formation (6). Bone loss induced
by breast cancer bone metastasis is based on activated osteoclastic
bone resorption and suppressed osteoblastic bone formation.
Bisphosphonate or anti-RANKL antibody (denosumab) is
used as the current standard care for patients with bone metastasis
(9). Bisphosphonate inhibits
osteoclastic bone resorption, but does not possess osteogenic
effects. Denosumab suppresses osteoclast maturation by inhibiting
the binding of RANKL to RANK, which is the receptor of RANKL in
preosteoclasts and mature osteoclasts. These drugs target bone
resorption mediated through osteoclasts. However, agents that
stimulate osteogenic bone formation to repair bone destruction have
been poorly developed.
Gentian violet (GV), a triaminophenylmethane dye,
has been used extensively in medicine for a century, and it has a
potent anti-microbial action (10).
Furthermore, recent studies have suggested the angiogenic and
anticancer properties of GV, and this chemical is currently
experiencing renewed interest in medical applications (11,12). Our
recent study demonstrated that GV inhibits nuclear factor-κB
(NF-κB) activity, and that this agent can potently enhance
osteoblast differentiation and mineralization, but suppress the
differentiation to osteoclasts (13).
Thus, GV may regulate the differentiation of bone cells in
vitro. Further development of GV as an anti-osteoporotic and/or
anti-inflammatory agent may be expected.
Moreover, GV may possess preventive effects on bone
loss induced by cancer cell bone metastasis. However, the
anticancer effects of GV on human breast cancer bone metastatic
cells have been poorly investigated. The present study was
undertaken to determine whether GV exhibits a suppressive effect on
the proliferation of human breast cancer MDA-MB-231 cells in
vitro. The results showed that GV potently suppresses the
proliferation of human breast cancer MDA-MB-231 cells.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) with 4.5
g/l glucose, L-glutamine and sodium pyruvate, and antibiotics
[penicillin and streptomycin (P/S); 5,000 U/ml and 5,000 µg/ml,
respectively] were purchased from Gibco Laboratories (Grand Island,
NY, USA). Fetal bovine serum (FBS) was obtained from HyClone
(Logan, UT, USA). Gentian violet, sodium butyrate, roscovitine,
sulforaphane, PD98059, staurosporine, wortmannin,
5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB) and all other
reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA)
unless otherwise specified. Gemcitabine was obtained from Hospira,
Inc. (Lake Forest, IL, USA). Gemcitabine was diluted in
phosphate-buffered saline (PBS) and other reagents were dissolved
in 100% ethanol to use in the experiments.
Cancer cells
MDA-MB-231 human breast cancer cells lack the
receptors for progesterone, estrogen and human epithelial growth
factor receptor 2, and are therefore considered as triple negative
(14). However, MDA-MB-231 cells do
express epithelial growth factor receptor (EGFR) at high levels,
and activation of this receptor and its downstream signaling events
enhance the migration, proliferation, invasion and progression of
the malignant phenotype of breast cancer cells (14). The present study used
estrogen-independent bone-seeking triple negative human breast
cancer MDA-MB-231 cells (1×106 cells/ml of DMEM
containing 10% FBS and 0.1% P/S), which were stored at −80°C. The
cells were obtained from the American Type Culture Collection
(Rockville, MD, USA).
Proliferation in cancer cells
The breast cancer MDA-MB-231 cells
(1×105/ml per well) were cultured in a 24-well plate
using DMEM containing 10% FBS and 1% P/S in the presence or absence
of GV (1, 10, 50, 100 or 200 nM) for 1, 3, 7 or 14 days in a
water-saturated atmosphere containing 5% CO2 and 95% air
at 37°C (15–17). In separate experiments, the MDA-MB-231
cells (1×105/ml per well) were cultured in DMEM
containing 10% FBS and 1% P/S in the presence of either ethanol
(0.1% final concentration; control), sodium butyrate (10 and 100
µM), roscovitine (10 and 100 nM), sulforaphane (1 and 10 nM),
PD98059 (1 µM), staurosporin (0.1 µM), wortmannin (1 µM), DRB (1
µM) or gemcitabine (100 nM) for 3–7 days. Subsequent to the culture
process, the cells were detached from each culture dish and counted
(16,17). In addition, to determine the effects
of GV on MDA-MB-231 cells that reached confluence, the cells
(1×105 cells/ml per well) were cultured using a 24-well
plate in DMEM containing 10% FBS and 1% P/S in the absence of GV
for 7 days until they reached confluence, and then the cells were
cultured in the presence of GV (1, 10, 50, 100 or 200 nM) for 3
days (18). Following this, the cells
were detached from each culture dish and counted.
Cell counting
Following trypsinization of each culture dish using
0.2% trypsin plus 0.02% EDTA in
Ca2+/Mg2+-free PBS for 2 min at 37°C,
detached cells from the dishes were collected after centrifugation
at 150 × g for 5 min (16–18). The cells were resuspended in PBS
solution and stained with eosin. Cell numbers were counted under a
microscope (Olympus MTV-3; Olympus, Tokyo, Japan) using a
hemocytometer plate. For each dish, the average of two counts was
used. Cell number is shown as the number per well of each
plate.
Statistical analysis
Statistical significance was determined using
GraphPad InStat version 3 for Windows XP (GraphPad Software Inc.,
La Jolla, CA, USA). Multiple comparisons were performed by one-way
analysis of variance with Tukey-Kramer multiple comparisons
post-hoc test for parametric data as indicated. P<0.05 was
considered to indicate a statistically significant difference.
Results
To determine the effects of GV on the proliferation
of human breast cancer MDA-MB-231 cells in vitro, the cancer
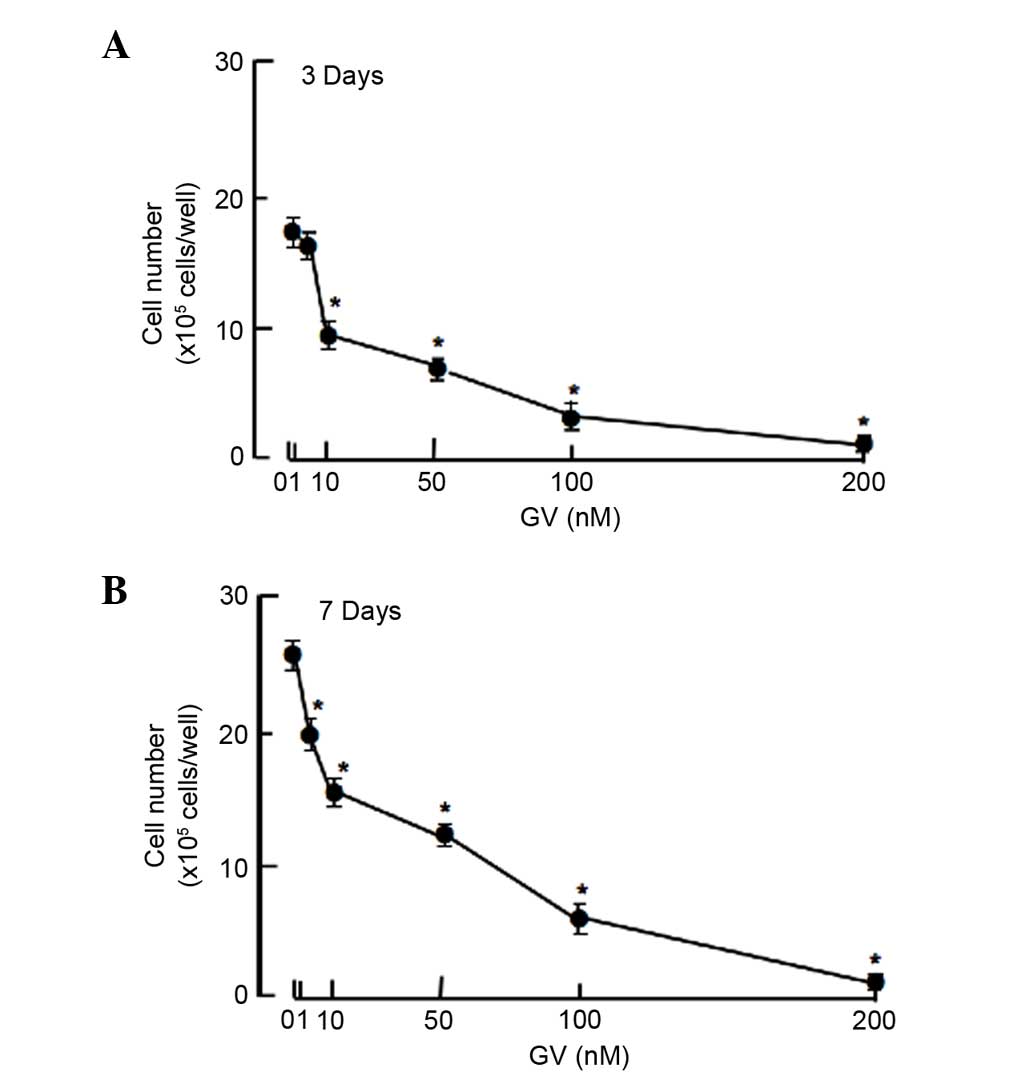
cells were cultured in the presence of GV for 3 or 7 days. Cell
numbers were increased with increasing culture periods. This
increase was suppressed after culture with GV (1–200 nM) for 3
(Fig. 1A) and 7 (Fig. 1B) days. Thus, GV was found to exhibit
suppressive effects on the proliferation of the MDA-MB-231 cells
in vitro. In addition, the MDA-MB-231 cells that reached
confluence after culture for 7 days were cultured for an additional
3 days in the presence of GV (1–200 nM). Cell number was
significantly (P=0.001) decreased after culture with GV (10–200 nM)
(data not shown), suggesting that GV partly stimulates cell
death.
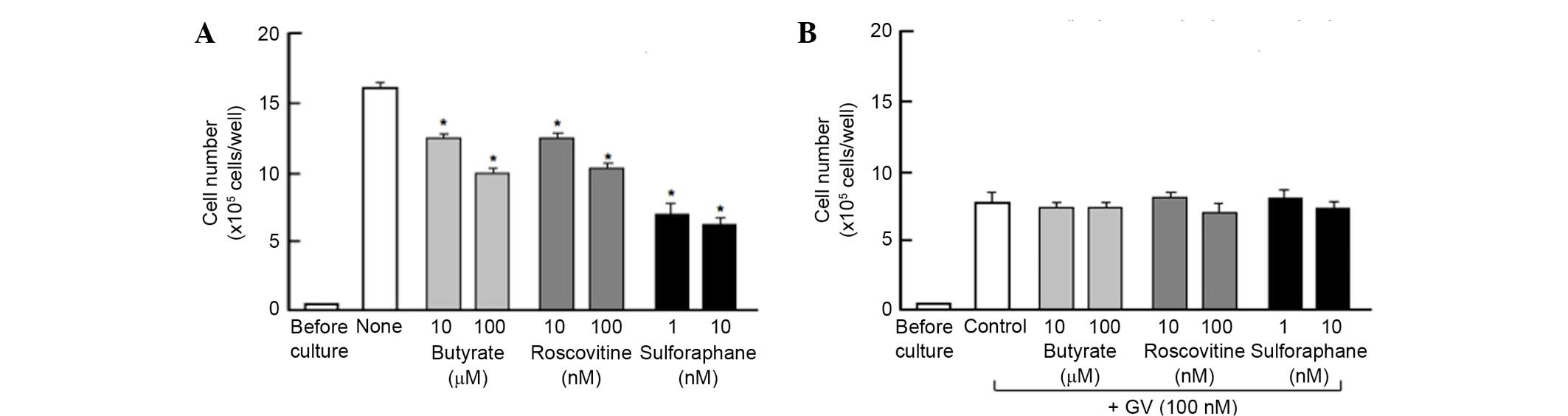
To determine a mechanistic characterization, the
present study determined whether the suppressive effects of GV on
the proliferation of MDA-MB-231 cells are altered using various
inhibitors that induce cell cycle arrest in vitro (Fig. 2). Cells were cultured for 3 days with
or without butyrate (10 and 100 µM), roscovitine (10 and 100 nM) or
sulforaphane (1 and 10 nM) (17,19,20). The
proliferation of the MDA-MB-231 cells, which were cultured in the
absence of GV, was suppressed in the presence of these inhibitors
(Fig. 2A). The suppressive effects of
these inhibitors on cell proliferation was not altered in the
presence of GV (100 nM) (Fig. 2B).
This finding suggested that GV induces G1 and G2/M phase cell cycle
arrest in MDA-MB-231 cells.
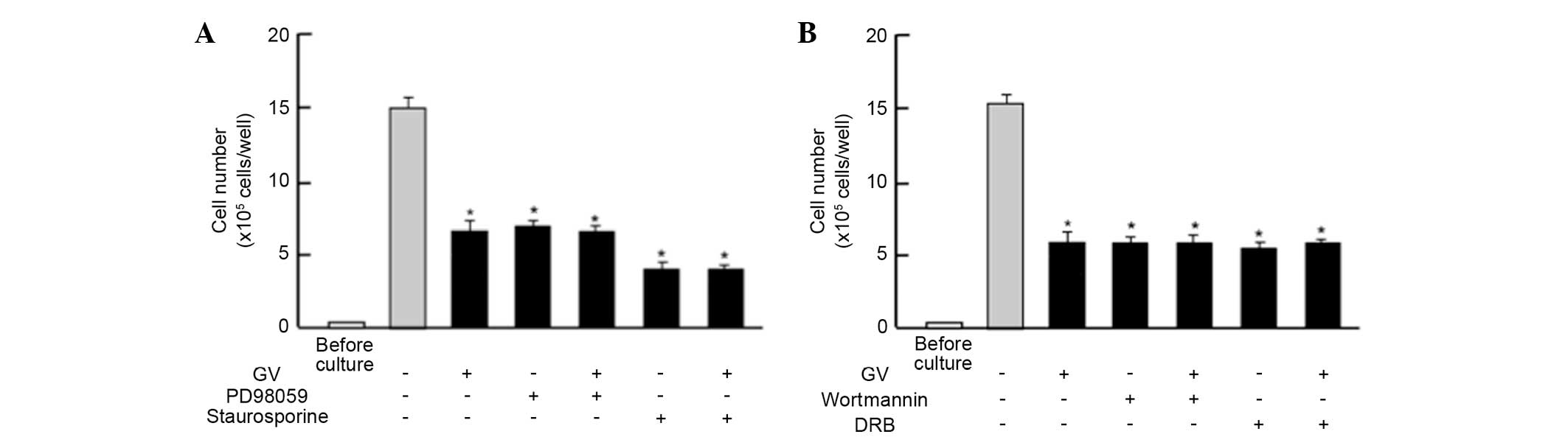
Next, the study determined whether the suppressive
effects of GV on the proliferation in MDA-MB-231 cells are changed
by various signaling factors that suppress proliferation. The
suppressive effects of GV (100 nM) on the proliferation of the
MDA-MB-231 cells were not altered in the presence of PD98059 (1
µM), an extracellular signal-regulated kinase (ERK) inhibitor
(21), or staurosporin (0.1 µM), an
inhibitor of protein kinase C (22)
(Fig. 3A). In addition, the
suppressive effects of GV on cell proliferation were not enhanced
in the presence of wortmannin (1 µM), an inhibitor of
phosphatidylinositol 3-kinase (PI3K) (23), or DRB (1 µM), an inhibitor of
transcriptional activity with RNA polymerase II inhibition
(24) (Fig.
3B).
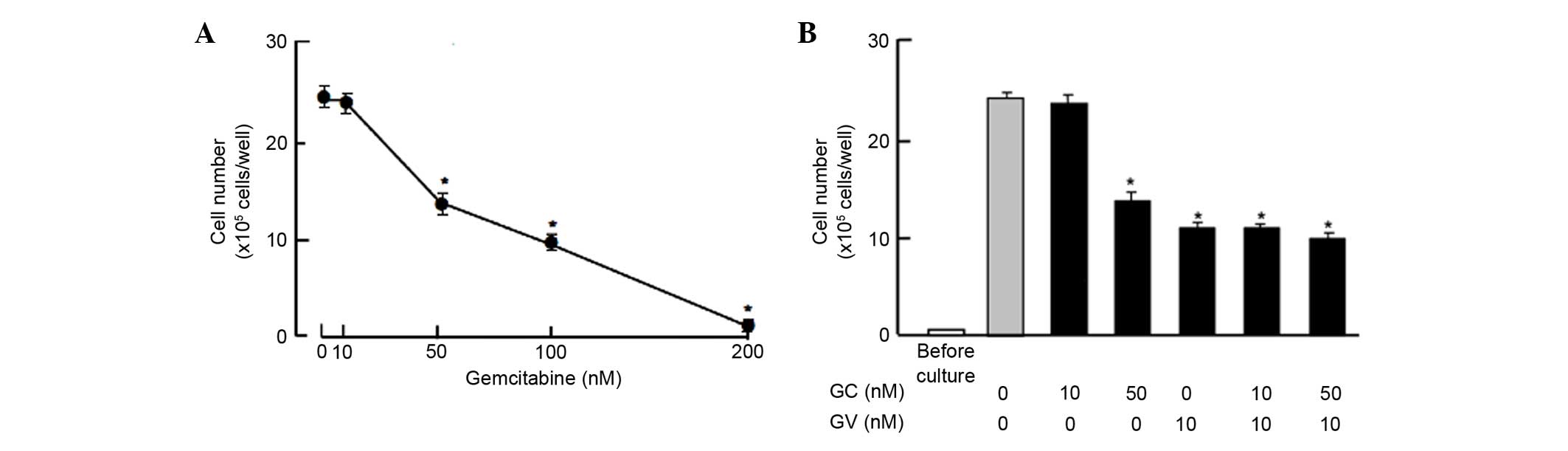
Moreover, the suppressive effects of GV on the
proliferation of the MDA-MB-231 cells were compared with those of
gemcitabine, a strong antitumor agent that induces nuclear DNA
damage (25). Culture with
gemcitabine (50, 100 and 300 nM) for 7 days suppressed cell
proliferation, while such effects were not observed at a
concentration of 10 nM gemcitabine (Fig.
4A). The suppressive effects of gemcitabine (10 and 50 nM) on
proliferation were not potentiated in the presence of GV (10 nM),
which exhibited suppressive effects on the proliferation of the
MDA-MB-231 cells (Fig. 4B).
Discussion
The present study demonstrated that GV exhibits a
potent suppressive effect on the proliferation of human breast
cancer MDA-MB-231 cells in vitro. The suppressive effects of
GV on cell proliferation were characterized using various factors
that inhibit cell cycle-related signaling processes. The
suppressive effects of GV on the proliferation of the MDA-MB-231
cells were not changed by the presence of butyrate, roscovitine or
sulforaphan, which induce cell cycle arrest. Roscovitine is a
potent and selective inhibitor of the cyclin-dependent kinases
cdc2, cdk2m and cdk5 (19),
sulforaphane induces G2/M phase cell cycle arrest (20) and butyrate induces the inhibition of
G1 progression (17). In the present
study, GV was suggested to induce G1 and G2/M phase cell cycle
arrest in the MDA-MB-231 cells.
The suppressive effects of GV on the proliferation
of the MDA-MB-231 cells were not altered in the presence of various
inhibitors that regulate intracellular signaling pathways in
vitro. The suppressive effects of GV on cell proliferation were
not potentiated in the presence of PD98059, an inhibitor of the
ERK/mitogen-activated protein kinase (MAPK) signaling pathway
(21), staurosporin, an inhibitor of
the calcium-dependent protein kinase C signaling pathway (22), or wortmannin, an inhibitor of the
PI3K/Akt signaling pathway (23). GV
appeared to suppress cell proliferation, which is mediated through
the inhibition of various signaling pathways associated with
ERK/MAPK, calcium and PI3/Akt in breast cancer MDA-MB-231
cells.
Moreover, the suppressive effects of GV on cell
proliferation were not altered in the presence of DRB, an inhibitor
of transcriptional activity with RNA polymerase II inhibition
(24). GV may also suppress
transcriptional activity in the nuclei of MDA-MB-231 cells.
Gemcitabine is an antitumor agent that induces nuclear DNA damage
(25). This agent suppresses cell
proliferation and stimulates apoptotic cell death in various types
of cancer cells. In the present study, the effects of GV on
proliferation and cell death were not enhanced in the presence of
gemcitabine in the MDA-MB-231 cells, suggesting that GV partly acts
in a process involved in the action of gemcitabine. Notably, GV
exhibited suppressive effects on cell proliferation at lower
concentrations compared with gemcitabine, indicating that GV
exhibits a potential effect in breast cancer cells. GV may provide
a useful tool as a novel antitumor agent.
GV has been shown to potently prevent TNF-α-induced
suppression of osteoblastic mineralization and RANKL-induced
stimulation of osteoclastogenesis by antagonizing the activation of
NF-κB signaling in preosteoblastic cells and RAW267.4
preosteoclastic cells in vitro (13). Moreover, GV has been demonstrated to
potently prevent suppressed osteoblastic mineralization and
enhanced osteoclastogenesis induced by MDA-MB-231 cells in bone
marrow culture in vitro (26).
From these findings, it has been suggested that GV exhibits a
potent suppressive effect on the activation of NF-κB signaling in
MDA-MB-231 cells.
In conclusion, the present study demonstrated that
GV potently suppresses the proliferation of human breast cancer
MDA-MB-231 cells in vitro, and that this effect of GV has
potential compared with that of gemcitabine, which is clinically
used as an anticancer drug (25). GV
may be a novel useful tool in the prevention and therapy of breast
cancer in vivo.
References
|
1
|
Boyce BF, Yoneda T and Guise TA: Factors
regulating the growth of metastasis cancer in bone. Endocr Relat
Cancer. 6:333–347. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roodman CD: Mechanism of bone metastasis.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akhtari M, Mansuri J, Newman KA, Guise TM
and Seth P: Biology of brest cancer bone metastasis. Cancer Biol
Ther. 7:3–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen YC, Sosnoski DM and Mastro AM: Breast
cancer metastasis to the bone: Mechanisms of bone loss. Breast
Cancer Res. 12:2152010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park BK, Zhang H, Zeng Q, Dai J, Keller
ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, et al: NF-kappaB in
breast cancer cells promotes osteolytic bone metastasis by inducing
osteoclastogenesis via GM-CSF. Nat Med. 13:62–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gonzalez-Suarez E, Jacob AP, Jones J,
Miller R, Roudier-Meyer MP, Enwert R, Pinkas J, Branstetter D and
Dougall WC: RANK ligand mediates progestin-induced mammary
epithelial proliferation and carcinogenesis. Nature. 468:103–107.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berrios RL and Arbiser JL: Effectiveness
of gentian violet and similar products commonly used to treat
pyodermas. Dermatol Clin. 29:69–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perry BN, Govindarajan B, Bhandarkar SS,
Knaus UG, Valo M, Sturk C, Carrillo CO, Sohn A, Cerimele F, Dumont
D, et al: Pharmacologic blockade of angiopoietin-2 is efficacious
against model hemangiomas in mice. J Invest Dermatol.
126:2316–2322. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Zheng Y, Fried LE, Du Y, Montano
SJ, Sohn A, Lefkove B, Holmgren L, Arbiser JL, Holmgren A and Lu J:
Disruption of the mitochondrial thioredoxin system as a cell death
mechanism of cationic triphenylmethanes. Free Radic Biol Med.
50:811–820. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamaguchi M, Vikulina1 T, Arbiser JL and
Weitzmann MN: Suppression of NF-κB activation by gentian violet
promotes osteoblastogenesis and suppresses osteoclastogenesis. Curr
Mol Med. 14:783–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoneda T, Williams PJ, Hiraga T, Niewolna
M and Nishimura R: A bone-seeking clone exhibits different
biological properties from the MDA-MB-231 parental human breast
cancer cells and a brain-seeking clone in vivo and in vitro. J Bone
Miner Res. 16:1486–1495. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi M, Zhu S, Weitzmann MN, Snyder
JP and Shoji M: Curcumin analog UBS109 prevents bone marrow
osteoblastogenesis and osteoclastogenesis disordered by coculture
with breast cancer MDA-MB-231 bone metastatic cells in vitro. Mol
Cell Biochem. 401:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Misawa H, Inagaki S and Yamaguchi M:
Suppression of cell proliferation and deoxyribonucleic acid
synthesis in cloned rat hepatoma H4-II-E cells overexpressing
regucalcin. J Cell Biochem. 84:143–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamaguchi M and Daimon Y: Overexpression
of regucalcin suppresses cell proliferation in cloned rat hepatoma
H4-II-E cells: Involvement of intracellular signaling factors and
cell cycle-related genes. J Cell Biochem. 95:1169–1177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Izumi T and Yamaguchi M: Overexpression of
regucalcin suppresses cell death in cloned rat hepatoma H4-II-E
cells induced by tumor necrosis factor-alpha or thapsigargin. J
Cell Biochem. 92:296–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meijer L, Borgne A, Mulner O, Chong JP,
Blow JJ, Inagaki N, Inagaki M, Deleros JG and Moulinoux JP:
Biochemical and cellular effects of roscovitine, a potent and
selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and
cdk5. Eur J Biochem. 243:527–536. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh SV, Herman-Antosiewice A, Singh AV,
Lew KL, Strivastava SK, Kamath R, Brown KD, Zhang L and Baskaran R:
Sulforaphan-induced G2/M phase cell cycle arrest involves
checkpoint kinase 2-mediated phosphorylation of cell division cycle
25C. J Biol Chem. 279:25813–25822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Wang Y, Ruan W, Wang X and Pan C:
Reversing multidrug resistance in hepatocellular carcinoma cells by
inhibiting extracellular signal-regulated kinase/mitogen-activated
protein kinase signaling pathway activity. Oncol Lett. 8:2333–2339.
2014.PubMed/NCBI
|
|
22
|
Chen QW, Edvinsson L and Xu CB: Role of
ERK/MAPK in endothelin receptor signaling in human aortic smoth
muscle cells. BMC Cell Biol. 10:522009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Serrano-Nascimento C, da Silva Teixeira S,
Nicola JP, Nachbar RT, Masini-Repiso AM and Nunes MT: The acute
inhibitory effect of iodide excess on sodium/iodide symporter
expression and activity involves the PI3K/Akt signaling pathway.
Endocrinology. 155:1145–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palangat M, Grass JA, Langelier MF,
Coulombe B and Landick R: The RPB2 flap loop of human RNA
polymerase II is dispensable for transcription initiation and
elongation. Mol Cell Biol. 31:3312–3325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang SC and Chen YC: Novel therapeutic
targets for pancreatic cancer. World J Gastroenterol.
20:10825–10844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaguchi M, Vikulina T and Weitzmann MN:
Gentian violet inhibits MDA-MB-231 human breast cancer cells
proliferation, and reverses the stimulation of osteoclastogenesis
and suppression of osteoblast activity induced by cancer cells.
Oncol Rep. 34:2156–2162. 2015.PubMed/NCBI
|